Ultrasound for Intra-Operative Detection of Peri-Centimetric Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery (VATS): A Comparison with Conventional Techniques in Multiportal VATS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
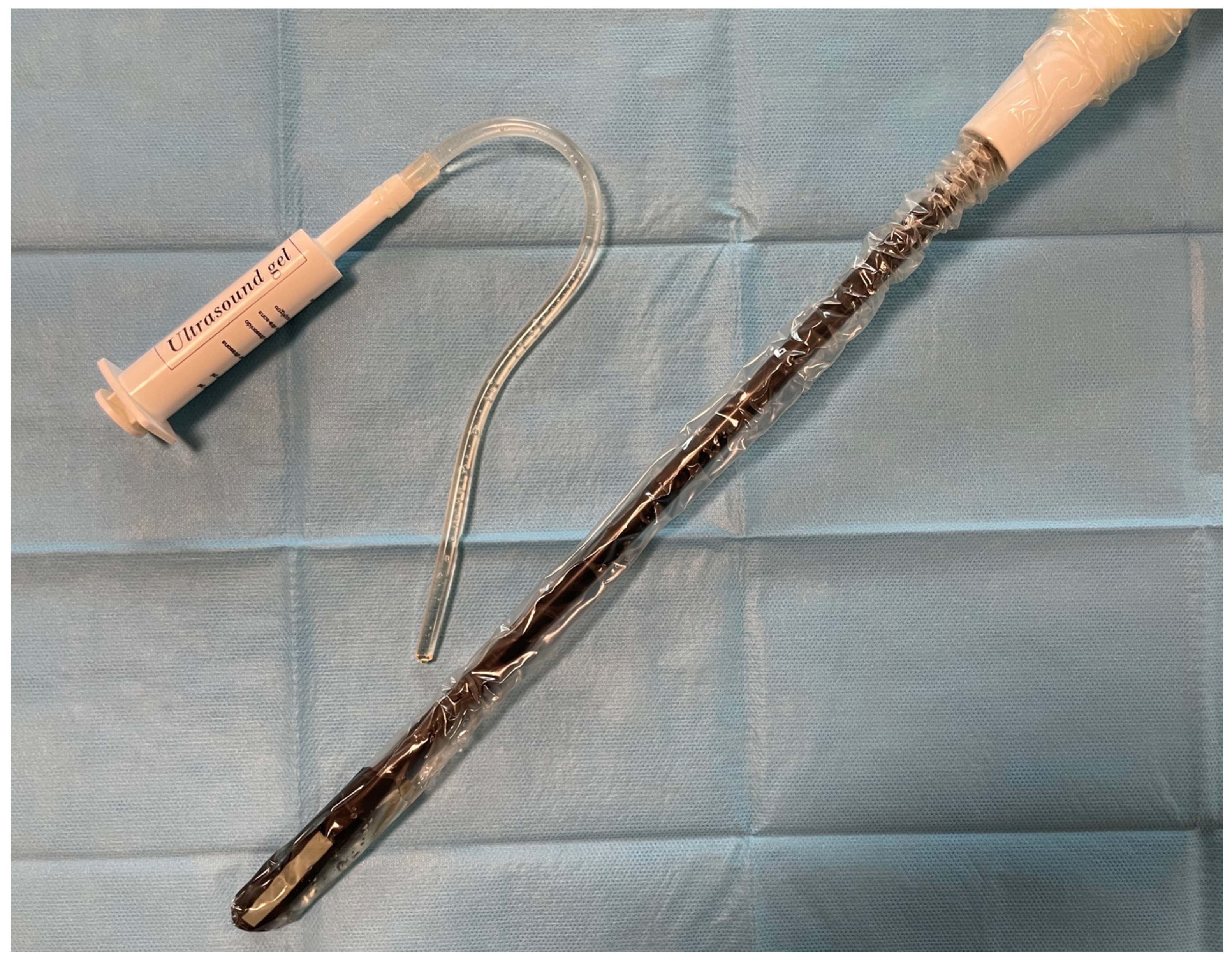
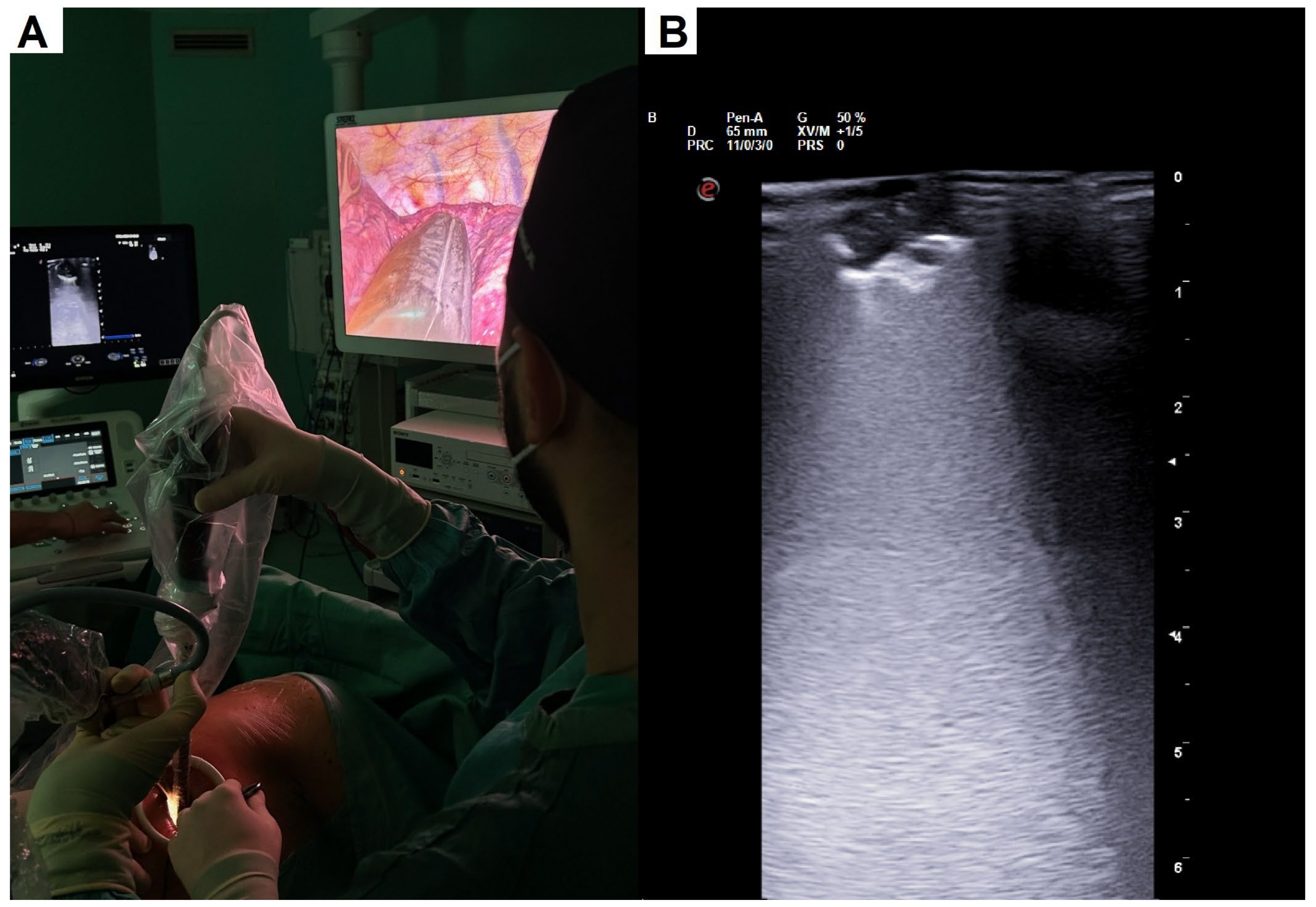
2.2. Surgical Details
2.3. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Intra-Operative Results and Post-Operative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung cancer screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, R.; Shi, Z.; Cheng, S. Uniport video assisted thoracoscopic surgery (U-VATS) exhibits increased feasibility, non-inferior tolerance, and equal efficiency compared with multiport VATS and open thoracotomy in the elderly non-small cell lung cancer patients at early stage. Medicine 2019, 98, e16137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sui, X.; Zhao, H.; Yang, F.; Li, J.L.; Wang, J. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J. Thorac. Dis. 2015, 7, 1580–1587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mogi, A.; Yajima, T.; Tomizawa, K.; Onozato, R.; Tanaka, S.; Kuwano, H. Video-Assisted Thoracoscopic Surgery after Preoperative CT-Guided Lipiodol Marking of Small or Impalpable Pulmonary Nodules. Ann. Thorac. Cardiovasc. Surg. 2015, 21, 435–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellomi, M.; Veronesi, G.; Trifirò, G.; Brambilla, S.; Bonello, L.; Preda, L.; Casiraghi, M.; Borri, A.; Paganelli, G.; Spaggiari, L. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann. Thorac. Surg. 2010, 90, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kuriyama, K.; Inoue, A.; Yoshida, Y.; Takami, K. Computed tomography-guided preoperative localization of small lung nodules with indocyanine green. Acta Radiol. 2018, 59, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Findik, G.; Demiröz, S.M.; Apaydın, S.M.K.; Ertürk, H.; Biri, S.; Incekara, F.; Aydogdu, K.; Kaya, S. Computed Tomography-Guided Methylene Blue Labeling Prior to Thoracoscopic Resection of Small Deeply Placed Pulmonary Nodules. Do We Really Need Palpation? Thorac. Cardiovasc. Surg. 2017, 65, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hirata, T.; Ogawa, E.; Fukuse, T.; Ueda, H.; Koyama, T.; Nakamura, T.; Wada, H. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS). Eur. J. Cardiothorac. Surg. 2004, 26, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Partlow, J.; Thomas, S.; Nicolini, M.; Greeno, S.; Schroeder, C. Image-Guided VATS in the Hybrid Operation Room Facilitates Early Diagnosis and Concurrent Treatment of Subcentimeter Nonpalpable Lung Nodules. Innovations 2024, 19, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Machi, J.; Sigel, B.; Zaren, H.A.; Schwartz, J.; Hosokawa, T.; Kitamura, H.; Kolecki, R.V. Technique of ultrasound examination during laparoscopic cholecystectomy. Surg. Endosc. 1993, 7, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Rotondo, A.; Guglielmi, G.; Catalano, D.; Feragalli, B.; Trovato, G.M. Transthoracic ultrasound in the assessment of pleural and pulmonary diseases: Use and limitations. Radiol. Med. 2014, 119, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, A.L.; Steiner, R.M.; Liu, J.B.; Cohn, H.E.; Goldberg, B.B.; Rawool, N.M.; Merton, D.A. Sonographic guidance for the localization of peripheral pulmonary nodules during thoracoscopy. Am. J. Roentgenol. 1997, 168, 1057–1060, Erratum in Am. J. Roentgenol. 1997, 168, 1625. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, R.; Montorsi, M.; Bianchi, P.; Mantovani, A.; Ghelma, F.; Mezzetti, M. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann. Thorac. Surg. 1999, 68, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Rocco, G.; Cicalese, M.; La Manna, C.; La Rocca, A.; Martucci, N.; Salvi, R. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann. Thorac. Surg. 2011, 92, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Taurchini, M.; Quarato, C.M.I.; Frongillo, E.M.; Ferretti, G.M.; Cipriani, C.; Bizzarri, M.; Foschino Barbaro, M.P.; Lacedonia, D.; Simeone, A.; Graziano, P.; et al. Intraoperative Lung Ultrasound (ILU) for the Assessment of Pulmonary Nodules. Diagnostics 2021, 11, 1691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.H.; Chen, K.C.; Chen, J.S. Ultrasound for intraoperative localization of lung nodules during thoracoscopic surgery. Ann. Transl. Med. 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khereba, M.; Ferraro, P.; Duranceau, A.; Martin, J.; Goudie, E.; Thiffault, V.; Liberman, M. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J. Thorac. Cardiovasc. Surg. 2012, 144, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Messina, G.; Pica, D.G.; Bove, M.; Capasso, F.; Mirra, R.; Natale, G.; D’Alba, F.P.; Caputo, A.; Leonardi, B.; et al. Intraoperative lung ultrasound improves subcentimetric pulmonary nodule localization during VATS: Results of a retrospective analysis. Thorac. Cancer. 2023, 14, 2558–2566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schauer, M.I.; Jung, E.M.; Platz Batista da Silva, N.; Akers, M.; Loch, E.; Markowiak, T.; Piler, T.; Larisch, C.; Neu, R.; Stroszczynski, C.; et al. Intraoperative Contrast-Enhanced Ultrasonography (Io-CEUS) in Minimally Invasive Thoracic Surgery for Characterization of Pulmonary Tumours: A Clinical Feasibility Study. Cancers 2023, 15, 3854. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sperandeo, M.; Venuti, M.; Quarato, C.M.I. Uniportal versus multiportal video-assisted thoracic surgery for lung cancer: Safety and advantages in employing complementary intraoperative lung ultrasound. J. Thorac. Dis. 2020, 12, 3013–3017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messina, G.; Bove, M.; Natale, G.; Noro, A.; Martone, M.; Opromolla, G.; Di Filippo, V.; Leonardi, B.; Fasano, M.; Polito, R.; et al. Ultrasound location of ground-glass opacity during thoracoscopic surgery. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variable | Group A (n = 31) | Group B (n = 33) | p-Value |
|---|---|---|---|
| Median age, years (IQR) | 70 (66–76) | 71 (65.7–75.2) | 0.99 |
| Gender, n (%) | 0.81 | ||
| male | 16 (51.6%) | 18 (54.5%) | |
| female | 15 (48.4%) | 15 (45.5%) | |
| Nodule diameter, mm (IQR) | 11 (9–13) | 11 (9–12) | 0.92 |
| Nodule location, n (%) | 0.85 | ||
| right upper lobe | 8 (25.8%) | 9 (27.3%) | |
| middle lobe | 2 (6.5%) | 3 (9.1%) | |
| right inferior lobe | 4 (12.9%) | 6 (18.2%) | |
| left upper lobe | 9 (29.0%) | 10 (30.3%) | |
| left inferior lobe | 8 (25.8%) | 5 (15.1%) | |
| Final histology, n (%) | 0.47 | ||
| Adenocarcinoma | 14 (45.2%) | 18 (54.5%) | |
| Squamous cell carcinoma | 9 (29.0%) | 5 (15.2%) | |
| Pulmonary metastasis | 2 (6.5%) | 1 (3.0%) | |
| Benign lesion | 6 (19.3%) | 9 (27.3%) |
| Variable | Group A (n = 31) | Group B (n = 33) | p-Value |
|---|---|---|---|
| Detection time, minutes (IQR) | 9 (8–10) | 14 (12.5–15) | <0.001 |
| Operative time, minutes (IQR) | 33 (29–38) | 43 (39–47) | <0.001 |
| Nodule distance from pleural surface, mm (IQR) | 27 (25–28) | 26 (24–28) | 0.54 |
| Need of additional wedge, n (%) | 0.086 | ||
| yes | 0 (0.0%) | 3 (9.1%) | |
| no | 31 (100.0%) | 30 (90.9%) | |
| Post-operative complication, n (%) | 0.16 | ||
| yes | 0 (0.0%) | 2 (6.1%) | |
| no | 31 (100.0%) | 31 (93.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastone, S.A.; Patirelis, A.; Luppichini, M.; Ambrogi, V. Ultrasound for Intra-Operative Detection of Peri-Centimetric Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery (VATS): A Comparison with Conventional Techniques in Multiportal VATS. J. Clin. Med. 2024, 13, 4448. https://doi.org/10.3390/jcm13154448
Bastone SA, Patirelis A, Luppichini M, Ambrogi V. Ultrasound for Intra-Operative Detection of Peri-Centimetric Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery (VATS): A Comparison with Conventional Techniques in Multiportal VATS. Journal of Clinical Medicine. 2024; 13(15):4448. https://doi.org/10.3390/jcm13154448
Chicago/Turabian StyleBastone, Sebastiano Angelo, Alexandro Patirelis, Matilde Luppichini, and Vincenzo Ambrogi. 2024. "Ultrasound for Intra-Operative Detection of Peri-Centimetric Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery (VATS): A Comparison with Conventional Techniques in Multiportal VATS" Journal of Clinical Medicine 13, no. 15: 4448. https://doi.org/10.3390/jcm13154448
APA StyleBastone, S. A., Patirelis, A., Luppichini, M., & Ambrogi, V. (2024). Ultrasound for Intra-Operative Detection of Peri-Centimetric Pulmonary Nodules in Uniportal Video-Assisted Thoracic Surgery (VATS): A Comparison with Conventional Techniques in Multiportal VATS. Journal of Clinical Medicine, 13(15), 4448. https://doi.org/10.3390/jcm13154448






