Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Cantu, E.; Diamond, J.M.; Suzuki, Y.; Lasky, J.; Schaufler, C.; Lim, B.; Shah, R.; Porteous, M.; Lederer, D.J.; Kawut, S.M.; et al. Quantitative Evidence for Revising the Definition of Primary Graft Dysfunction after Lung Transplant. Am. J. Respir. Crit. Care Med. 2018, 197, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Nath, D.S.; Johnson, A.C.; Walker, A.R.; Prekker, M.E.; Radosevich, D.M.; Herrington, C.S.; Dahlberg, P.S. Risk factors for primary graft dysfunction after lung transplantation. J. Thorac. Cardiovasc. Surg. 2006, 131, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Prekker, M.E.; Herrington, C.S.; Whelan, T.P.; Radosevich, D.M.; Hertz, M.I.; Dahlberg, P.S. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J. Heart Lung Transplant. 2007, 26, 1004–1011. [Google Scholar] [CrossRef]
- Cantu, E.; Diamond, J.M.; Cevasco, M.; Suzuki, Y.; Crespo, M.; Clausen, E.; Dallara, L.; Ramon, C.V.; Harmon, M.T.; Bermudez, C.; et al. Contemporary trends in PGD incidence, outcomes, and therapies. J. Heart Lung Transplant. 2022, 41, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Gouchoe, D.A.; Whitson, B.A.; Rosenheck, J.; Henn, M.C.; Mokadam, N.A.; Ramsammy, V.; Kirkby, S.; Nunley, D.; Ganapathi, A.M. Long-Term Survival Following Primary Graft Dysfunction Development in Lung Transplantation. J. Surg. Res. 2024, 296, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan, C.; DeMissie, E.; Kimmel, S.E. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Sager, J.S.; Kimmel, S.E.; Ahya, V.N.; Gaughan, C.; Blumenthal, N.P.; Kotloff, R.M. Impact of primary graft failure on outcomes following lung transplantation. Chest 2005, 127, 161–165. [Google Scholar] [CrossRef]
- Benazzo, A.; Ali, A.; Aversam, M.; Keshavjee, S.; Cypel, M. Grade 3 primary graft dysfunction after ex-vivo lung perfusion is associated with better short-term outcomes compared to direct transplantation. In Proceedings of the Mechanical Support and Thoracic Transplantation Summit, Toronto, CA, USA, 9–10 June 2023. [Google Scholar]
- Warnecke, G.; Van Raemdonck, D.; Smith, M.A.; Massard, G.; Kukreja, J.; Rea, F.; Loor, G.; De Robertis, F.; Nagendran, J.; Dhital, K.K.; et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): A randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 2018, 6, 357–367. [Google Scholar] [CrossRef]
- Gouchoe, D.A.; Sanchez, P.G.; D’Cunha, J.; Bermudez, C.A.; Daneshmand, M.A.; Davis, R.D.; Hartwig, M.G.; Wozniak, T.C.; Kon, Z.N.; Griffith, B.P.; et al. Ex Vivo Lung Perfusion in Donation after Circulatory Death: A Post-Hoc Analysis of the NOVEL Trial. J. Thorac. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef]
- Gouchoe, D.A.; Lee, Y.G.; Kim, J.L.; Zhang, Z.; Marshall, J.M.; Ganapathi, A.; Zhu, H.; Black, S.M.; Ma, J.; Whitson, B.A. Mitsugumin 53 Mitigation of Ischemia Reperfusion Injury in a Mouse Model. J. Thorac. Cardiovasc. Surg. 2023, 167, e48–e58. [Google Scholar] [CrossRef] [PubMed]
- Gouchoe, D.A.; Yi, T.; Kim, J.L.; Lee, Y.G.; Black, S.M.; Breuer, C.; Ma, J.; Whitson, B.A. MG53 Mitigates Warm Ischemic Lung Injury in A Murine Model of Transplantation. J. Thorac. Cardiovasc. Surg. 2023, 168, e13–e26. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Howard, B.T.; Spratt, J.R.; Mattison, L.M.; Panoskaltsis-Mortari, A.; Brown, R.Z.; Iles, T.L.; Meyer, C.M.; Helms, H.R.; Price, A.; et al. Prolonged EVLP Using OCS Lung: Cellular and Acellular Perfusates. Transplantation 2017, 101, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Warnecke, G.; Villavicencio, M.A.; Smith, M.A.; Kukreja, J.; Ardehali, A.; Hartwig, M.; Daneshmand, M.A.; Hertz, M.I.; Huddleston, S.; et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): A single-arm, pivotal trial. Lancet Respir. Med. 2019, 7, 975–984. [Google Scholar] [CrossRef]
- Yeung, J.C.; Zamel, R.; Klement, W.; Bai, X.H.; Machuca, T.N.; Waddell, T.K.; Liu, M.; Cypel, M.; Keshavjee, S. Towards donor lung recovery-gene expression changes during ex vivo lung perfusion of human lungs. Am. J. Transplant. 2018, 18, 1518–1526. [Google Scholar] [CrossRef]
- Ferdinand, J.R.; Morrison, M.I.; Andreasson, A.; Charlton, C.; Chhatwal, A.K.; Scott, W.E., 3rd; Borthwick, L.A.; Clatworthy, M.R.; Fisher, A.J. Transcriptional analysis identifies potential novel biomarkers associated with successful ex-vivo perfusion of human donor lungs. Clin. Transplant. 2022, 36, e14570. [Google Scholar] [CrossRef]
- Wong, A.; Zamel, R.; Yeung, J.; Bader, G.D.; Dos Santos, C.C.; Bai, X.; Wang, Y.; Keshavjee, S.; Liu, M. Potential therapeutic targets for lung repair during human ex vivo lung perfusion. Eur. Respir. J. 2020, 55, 1902222. [Google Scholar] [CrossRef]
- Miggins, J.J.; Reul, R.M., Jr.; Barrett, S.; Rana, A.; Alnajar, A.; Dunson, J.; Shafii, A.; Garcha, P.; Goss, J.; Loor, G. Twenty-year survival following lung transplantation. J. Thorac. Dis. 2023, 15, 2997–3012. [Google Scholar] [CrossRef]
- Todd, J.L.; Neely, M.L.; Kopetskie, H.; Sever, M.L.; Kirchner, J.; Frankel, C.W.; Snyder, L.D.; Pavlisko, E.N.; Martinu, T.; Tsuang, W.; et al. Risk Factors for Acute Rejection in the First Year after Lung Transplant. A Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 576–585. [Google Scholar] [CrossRef]
- Puri, V.; Patterson, G.A.; Meyers, B.F. Single versus bilateral lung transplantation: Do guidelines exist? Thorac. Surg. Clin. 2015, 25, 47–54. [Google Scholar] [CrossRef]
- Hartwig, M.; van Berkel, V.; Bharat, A.; Cypel, M.; Date, H.; Erasmus, M.; Hoetzenecker, K.; Klepetko, W.; Kon, Z.; Kukreja, J.; et al. The American Association for Thoracic Surgery (AATS) 2022 Expert Consensus Document: The use of mechanical circulatory support in lung transplantation. J. Thorac. Cardiovasc. Surg. 2023, 165, 301–326. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Su, L.; Jiang, S.J. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: A systematic review and meta-analysis. PLoS ONE 2014, 9, e92773. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.J.; Valapour, M.; Gunsalus, P.R.; McKinney, W.T.; Berg, K.A.; Rose, J.; Dalton, J.E. Association of Socioeconomic Position with Racial and Ethnic Disparities in Survival after Lung Transplant. JAMA Netw. Open 2023, 6, e238306. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Weiss, E.S.; Merlo, C.A.; Baumgartner, W.A.; Conte, J.V.; Shah, A.S. Impact of Donor–Recipient Race Matching on Survival after Lung Transplantation: Analysis of over 11,000 Patients. J. Heart Lung Transplant. 2009, 28, 1063–1071. [Google Scholar] [CrossRef]
- Valapour, M.; Lehr, C.J.; Schladt, D.P.; Smith, J.M.; Goff, R.; Mupfudze, T.G.; Swanner, K.; Gauntt, K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Lung. Am. J. Transpl. 2023, 23, S379–S442. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Keshavjee, S.; Levvey, B.; Cherikh, W.S.; Snell, G.; Erasmus, M.; Simon, A.; Glanville, A.R.; Clark, S.; D’Ovidio, F.; et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J. Heart Lung Transplant. 2019, 38, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Hillinger, S.; Schneiter, D.; Opitz, I.; Schuurmans, M.; Benden, C.; Weder, W. Lung Transplantation with Controlled Donation after Circulatory Death Donors. Ann. Thorac. Cardiovasc. Surg. 2018, 24, 296–302. [Google Scholar] [CrossRef]
- Xia, Y.; Kim, S.T.; Dacey, M.; Sayah, D.; Biniwale, R.; Ardehali, A. Characteristics and Outcomes of Lung Transplants Performed with Ex-situ Lung Perfusion. J. Heart Lung Transplant. 2024, 43, 217–225. [Google Scholar] [CrossRef]

| ISHLT Definition | Study Definition | |
|---|---|---|
| No-PGD | No pulmonary edema on chest X-ray with P:F > 300 | P:F > 300 |
| Grade 1 | Pulmonary edema on chest X-ray with P:F > 300 | P:F 200–300 |
| Grade 2 | Pulmonary edema on chest X-ray with P:F 200–300 | P:F 200–300 |
| Grade 3 | Pulmonary edema on chest X-ray with P:F < 200 or ECMO use | P:F < 200 or ECMO at 72 h |
| Variable | Overall (n = 10,342) | No-PGD (n = 5089) | PGD1/2 (n = 3037) | PGD3 (n = 2216) | p-Value |
|---|---|---|---|---|---|
| Age | 61 (53, 66) | 61 (52, 66) | 61 (53, 66) | 61 (53, 67) | 0.002 |
| Male sex | 6079 (58.8%) | 2978 (58.5%) | 1796 (59.1%) | 1305 (58.9%) | 0.854 |
| Race | 0.035 | ||||
| White | 7439 (71.9%) | 3722 (73.1%) | 2161 (71.2%) | 1556 (70.2%) | |
| Black | 1171 (11.3%) | 545 (10.7%) | 343 (11.3%) | 283 (12.8%) | |
| Other | 1732 (16.7%) | 822 (16.2%) | 533 (17.6%) | 377 (17%) | |
| BMI (kg/m2) | 26.3 (22.8, 29.4) | 25.6 (22, 28.8) | 26.8 (23.4, 29.6) | 27.3 (23.8, 30.1) | <0.001 |
| Diabetes | 2072 (20.1%) | 989 (19.5%) | 617 (20.3%) | 466 (21%) | 0.271 |
| Former smoker >20 pack years | 5505 (53.2%) | 2672 (52.5%) | 1633 (53.8%) | 1200 (54.2%) | 0.33 |
| GFR (mL/min/1.73 m2) | 92 (72.2, 120.9) | 93.5 (73.5, 124.3) | 90.8 (71.4, 118.7) | 89.8 (70.7, 115.9) | <0.001 |
| Preoperative dialysis | 52 (13.1%) | 24 (11.8%) | 10 (9.3%) | 18 (20.7%) | 0.048 |
| mPAP (mm Hg) | 26 (21, 33) | 26 (21, 33) | 26 (20.1, 33) | 26 (21, 34) | 0.284 |
| Diagnosis | <0.001 | ||||
| Cystic fibrosis/immunodeficiency | 634 (6.1%) | 411 (8.1%) | 135 (4.4%) | 88 (4%) | |
| Obstructive lung disease | 2100 (20.3%) | 1193 (23.4%) | 549 (18.1%) | 358 (16.2%) | |
| Pulmonary vascular disease | 691 (6.7%) | 300 (5.9%) | 202 (6.7%) | 189 (8.5%) | |
| Restrictive lung disease | 6917 (66.9%) | 3185 (62.6%) | 2151 (70.8%) | 1581 (71.3%) | |
| LAS | 43.3 (36.5, 62.3) | 43.1 (36.1, 62.8) | 43.4 (36.7, 59.9) | 43.5 (37.1, 65.2) | 0.032 |
| Hospitalized prior to transplant | 0.074 | ||||
| Not hospitalized | 7308 (70.7%) | 3596 (70.7%) | 2175 (71.6%) | 1537 (69.4%) | |
| Hospitalized | 1184 (11.5%) | 608 (11.9%) | 334 (11%) | 242 (10.9%) | |
| In ICU | 1848 (17.9%) | 884 (17.4%) | 527 (17.4%) | 437 (19.7%) | |
| Preoperative ventilator | 814 (7.9%) | 397 (7.8%) | 226 (7.4%) | 191 (8.6%) | 0.284 |
| Preoperative ECMO | 978 (9.5%) | 478 (9.4%) | 288 (9.5%) | 212 (9.6%) | 0.971 |
| Days on wait list | 42 (13, 130) | 42 (13, 137) | 43 (13, 128) | 40 (13, 118) | 0.221 |
| Variable | Overall (n = 10,470) | No-PGD (n = 5140) | PGD1/2 (n = 3080) | PGD3 (n = 2250) | p-Value |
|---|---|---|---|---|---|
| Age | 35 (25, 47) | 34 (24, 47) | 35 (25, 48) | 36 (26, 48) | <0.001 |
| Male sex | 6073 (58.7%) | 2993 (58.8%) | 1798 (59.2%) | 1282 (57.9%) | 0.607 |
| Coronary artery disease | 718 (7%) | 337 (6.6%) | 221 (7.3%) | 160 (7.2%) | 0.442 |
| Smoking history | 777 (7.7%) | 326 (6.5%) | 254 (8.5%) | 197 (9.1%) | <0.001 |
| Recent cocaine use | 2053 (20.2%) | 970 (19.4%) | 617 (20.8%) | 466 (21.4%) | 0.099 |
| Diabetes | 898 (8.8%) | 461 (9.1%) | 248 (8.3%) | 189 (8.6%) | 0.376 |
| Hypertension | 2614 (25.5%) | 1306 (25.9%) | 761 (25.4%) | 547 (24.9%) | 0.677 |
| Alcohol abuse | 1785 (17.7%) | 816 (16.5%) | 543 (18.4%) | 426 (19.7%) | 0.002 |
| BMI (kg/m2) | 25.7 (22.6, 29.5) | 25.7 (22.6, 29.5) | 25.9 (22.8, 29.5) | 25.6 (22.5, 29.4) | 0.104 |
| PF ratio | 438 (379, 492) | 445 (388, 496) | 434.5 (375, 486) | 427 (367, 490) | <0.001 |
| Donor cause of death | 0.681 | ||||
| Neuro (seizure/CVA) | 3085 (29.8%) | 1497 (29.4%) | 922 (30.4%) | 666 (30.1%) | |
| Drug overdose | 1523 (14.7%) | 765 (15%) | 436 (14.4%) | 322 (14.5%) | |
| Asphyxiation | 554 (5.4%) | 267 (5.2%) | 175 (5.8%) | 112 (5.1%) | |
| Cardiovascular | 949 (9.2%) | 472 (9.3%) | 280 (9.2%) | 197 (8.9%) | |
| Trauma (GSW/stab/blunt) | 3859 (37.3%) | 1891 (37.2%) | 1121 (36.9%) | 847 (38.2%) | |
| Drowning | 26 (0.3%) | 18 (0.4%) | 6 (0.2%) | 2 (0.1%) | |
| Other | 344 (3.3%) | 178 (3.5%) | 96 (3.2%) | 70 (3.2%) | |
| DCD | 685 (6.6%) | 334 (6.6%) | 207 (6.8%) | 144 (6.5%) | 0.875 |
| Variable | Overall (n = 10,470) | No-PGD (n = 5140) | PGD1/2 (n = 3080) | PGD3 (n = 2250) | p-Value |
|---|---|---|---|---|---|
| Center volume yearly | 26.9 (14.9, 40.7) | 32.1 (15.5, 41) | 24.7 (14.9, 40.7) | 22.8 (13.7, 34.4) | <0.001 |
| Bilateral lung transplant | 8304 (80.3%) | 4245 (83.4%) | 2340 (77%) | 1719 (77.6%) | <0.001 |
| Distance traveled | 163 (45, 311) | 162 (45, 304) | 158 (44, 302) | 173 (51.8, 351) | 0.017 |
| Ischemic time | 5.6 (4.6, 6.8) | 5.6 (4.6, 6.9) | 5.6 (4.5, 6.7) | 5.5 (4.5, 6.6) | <0.001 |
| Length of stay (days) | 23 (15, 41) | 21 (14, 36) | 24 (15, 41) | 27 (17, 51) | <0.001 |
| In-hospital mortality | 607 (6%) | 242 (4.9%) | 168 (5.7%) | 197 (9.2%) | <0.001 |
| Postoperative dialysis | 1260 (12.2%) | 512 (10.1%) | 362 (11.9%) | 386 (17.4%) | <0.001 |
| Postoperative stroke | 338 (3.3%) | 178 (3.5%) | 83 (2.7%) | 77 (3.5%) | 0.14 |
| Airway dehiscence | 221 (2.1%) | 95 (1.9%) | 63 (2.1%) | 63 (2.9%) | 0.029 |
| Postoperative ECMO | 1377 (13.3%) | 626 (12.3%) | 345 (11.4%) | 406 (18.3%) | <0.001 |
| Postoperative ventilator | <0.001 | ||||
| <2 days | 4037 (39.4%) | 2286 (45.4%) | 1112 (36.9%) | 639 (29.1%) | |
| 2–5 days | 2307 (22.5%) | 1129 (22.4%) | 692 (23%) | 486 (22.1%) | |
| 5+ days | 3757 (36.7%) | 1557 (30.9%) | 1164 (38.6%) | 1036 (47.2%) | |
| None | 140 (1.4%) | 62 (1.2%) | 44 (1.5%) | 34 (1.5%) | |
| Acute rejection (hospitalization) | <0.001 | ||||
| Yes and treated with immunosuppressant | 777 (7.5%) | 322 (6.3%) | 244 (8%) | 211 (9.5%) | |
| Yes and not treated with immunosuppressant | 127 (1.2%) | 56 (1.1%) | 35 (1.2%) | 36 (1.6%) | |
| No | 9435 (91.3%) | 4710 (92.6%) | 2757 (90.8%) | 1968 (88.8%) | |
| Treated rejection (1st year) | 1512 (19.8%) | 709 (18.7%) | 472 (20.6%) | 331 (21.4%) | 0.041 |
| Cause of death | 0.102 | ||||
| Graft failure | 516 (16.4%) | 237 (16.3%) | 145 (15.8%) | 134 (17.3%) | |
| Malignancy | 200 (6.4%) | 103 (7.1%) | 57 (6.2%) | 40 (5.2%) | |
| Cardio/cerebrovascular | 415 (13.2%) | 188 (13%) | 125 (13.7%) | 102 (13.2%) | |
| Pulmonary | 647 (20.6%) | 289 (19.9%) | 199 (21.7%) | 159 (20.6%) | |
| Infection | 758 (24.1%) | 339 (23.4%) | 244 (26.7%) | 175 (22.6%) | |
| Other | 603 (19.2%) | 295 (20.3%) | 145 (15.8%) | 163 (21.1%) |
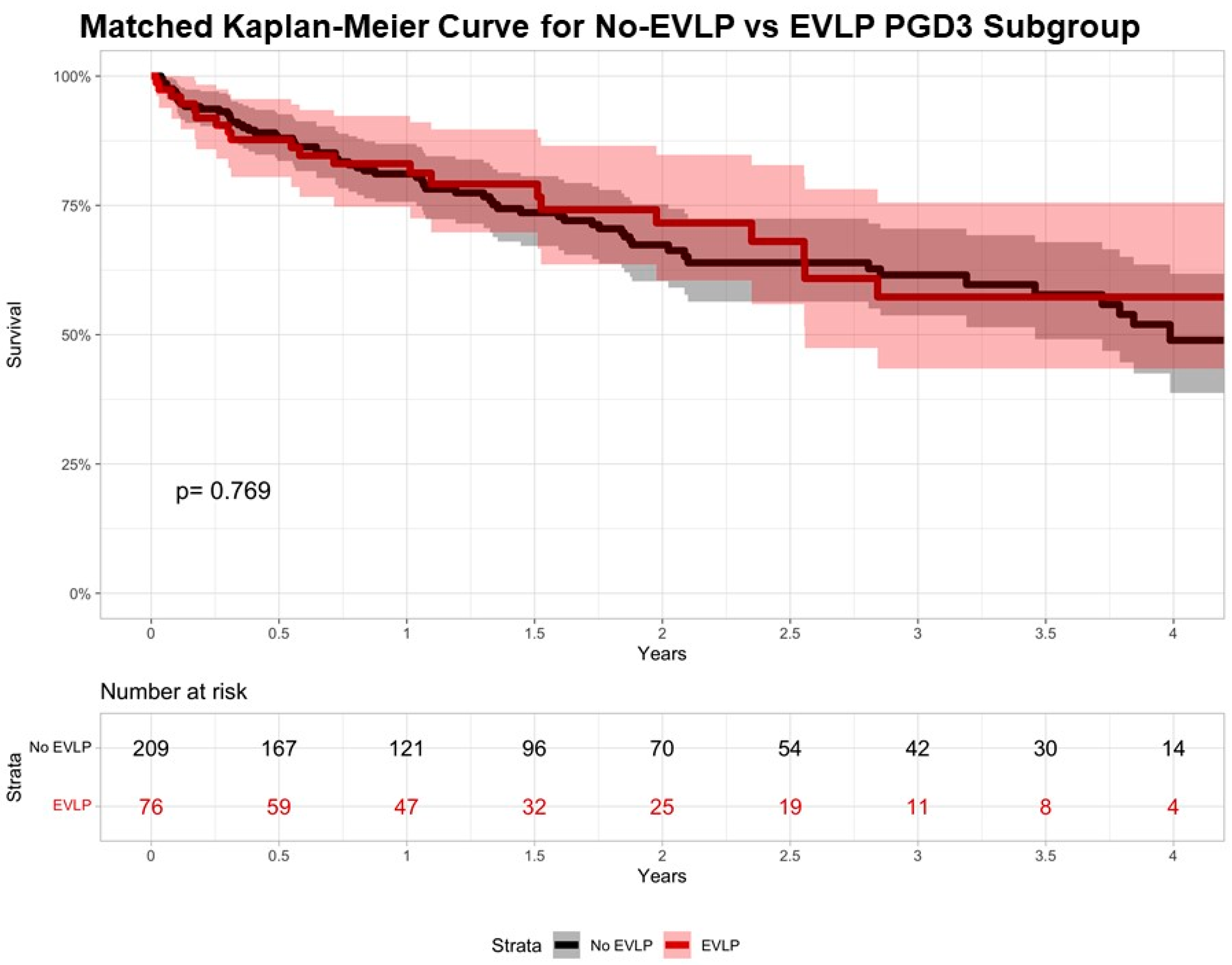
| Variable | Overall (n = 285) | No EVLP (n = 209) | EVLP (n = 76) | p-Value |
|---|---|---|---|---|
| Center volume yearly | 16.7 (11.5, 23.3) | 16.7 (12.3, 23.1) | 16.5 (9.1, 31.1) | 0.561 |
| Bilateral lung transplant | 244 (85.6%) | 179 (85.6%) | 65 (85.5%) | 0.707 |
| Distance traveled | 180 (94, 428) | 157 (76, 314) | 312 (145, 536.8) | <0.001 |
| Ischemic time | 6.4 (5, 8.7) | 5.8 (4.7, 6.9) | 12 (8.3, 15.3) | <0.001 |
| Length of stay (days) | 35 (18, 62) | 36 (18, 64) | 29 (18, 47.8) | 0.29 |
| In-hospital mortality | 23 (8.4%) | 16 (8%) | 7 (9.5%) | 0.887 |
| Postop dialysis | 65 (22.8%) | 47 (22.5%) | 18 (23.7%) | 0.958 |
| Postop stroke | 11 (3.9%) | 9 (4.3%) | 2 (2.6%) | 0.758 |
| Airway dehiscence | 5 (1.8%) | 4 (1.9%) | 1 (1.3%) | 0.999 |
| Postoperative ECMO | 70 (24.6%) | 41 (19.6%) | 29 (38.2%) | 0.002 |
| Postoperative ventilator | 0.869 | |||
| <2 Days | 62 (22.1%) | 48 (23.3%) | 14 (18.9%) | |
| 2–5 Days | 55 (19.6%) | 39 (18.9%) | 16 (21.6%) | |
| 5+ Days | 155 (55.4%) | 113 (54.9%) | 42 (56.8%) | |
| None | 8 (2.9%) | 6 (2.9%) | 2 (2.7%) | |
| Acute rejection (hospitalization) | 0.369 | |||
| Yes and treated with immunosuppressant | 32 (11.2%) | 22 (10.5%) | 10 (13.2%) | |
| Yes and not treated with immunosuppressant | 9 (3.2%) | 5 (2.4%) | 4 (5.3%) | |
| No | 244 (85.6%) | 182 (87.1%) | 62 (81.6%) | |
| Treated rejection (1st year) | 42 (21.4%) | 27 (18.8%) | 15 (28.8%) | 0.186 |
| Cause of death | 0.694 | |||
| Graft failure | 15 (17.2%) | 12 (18.2%) | 3 (14.3%) | |
| Malignancy | 2 (2.3%) | 2 (3%) | 0 (0%) | |
| Cardio/cerebrovascular | 11 (12.6%) | 8 (12.1%) | 3 (14.3%) | |
| Pulmonary | 25 (28.7%) | 21 (31.8%) | 4 (19%) | |
| Infection | 24 (27.6%) | 16 (24.2%) | 8 (38.1%) | |
| Other | 10 (11.5%) | 7 (10.6%) | 3 (14.3%) | |
| Perfused by | ||||
| Organ procurement organization | 2 (2.7%) | |||
| Transplant program | 49 (65.3%) | |||
| External perfusion center | 24 (32%) | |||
| Perfusion time (minutes) | 254.5 (219.8, 345.8) |
| Variable | Odds Ratio | Lower Bound | Upper Bound | p-Value |
|---|---|---|---|---|
| EVLP use | ||||
| No | Reference | |||
| Yes | 0.70 | 0.57 | 0.86 | <0.001 |
| Recipient age | 1.00 | 0.99 | 1.00 | 0.81 |
| Recipient BMI | 1.06 | 1.05 | 1.07 | <0.001 |
| Recipient glomerular filtration rate | 1.00 | 1.00 | 1.00 | 0.49 |
| Recipient medical condition | ||||
| Not hospitalized | Reference | |||
| Hospitalized | 0.91 | 0.77 | 1.08 | 0.28 |
| In intensive care unit | 1.05 | 0.88 | 1.24 | 0.61 |
| Lung allocation score | 1.00 | 1.00 | 1.01 | 0.08 |
| Recipient days on waitlist | 1.00 | 1.00 | 1.00 | 0.37 |
| Donor age | 1.01 | 1.00 | 1.01 | 0.001 |
| Donor race | ||||
| White | Reference | |||
| Black | 0.67 | 0.59 | 0.77 | <0.001 |
| Hispanic | 0.79 | 0.69 | 0.89 | <0.001 |
| Asian | 0.69 | 0.53 | 0.91 | 0.008 |
| Other | 0.67 | 0.42 | 1.07 | 0.09 |
| Donor cigarette use | ||||
| No | Reference | |||
| Yes | 1.16 | 0.96 | 1.39 | 0.12 |
| Donor PaO2/FiO2 ratio | 1.00 | 1.00 | 1.00 | 0.15 |
| Transplant laterality | ||||
| Bilateral | Reference | |||
| Right-single | 1.50 | 1.25 | 1.79 | <0.001 |
| Left-single | 1.39 | 1.17 | 1.65 | <0.001 |
| Transplant year | 0.97 | 0.94 | 1.01 | 0.11 |
| Yearly center volume | 1.00 | 1.00 | 1.00 | <0.001 |
| Variable | Odds Ratio | Lower Bound | Upper Bound | p-Value |
|---|---|---|---|---|
| EVLP use | ||||
| No | Reference | |||
| Yes | 1.16 | 0.95 | 1.43 | 0.15 |
| Primary graft dysfunction | ||||
| No-PGD | Reference | |||
| PGD 1/2 | 0.99 | 0.88 | 1.11 | 0.89 |
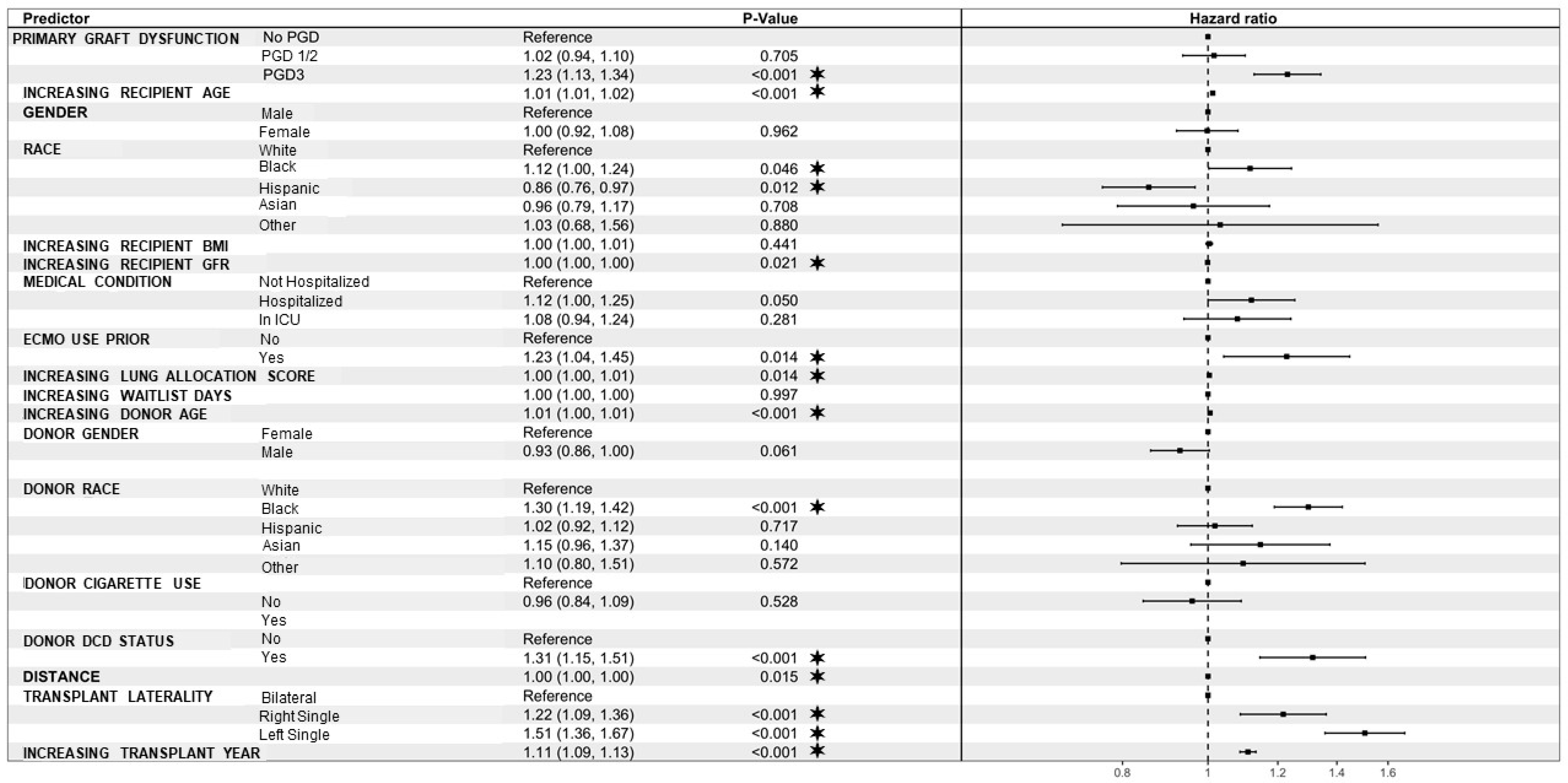
| PGD 3 | 1.13 | 0.99 | 1.28 | 0.07 |
| Recipient age | 1.01 | 1.01 | 1.02 | <0.001 |
| Recipient BMI | 1.01 | 1.00 | 1.02 | 0.06 |
| Recipient smoking history | ||||
| No | Reference | |||
| Yes | 0.86 | 0.78 | 0.96 | 0.01 |
| Recipient medical condition | ||||
| Not hospitalized | Reference | |||
| Hospitalized | 1.26 | 1.07 | 1.48 | 0.01 |
| In intensive care unit | 1.11 | 0.92 | 1.35 | 0.28 |
| ECMO use prior | ||||
| No | Reference | |||
| Yes | 1.26 | 1.01 | 1.58 | 0.04 |
| Lung allocation score | 1.00 | 1.00 | 1.01 | 0.50 |
| Recipient days on waitlist | 1.00 | 1.00 | 1.00 | 0.02 |
| Donor age | 1.01 | 1.00 | 1.01 | <0.001 |
| Donor race | ||||
| White | Reference | |||
| Black | 1.30 | 1.14 | 1.49 | <0.001 |
| Hispanic | 1.06 | 0.93 | 1.21 | 0.37 |
| Asian | 1.25 | 0.95 | 1.64 | 0.11 |
| Other | 1.11 | 0.70 | 1.78 | 0.65 |
| Donation after circulatory death donor | ||||
| No | Reference | |||
| Yes | 1.35 | 1.12 | 1.63 | <0.001 |
| Donor PaO2/FiO2 ratio | 1.00 | 1.00 | 1.00 | 0.02 |
| Donor cocaine use | ||||
| No | Reference | |||
| Yes | 1.11 | 0.98 | 1.25 | 0.09 |
| Transplant laterality | ||||
| Bilateral | Reference | |||
| Right-single | 0.83 | 0.69 | 0.99 | 0.04 |
| Left-single | 1.04 | 0.87 | 1.23 | 0.68 |
| Distance | 1.00 | 1.00 | 1.00 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouchoe, D.A.; Cui, E.Y.; Satija, D.; Henn, M.C.; Choi, K.; Rosenheck, J.P.; Nunley, D.R.; Mokadam, N.A.; Ganapathi, A.M.; Whitson, B.A. Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis. J. Clin. Med. 2024, 13, 4440. https://doi.org/10.3390/jcm13154440
Gouchoe DA, Cui EY, Satija D, Henn MC, Choi K, Rosenheck JP, Nunley DR, Mokadam NA, Ganapathi AM, Whitson BA. Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis. Journal of Clinical Medicine. 2024; 13(15):4440. https://doi.org/10.3390/jcm13154440
Chicago/Turabian StyleGouchoe, Doug A., Ervin Y. Cui, Divyaam Satija, Matthew C. Henn, Kukbin Choi, Justin P. Rosenheck, David R. Nunley, Nahush A. Mokadam, Asvin M. Ganapathi, and Bryan A. Whitson. 2024. "Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis" Journal of Clinical Medicine 13, no. 15: 4440. https://doi.org/10.3390/jcm13154440
APA StyleGouchoe, D. A., Cui, E. Y., Satija, D., Henn, M. C., Choi, K., Rosenheck, J. P., Nunley, D. R., Mokadam, N. A., Ganapathi, A. M., & Whitson, B. A. (2024). Ex Vivo Lung Perfusion and Primary Graft Dysfunction Following Lung Transplantation: A Contemporary United Network for Organ Sharing Database Analysis. Journal of Clinical Medicine, 13(15), 4440. https://doi.org/10.3390/jcm13154440






