Is Conduction System Pacing Going to Be the New Gold Standard for Cardiac Resynchronization Therapy?
Abstract
:1. Introduction
2. Comparison of Implant Procedures
3. Evidence on BiV-CRT
4. Evidence on CSP
5. Direct Comparison of CSP vs. BiV-CRT
6. Future of Role of Conduction System Pacing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Shan, P.; Su, L.; Zhou, X.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A.; Huang, W. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm 2018, 15, 405–412. [Google Scholar] [CrossRef]
- Ye, Y.; Wu, S.; Su, L.; Sheng, X.; Zhang, J.; Wang, B.; Sharma, P.S.; Ellenbogen, K.A.; Su, Y.; Chen, X.; et al. Feasibility and outcomes of upgrading to left bundle branch pacing in patients with pacing-induced cardiomyopathy and infranodal atrioventricular block. Front. Cardiovasc. Med. 2021, 8, 674452. [Google Scholar] [CrossRef]
- Kaza, N.; Htun, V.; Miyazawa, A.; Simader, F.; Porter, B.; Howard, J.P.; Arnold, A.D.; Naraen, A.; Luria, D.; Glikson, M.; et al. Upgrading right ventricular pacemakers to biventricular pacing or conduction system pacing: A systematic review and meta-analysis. EP Europace 2023, 25, 1077–1086. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Schalij, M.J.; Bleeker, G.B.; Holman, E.R.; Bax, J.J. Acceleration-dependent left bundle branch block with severe left ventricular dyssynchrony results in acute heart failure: Are there more patients who benefit from cardiac resynchronization therapy? J. Cardiovasc. Electrophysiol. 2006, 17, 101–103. [Google Scholar] [CrossRef]
- Scolari, F.L.; Silveira, A.D.; Menegazzo, W.R.; Mendes, A.P.C.; Pimentel, M.; Clausell, N.; Goldraich, L.A. Expanding benefits from cardiac resynchronization therapy to exercise-induced left bundle branch block in advanced heart failure. ESC Heart Fail. 2020, 7, 329–333. [Google Scholar] [CrossRef]
- Brignole, M.; Pentimalli, F.; Palmisano, P.; Landolina, M.; Quartieri, F.; Occhetta, E.; Calò, L.; Mascia, G.; Mont, L.; Vernooy, K.; et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: The APAF-CRT mortality trial. Eur. Heart J. 2021, 42, 4731–4739. [Google Scholar] [CrossRef]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023, 20, e17–e91, Advance online publication. [Google Scholar] [CrossRef]
- Deshmukh, P.; Casavant, D.A.; Romanyshyn, M.; Anderson, K. Permanent, direct His-bundle pacing: A novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000, 101, 869–877. [Google Scholar] [CrossRef]
- Dandamudi, G.; Vijayaraman, P. How to perform permanent His bundle pacing in routine clinical practice. Heart Rhythm 2016, 13, 1362–1366. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A novel pacing strategy with low and stable output: Pacing the left bundle branch immediately beyond the conduction block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef]
- Teigeler, T.; Kolominsky, J.; Vo, C.; Shepard, R.K.; Kalahasty, G.; Kron, J.; Huizar, J.F.; Kaszala, K.; Tan, A.Y.; Koneru, J.N.; et al. Intermediate-term performance and safety of His-bundle pacing leads: A single-center experience. Heart Rhythm 2021, 18, 743–749. [Google Scholar] [CrossRef]
- Frausing, M.H.J.P.; Bæk, A.L.; Kristensen, J.; Gerdes, C.; Nielsen, J.C.; Kronborg, M.B. Long-term follow-up of selective and non-selective His bundle pacing leads in patients with atrioventricular block. J. Interv. Card. Electrophysiol. 2023, 66, 1849–1857. [Google Scholar] [CrossRef]
- Grigoryan, A.; Foeldvary, M.; Tribunyan, S.; Bachour, C.; Feldmann, T.; Israel, C. Development of chronic thresholds in His bundle pacing: Single-center experience over 7 years in 426 patients. EP Europace 2024, 26 (Suppl. S1), euae102.433. [Google Scholar] [CrossRef]
- Perino, A.C.; Wang, P.J.; Lloyd, M.; Zanon, F.; Fujiu, K.; Osman, F.; Briongos-Figuero, S.; Sato, T.; Aksu, T.; Jastrzebski, M.; et al. Worldwide survey on implantation of and outcomes for conduction system pacing with His bundle and left bundle branch area pacing leads. J. Interv. Card. Electrophysiol. 2023, 66, 1589–1600. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Ning, X.; Liang, E.; Pang, K.; Yao, Y.; Hua, W.; Zhang, S.; Fan, X. Permanent left bundle branch area pacing for atrioventricular block: Feasibility, safety, and acute effect. Heart Rhythm 2019, 16, 1766–1773. [Google Scholar] [CrossRef]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019, 16, 1791–1796. [Google Scholar] [CrossRef]
- Chahine, J.; Baranowski, B.; Tarakji, K.; Gad, M.M.; Saliba, W.; Rickard, J.; Cantillon, D.J.; Diab, M.; Kanj, M.; Callahan, T.; et al. Cardiac venous injuries: Procedural profiles and outcomes during left ventricular lead placement for cardiac resynchronization therapy. Heart Rhythm 2020, 17, 1298–1303. [Google Scholar] [CrossRef]
- Witt, C.T.; Ng Kam Chuen, M.J.; Kronborg, M.B.; Kristensen, J.; Gerdes, C.; Nielsen, J.C. Non-infective left ventricular lead complications requiring re-intervention following cardiac resynchronization therapy: Prevalence, causes and outcomes. J. Interv. Card. Electrophysiol. 2022, 63, 69–75. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xu, T.; Cai, M.; Xu, L.; Vijayaraman, P.; Sharma, P.S.; Chen, X.; Zheng, R.; Wu, S.; Huang, W. Electrophysiological characteristics and clinical values of left bundle branch current of injury in left bundle branch pacing. J. Cardiovasc. Electrophysiol. 2020, 31, 834–842. [Google Scholar] [CrossRef]
- Chung, W.H.; Wu, H.P.; Wu, M.Y.; Lin, Y.N.; Chen, J.Y.; Lin, K.H.; Chang, K.C. Correlations between myocardial injury current and lead performance in His bundle pacing compared with left bundle branch area pacing and right ventricular septum pacing. J. Interv. Card. Electrophysiol. 2023, 66, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A.; Panikkath, R.; John, K.; Mascarenhas, V.; Bauch, T.D.; Huang, W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019, 16, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Kiełbasa, G.; Curila, K.; Moskal, P.; Bednarek, A.; Rajzer, M.; Vijayaraman, P. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm 2021, 18, 935–943. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Burri, H.; Kiełbasa, G.; Curila, K.; Moskal, P.; Bednarek, A.; Rajzer, M.; Vijayaraman, P. The V6-V1 interpeak interval: A novel criterion for the diagnosis of left bundle branch capture. EP Europace 2022, 24, 40–47. [Google Scholar] [CrossRef]
- Jastrzębski, M. ECG and Pacing Criteria for Differentiating Conduction System Pacing from Myocardial Pacing. Arrhythmia Electrophysiol. Rev. 2021, 10, 172–180. [Google Scholar] [CrossRef]
- Rijks, J.; Sandgren, E.; Lankveld, T.; Van Stipdonk, A.M.W.; Timmer, S.A.J.; Vernooy, K.; Luermans, J.G.L.M. Assessing V6 RWPT and V6-V1 interpeak time as conduction system capture criteria in non-basal LBBAP lead positions. EP Europace 2024, 26, euae102.417. [Google Scholar] [CrossRef]
- Keene, D.; Anselme, F.; Burri, H.; Pérez, Ó.C.; Čurila, K.; Derndorfer, M.; Foley, P.; Gellér, L.; Glikson, M.; Huybrechts, W.; et al. Conduction system pacing, a European survey: Insights from clinical practice. EP Europace 2023, 25, euad019. [Google Scholar] [CrossRef]
- Liu, X.; Gu, M.; Hua, W.; Hu, Y.; Niu, H.X.; Cai, M.; Zhang, N.; Zhang, S. Comparison of electrical characteristics and pacing parameters of pacing different parts of the His-Purkinje system in bradycardia patients. J. Interv. Card. Electrophysiol. 2022, 63, 175–183. [Google Scholar] [CrossRef]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Sinner, M.F.; Joghetaei, N.; Fichtner, S. Early sudden distal conductor fracture of a stylet-driven lead implanted for left bundle branch area pacing. HeartRhythm Case Rep. 2022, 9, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Agudo, C.A.; Jaén, E.G.; Sánchez, D.J.; Urda, V.C.; Ramos, J.T.; Lozano, I.F. Extraction of a fractured pacemaker lead in the left bundle branch area using a snare via a femoral approach. J. Interv. Card. Electrophysiol. 2023, 66, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, V.V.; Ponnusamy, S.S. Late distal conductor fracture of the lumenless pacing lead after left bundle branch area pacing. Heart Rhythm 2024, 21, 490–491. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.; Gillis, K.; Wielandts, J.Y.; De Becker, B.; Knecht, S.; Duytschaever, M.; Tavernier, R.; Le Polain De Waroux, J.B. Septal coronary vein infringement during LBBAP. J. Interv. Card. Electrophysiol. 2023, 66, 507. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yanagisawa, S.; Ota, R.; Murakami, H.; Kada, K.; Tsuboi, N.; Inden, Y.; Murohara, T. Septal coronary artery fistula after left bundle branch area pacing assessed by multi-imaging modalities and shunt volume quantification. Pacing Clin. Electrophysiol. 2022, 45, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Pastori, P.; De Rosa, F.; Vitali, F.; Fasulo, A.; Tortorella, G.; Pastore, M.; Malagù, M.; Bertini, M. Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report-The Devil Is Not So Black as He Is Painted. J. Cardiovasc. Dev. Dis. 2024, 11, 52. [Google Scholar] [CrossRef]
- Cazeau, S.; Ritter, P.; Bakdach, S.; Lazarus, A.; Limousin, M.; Henao, L.; Mundler, O.; Daubert, J.C.; Mugica, J. Four chamber pacing in dilated cardiomyopathy. Pacing Clin. Electrophysiol. 1994, 17, 1974–1979. [Google Scholar] [CrossRef]
- Daubert, J.C.; Ritter, P.; Le Breton, H.; Gras, D.; Leclercq, C.; Lazarus, A.; Mugica, J.; Mabo, P.; Cazeau, S. Permanent Left Ventricular Pacing With Transvenous Leads Inserted Into The Coronary Veins. Pacing Clin. Electrophysiol. 1998, 21, 239–245. [Google Scholar] [CrossRef]
- Cazeau, S.; Leclercq, C.; Lavergne, T.; Walker, S.; Varma, C.; Linde, C.; Garrigue, S.; Kappenberger, L.; Haywood, G.A.; Santini, M.; et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N. Engl. J. Med. 2001, 344, 873–880. [Google Scholar] [CrossRef]
- Auricchio, A.; Stellbrink, C.; Sack, S.; Block, M.; Vogt, J.; Bakker, P.; Huth, C.; Schöndube, F.; Wolfhard, U.; Böcker, D.; et al. long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J. Am. Coll. Cardiol. 2002, 39, 2026–2033. [Google Scholar] [CrossRef]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Linde, C.; Abraham, W.T.; Gold, M.R.; Sutton, M.S.J.; Ghio, S.; Daubert, C.; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fang, F.; Luo, X.; Zhang, Q.; Azlan, H.; Razali, O. Long-term follow-up results of the Pacing to Avoid Cardiac Enlargement (PACE) trial. Eur. J. Heart Fail. 2014, 16, 1016–1025. [Google Scholar] [CrossRef]
- Stockburger, M.; Gómez-Doblas, J.J.; Lamas, G.; Alzueta, J.; Fernández-Lozano, I.; Cobo, E.; Wiegand, U.; de la Concha, J.F.; Navarro, X.; Navarro-López, F.; et al. Preventing ventricular dysfunction in pacemaker patients without advanced heart failure: Results from a multicentre international randomized trial (PREVENT-HF). Eur. J. Heart Fail. 2011, 13, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ploux, S.; Eschalier, R.; Whinnett, Z.I.; Lumens, J.; Derval, N.; Sacher, F.; Hocini, M.; Jaïs, P.; Dubois, R.; Ritter, P.; et al. Electrical dyssynchrony induced by biventricular pacing: Implications for patient selection and therapy improvement. Heart Rhythm 2015, 12, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Thibault, B.; Harel, F.; Ducharme, A.; White, M.; Ellenbogen, K.A.; Frasure-Smith, N.; Roy, D.; Philippon, F.; Dorian, P.; Talajic, M.; et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex< 120 milliseconds: The Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) trial. Circulation 2013, 127, 873–881. [Google Scholar]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J., III; Gras, D. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, C.; Kwok, C.S.; Satchithananda, D.K.; Patwala, A.; Khan, M.A.; Zaidi, A.; Ahmed, F.Z.; Mamas, M.A. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: Meta-analysis of randomized controlled trials. Heart 2015, 101, 14561462. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Sha, Y.Q.; Sun, Q.Y.; Qiu, Y.; Shao, B.; Ni, Y.H.; Mei, Y.K.; Zhang, C.Y.; Wang, R.X. The long-term therapeutic effects of His-Purkinje system pacing on bradycardia and cardiac conduction dysfunction compared with right ventricular pacing: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Naperkowski, A.; Ellenbogen, K.A.; Dandamudi, G. Electrophysiologic insights into site of atrioventricular block: Lessons from permanent His bundle pacing. JACC Clin. Electrophysiol. 2015, 1, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Patel, N.R.; Ravi, V.; Zalavadia, D.V.; Dommaraju, S.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm 2022, 19, 3–11. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Qiu, C.; Wang, Z.; Li, H.; Pang, K.; Yao, Y.; Liu, Z.; Xie, R.; Chen, Y.; et al. Clinical Outcomes in Patients With Left Bundle Branch Area Pacing vs. Right Ventricular Pacing for Atrioventricular Block. Front. Cardiovasc. Med. 2021, 8, 685253. [Google Scholar] [CrossRef]
- Zheng, R.; Yao, H.; Lian, L. His-Purkinje conduction system pacing for pacing-induced cardiomyopathy: A systematic literature review and meta-analysis. J. Interv. Card. Electrophysiol. 2023, 66, 1005–1013. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Travlos, C.; Leventopoulos, G.; Anagnostopoulou, V.; Patrinos, P.; Perperis, A.; Koros, R.; Papageorgiou, A.; Davlouros, P. Left bundle brunch pacing versus conventional right ventricular pacing in patients with bradycardia and conduction system disorders: A systematic review and meta-analysis. EP Europace 2023, 25 (Suppl. S1), euad122.355. [Google Scholar] [CrossRef]
- Teng, A.E.; Massoud, L.; Ajijola, O.A. Physiological mechanisms of QRS narrowing in bundle branch block patients undergoing permanent His bundle pacing. J. Electrocardiol. 2016, 49, 644–648. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Cherian, T.; Shatz, D.Y.; Beaser, A.D.; Aziz, Z.; Ozcan, C.; Broman, M.T.; Nayak, H.M.; Tung, R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation 2019, 139, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova-Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; Liberman, E.; Sheldon, T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm 2015, 12, 1548–1557. [Google Scholar] [CrossRef]
- Vinther, M.; Risum, N.; Svendsen, J.H.; Møgelvang, R.; Philbert, B.T. A randomized trial of His pacing versus biventricular pacing in symptomatic HF patients with left bundle branch block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Ponnusamy, S.; Cano, Ó.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Dal Forno, A.R.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized trial of left bundle branch vs. biventricular pacing for cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Vijayaraman, P.; Zalavadia, D.; Zalavadia, D.; Haseeb, A.; Haseeb, A.; Dye, C.; Dye, C.; Madan, N.; Madan, N.; et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef]
- Herweg, B.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; Whinnett, Z.I.; et al. Arrhythmic Risk in Biventricular Pacing Compared With Left Bundle Branch Area Pacing: Results From the I-CLAS Study. Circulation 2024, 149, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Gabr, M.; Tedrow, U.B.; Duque, M.; Aristizabal, J.; Marin, J.; Niño, C.; Bastidas, O.; Koplan, B.A.; Hoyos, C.; et al. Improved all-cause mortality with left bundle branch area pacing compared to biventricular pacing in cardiac resynchronization therapy: A meta-analysis. J. Interv. Card. Electrophysiol. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tedrow, U.B.; Miranda-Arboleda, A.F.; Sauer, W.H.; Duque, M.; Koplan, B.A.; Marín, J.E.; Aristizabal, J.M.; Niño, C.D.; Bastidas, O.; Martinez, J.M.; et al. Sex Differences in Left Bundle Branch Area Pacing Versus Biventricular Pacing for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2024. [Google Scholar] [CrossRef] [PubMed]

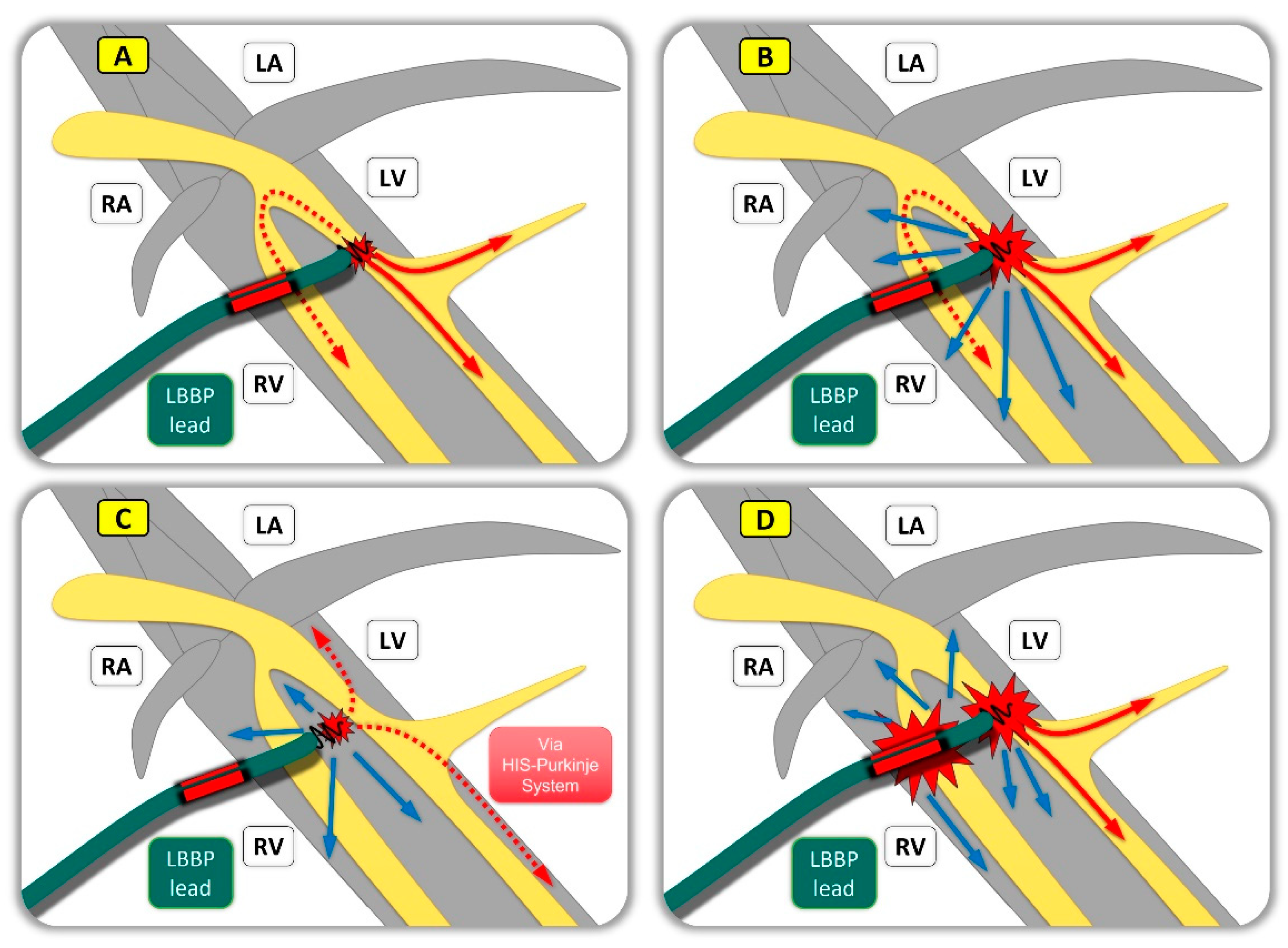
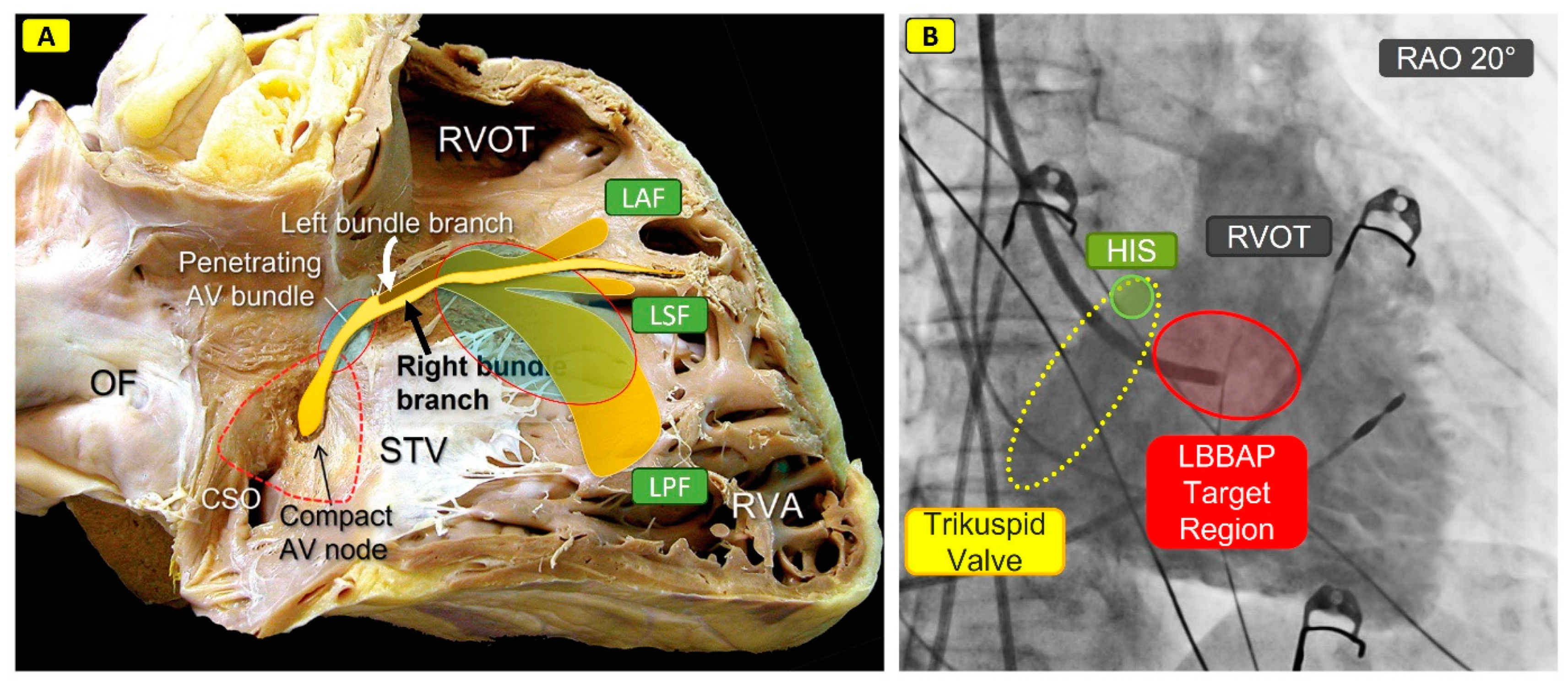
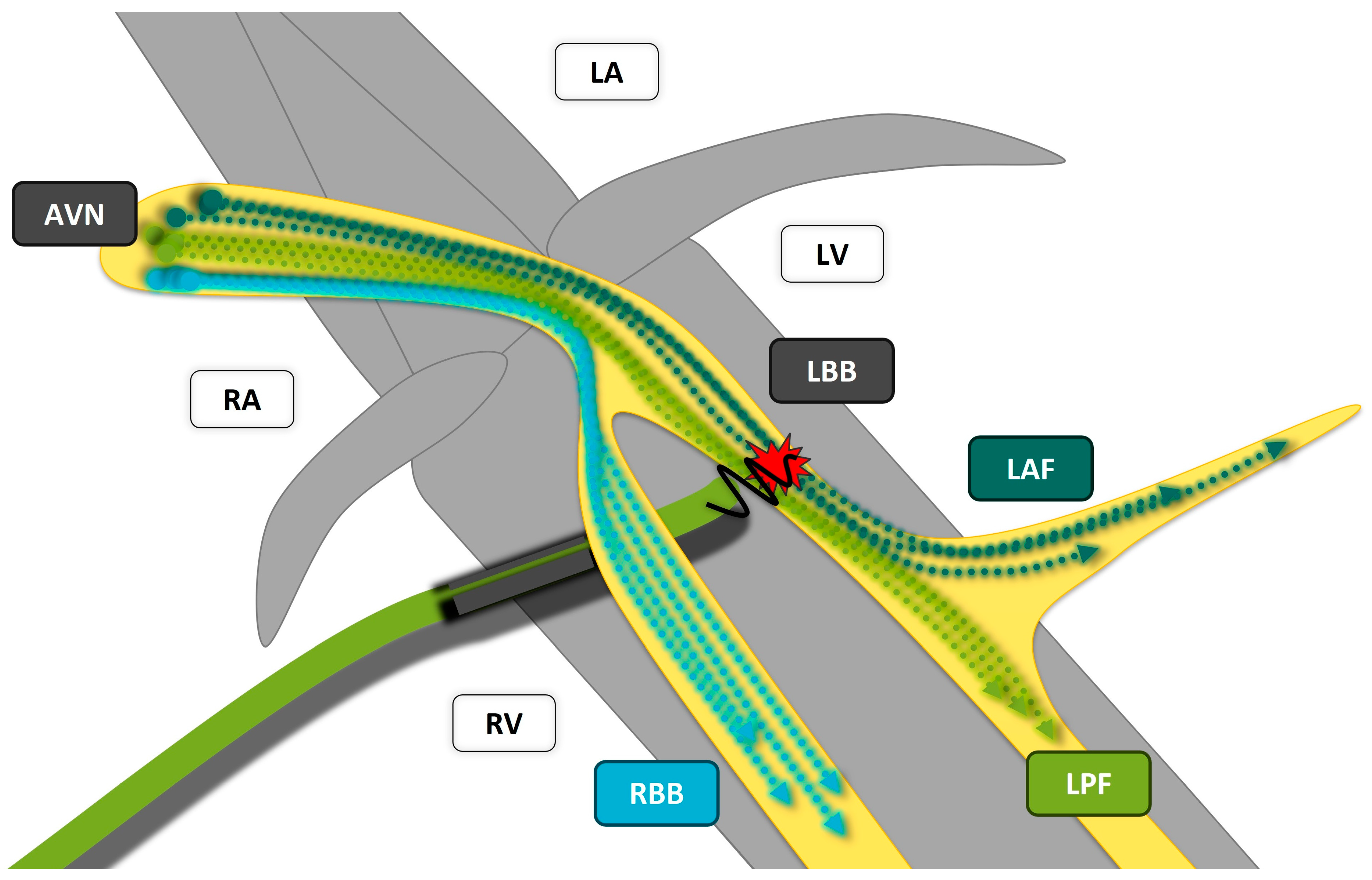

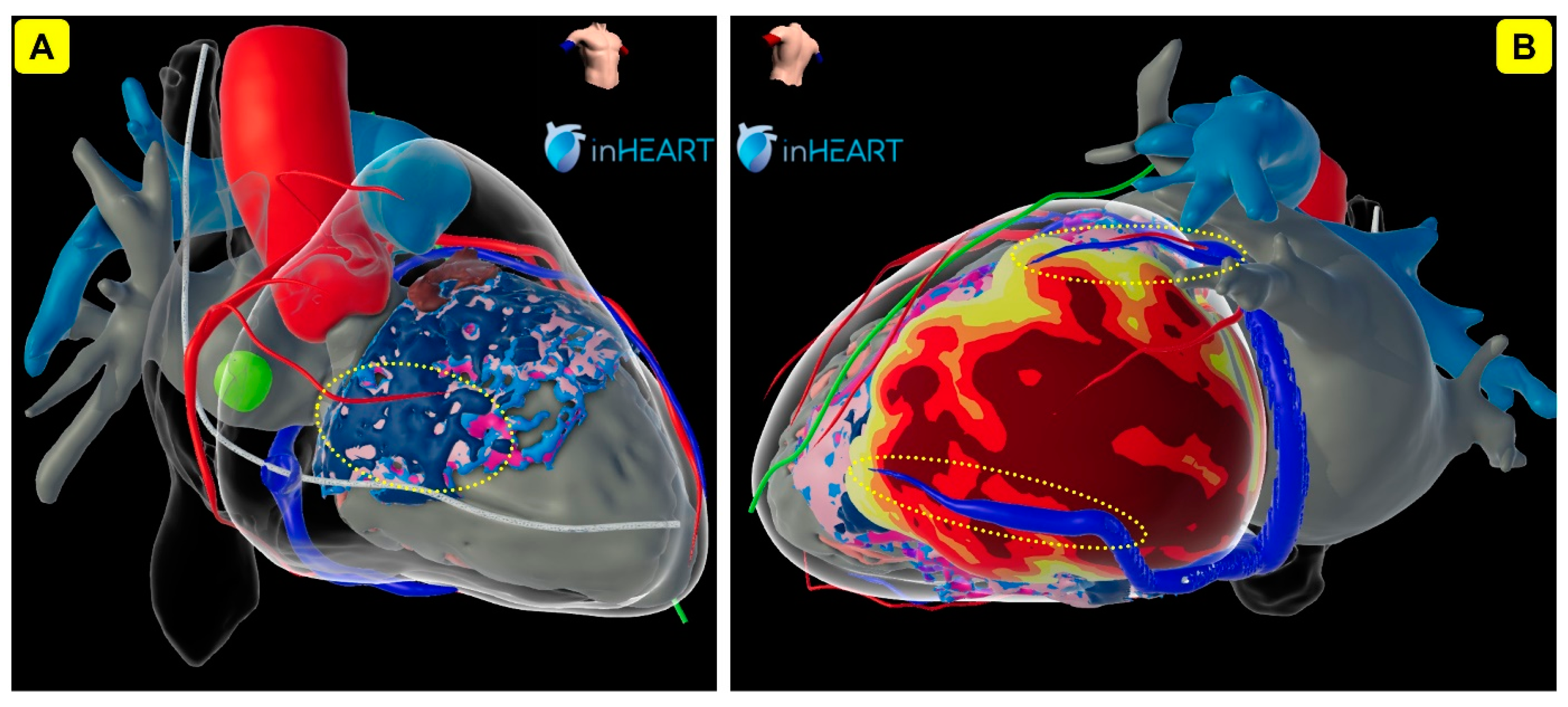
| Study | Study Design | Patients‘ Allocation | Mean FU | Outcomes |
|---|---|---|---|---|
| Lustgarten et al. Heart Rhythm 2015 [65] | Randomize crossover multicenter | HBP: 29 BiV-CRT: 29 pts | 12 month | HBP and BiV-CRT both improved LVEF, NYHA class, 6MWT and QoL significantly |
| “HIS-Alternative” Vinther et al. JACC EP 2021 [66] | Randomized prospective single-center | HBP: 25 pts BiV-CRT: 25 pts | 6 month | LVEF significantly higher and LVESV significantly lower in HBP group at 6 months |
| Vijayaraman et al. JACC EP 2021 [67] | Observational retrospective multicenter | LBBAP: 325 pts | 6 ± 5 month | QRS narrowing; LVEF and NYHA class improvement |
| “LBBP-RESYNC” Wang Y et al. JACC EP 2022 [68] | Randomized prospective multicentre | LBBAP: 20 pts BiV-CRT: 20 pts | 6 month | Greater LVEF improvement and higher reduction in LVESV and NT-proBNP with LBBAP |
| Vijayaraman et al. Heart Rhythm 2022 [69] | Observational retrospective multicenter | HBP: 87 pts LBBAP: 171 pts BiV-CRT: 219 pts | 27 ± 12 month | Superior increase in LVEF with CSP; significantly lower primary outcome (death or HF-related hospitalization with CSP vs. BiV-CRT |
| Vijayaraman et al. JACC 2023 [70] | Observational retrospective multicenter | LBBAP: 797 pts BiV-CRT: 981 pts | 33 ± 16 month | Significant improvement in LVEF with both LBBAP and BiV-CRT but significantly better with LBBAP; Primary outcome (composite of death or HF-related hospitalization) significantly better with LBBAP |
| “I-CLAS” Herweg et al. Circulation 2024 [71] | Observational retrospective multicenter | LBBAP: 797 pts BiV-CRT: 981 pts | 25.2 ± 15 month | Occurrence of VT/VF, VT-storm, new-onset AF > 30 s and AF lasting > 24 h was significantly lower with LBBAP compared with BiV-CRT |
| Diaz et al. JICE, 2024 [72] | Meta-analysis | LBBAP: 1338 pts BiV-CRT: 1901 pts | 25.8 month | Significant reduction in all cause mortality, HF-related hospitalization when compared to BiV-CRT |
| Tedrow et al. JACC 2024 [73] | Observational prospective multicenter | women: LBBAP: 58 pts women: BiV-CRT: 105 pts men: LBBAP: 127 pts men: BiV-CRT: 249 pts | 400.5 days | No sex differences in patients undergoing LBBAP for CRT; Lower risk of HF-hospitalization and all-cause mortality with LBBAP vs. BiV-CRT in men; No difference between LBBAP and BiV-CRT in women |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derndorfer, M.; Kollias, G.; Martinek, M.; Pürerfellner, H. Is Conduction System Pacing Going to Be the New Gold Standard for Cardiac Resynchronization Therapy? J. Clin. Med. 2024, 13, 4320. https://doi.org/10.3390/jcm13154320
Derndorfer M, Kollias G, Martinek M, Pürerfellner H. Is Conduction System Pacing Going to Be the New Gold Standard for Cardiac Resynchronization Therapy? Journal of Clinical Medicine. 2024; 13(15):4320. https://doi.org/10.3390/jcm13154320
Chicago/Turabian StyleDerndorfer, Michael, Georgios Kollias, Martin Martinek, and Helmut Pürerfellner. 2024. "Is Conduction System Pacing Going to Be the New Gold Standard for Cardiac Resynchronization Therapy?" Journal of Clinical Medicine 13, no. 15: 4320. https://doi.org/10.3390/jcm13154320





