Transoral Robotic Surgery and Human Papillomavirus Infection: Impact on Oropharyngeal Cancer Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Abstraction
2.3. Specimen Collection, Processing, and DNA Isolation
2.4. Nested PCR Amplification and Genome Sequencing
2.5. Protein Structure Prediction and Visualization
2.6. Affinity Prediction of MHC Binding to HPV Protein
2.7. Statistical Analyses
2.8. Ethical Approval
3. Results
3.1. Participant Characteristics
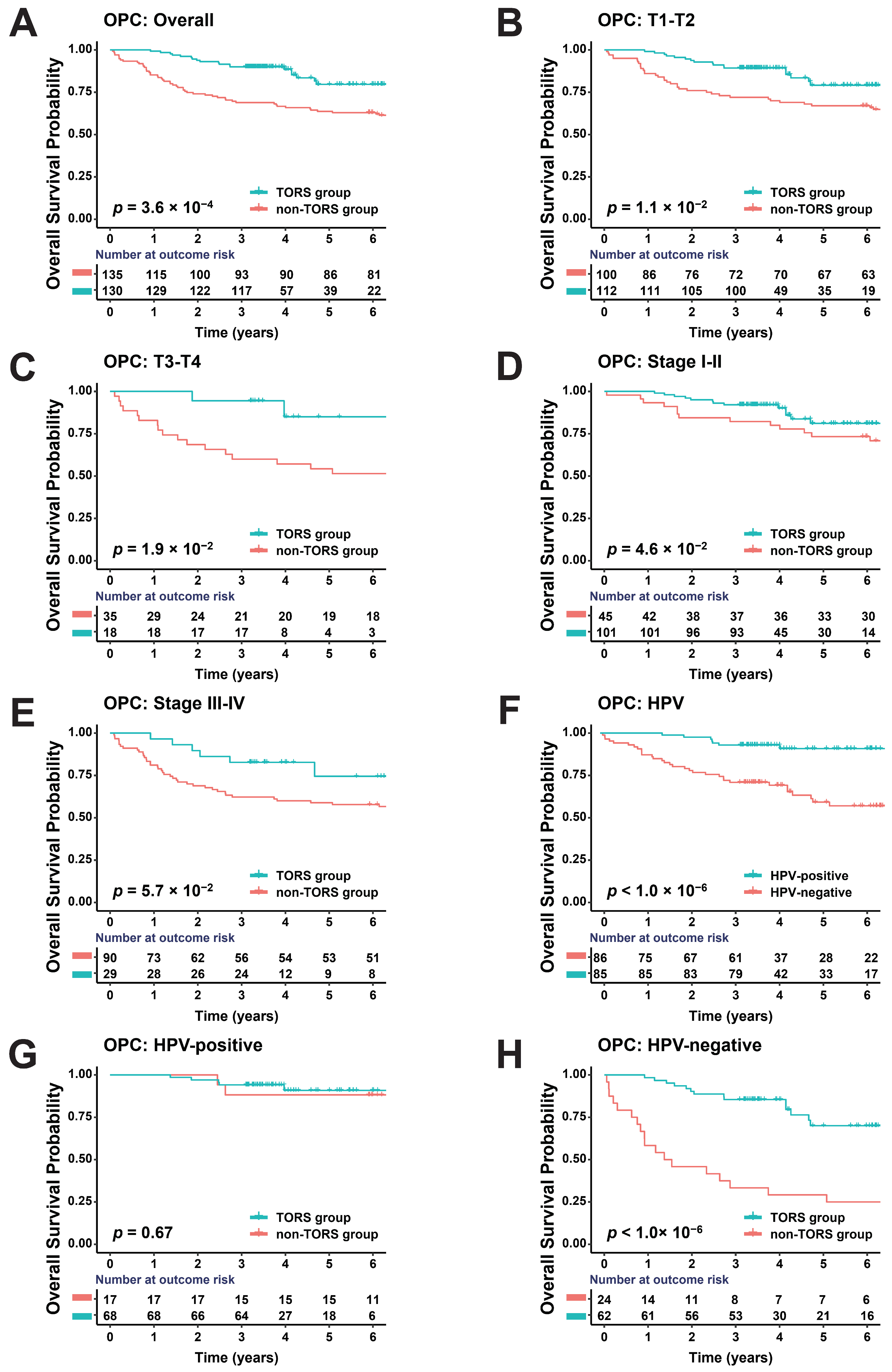
3.2. TORS Can Improve Prognosis of OPC
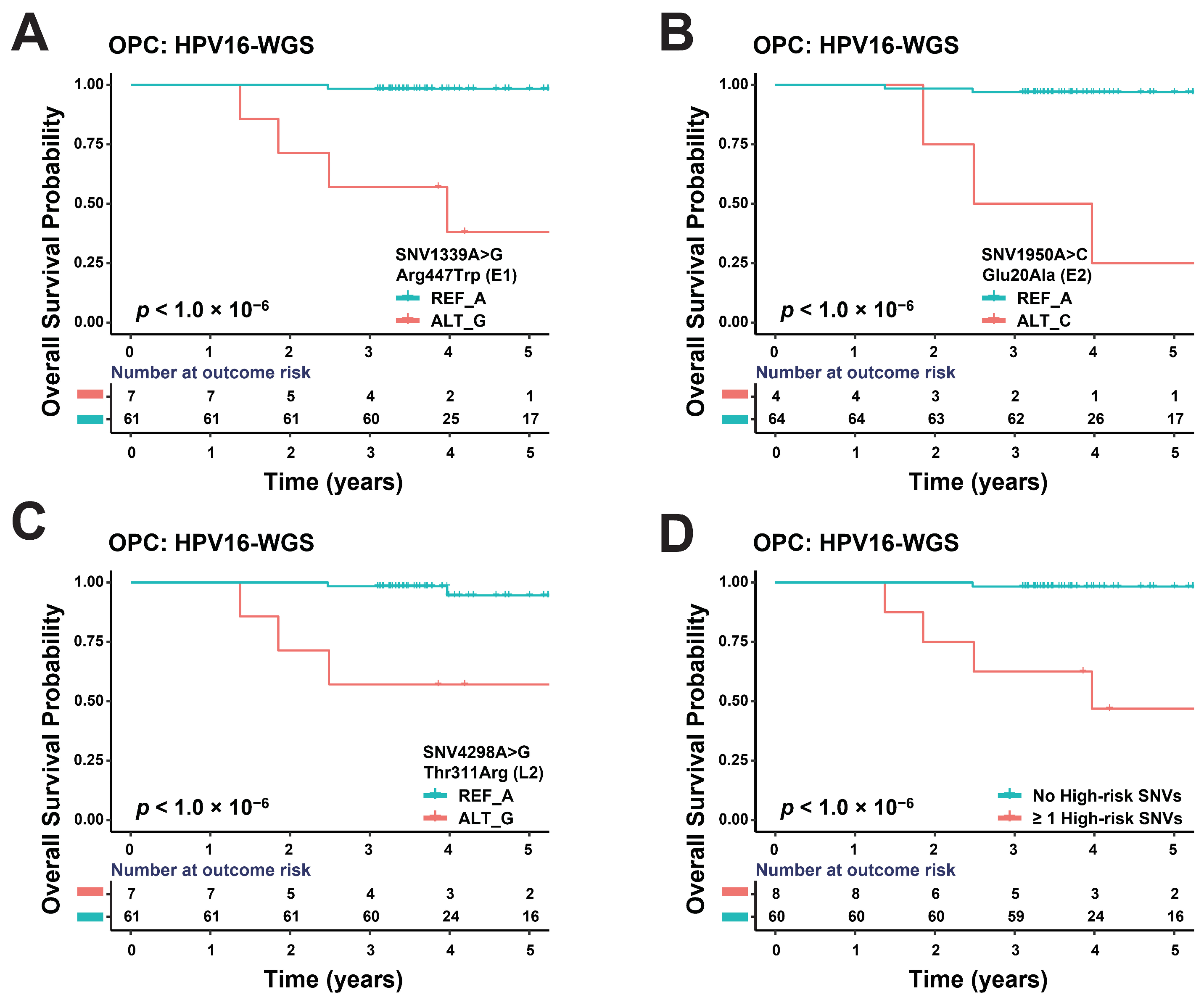
3.3. Some SNVs of HPV16 Genome May Affect OPC Prognosis
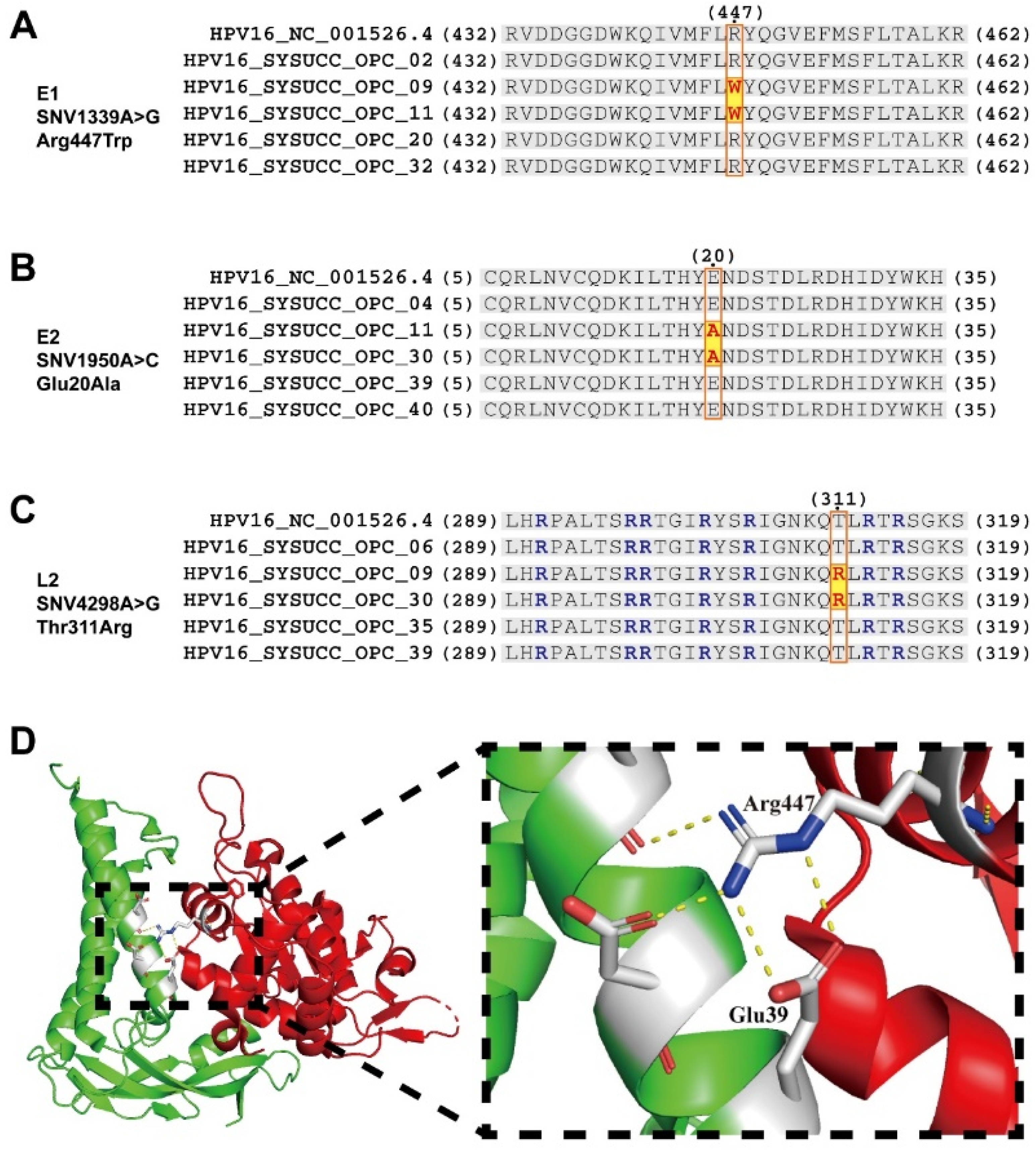
3.4. Prognosis-Associated SNVs May Alter Interactions and Functions between Proteins
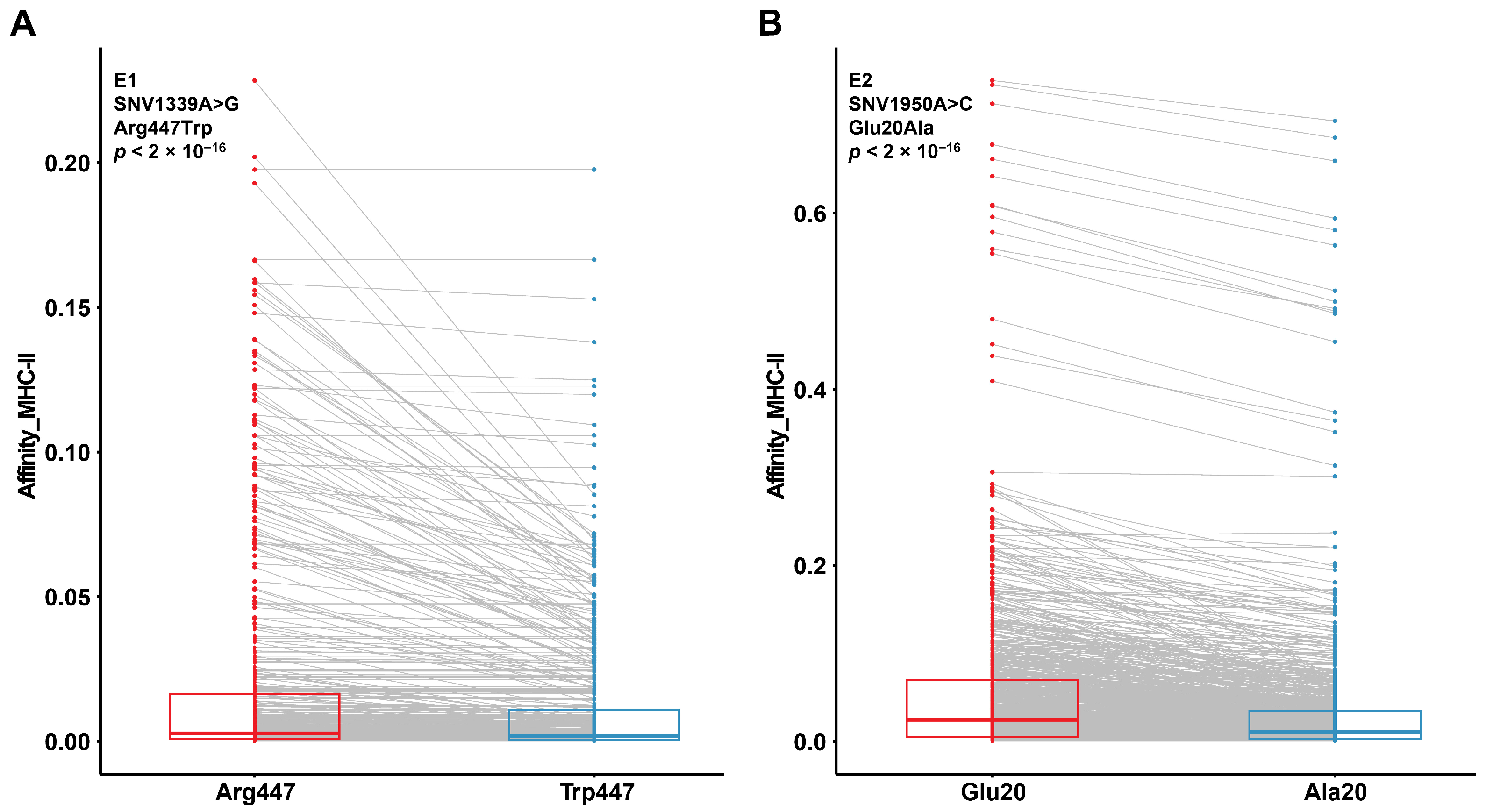
3.5. Prognosis-Associated SNVs May Alter Protein Affinity for MHC-II Binding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Stein, A.; Conway, E.L.; Regan, D.; Grulich, A.; Law, M.; Brotherton, J.M. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br. J. Cancer 2011, 104, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Alemany, L.; Snijders, P.J.; Chaturvedi, A.; Steinberg, B.M.; Schwartz, S.; Castellsague, X. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine 2012, 30 (Suppl. S5), F34–F54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Li, H.; Yang, Z.; Zhang, X.; Li, X.; Wang, J.; Zhang, Y.; Chen, S.; Song, M. Single-cell transcriptomics reveal the intratumoral landscape of infiltrated T-cell subpopulations in oral squamous cell carcinoma. Mol. Oncol. 2021, 15, 866–886. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsague, X.; Laporte, L.; Bosch, F.X.; de Sanjose, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Nasman, A.; Attner, P.; Hammarstedt, L.; Du, J.; Eriksson, M.; Giraud, G.; Ahrlund-Richter, S.; Marklund, L.; Romanitan, M.; Lindquist, D.; et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int. J. Cancer 2009, 125, 362–366. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Golia D’Augè, T.; Cuccu, I.; Etrusco, A.; D’Amato, A.; Laganà, A.S.; D’Oria, O.; Bogani, G.; Di Donato, V.; Muzii, L.; Giannini, A. State of the art on HPV-related cervical lesions. Ital. J. Gynaecol. Obstet. 2024, 36, 135–137. [Google Scholar] [CrossRef]
- Bogani, G.; Sopracordevole, F.; Ciavattini, A.; Vizza, E.; Vercellini, P.; Ghezzi, F.; Scambia, G.; Di Donato, V.; Giannini, A.; Raspagliesi, F. HPV persistence after cervical surgical excision of high-grade cervical lesions. Cancer Cytopathol. 2024, 132, 268–269. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Sallah, S.; Karlsson, U.; Antoine, J.E. Combined chemotherapy and radiation therapy for head and neck malignancies: Quality of life issues. Cancer 2002, 94, 1131–1141. [Google Scholar] [CrossRef]
- Warner, L.; O’Hara, J.T.; Lin, D.J.; Oozeer, N.; Fox, H.; Meikle, D.; Hamilton, D.; Iqbal, M.S.; Robinson, M.; Paleri, V. Transoral robotic surgery and neck dissection alone for head and neck squamous cell carcinoma: Influence of resection margins on oncological outcomes. Oral Oncol. 2022, 130, 105909. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Kabarriti, R.; Jiang, J.; Mehta, V.; Guha, C.; Kalnicki, S.; Smith, R.V.; Garg, M.K. Utilization of Transoral Robotic Surgery (TORS) in patients with Oropharyngeal Squamous Cell Carcinoma and its impact on survival and use of chemotherapy. Oral Oncol. 2018, 86, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Luu, M.; Mallen-St Clair, J.; Mita, A.C.; Scher, K.S.; Lu, D.J.; Shiao, S.L.; Ho, A.S.; Zumsteg, Z.S. Comparison of Survival After Transoral Robotic Surgery vs Nonrobotic Surgery in Patients With Early-Stage Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2020, 6, 1555–1562. [Google Scholar] [CrossRef]
- Fakhry, C.; Psyrri, A.; Chaturvedhi, A. HPV and head and neck cancers: State-of-the-science. Oral Oncol. 2014, 50, 353–355. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Yu, P.K.; Otremba, M.D.; Park, H.S.; Bhatia, A.; Zogg, C.K.; Mehra, S.; Yarbrough, W.G.; Judson, B.L. Treatment deintensification in human papillomavirus-positive oropharynx cancer: Outcomes from the National Cancer Data Base. Cancer 2018, 124, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Qualliotine, J.R.; Ha, P.K.; Califano, J.A.; Kim, Y.; Saunders, J.R.; Blanco, R.G.; D’Souza, G.; Zhang, Z.; Chung, C.H.; et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 2015, 121, 1977–1984. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; Desalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS ONE 2011, 6, e20183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Terai, M.; Fu, L.; Herrero, R.; DeSalle, R.; Burk, R.D. Diversifying selection in human papillomavirus type 16 lineages based on complete genome analyses. J. Virol. 2005, 79, 7014–7023. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.; Boland, J.F.; Schiffman, M.; Zhang, X.; Wentzensen, N.; Yang, Q.; Chen, Z.; Yu, K.; Mitchell, J.; Roberson, D.; et al. Deep sequencing of HPV16 genomes: A new high-throughput tool for exploring the carcinogenicity and natural history of HPV16 infection. Papillomavirus Res. 2015, 1, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-sites: Rapid efficient extraction of SNPs from multi-FASTA alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]
- Comeau, S.R.; Gatchell, D.W.; Vajda, S.; Camacho, C.J. ClusPro: An automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 2004, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- One-Way ANOVA Followed by Dunnett’s Multiple Comparisons Test Was Performed Using GraphPad Prism Version 8.0.2 for Windows. Available online: https://www.graphpad.com/guides/prism/latest/user-guide/citing_graphpad_prism.htm (accessed on 1 April 2024).
- Chen, A.M.; Felix, C.; Wang, P.C.; Hsu, S.; Basehart, V.; Garst, J.; Beron, P.; Wong, D.; Rosove, M.H.; Rao, S.; et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017, 18, 803–811. [Google Scholar] [CrossRef]
- Schache, A.G.; Liloglou, T.; Risk, J.M.; Filia, A.; Jones, T.M.; Sheard, J.; Woolgar, J.A.; Helliwell, T.R.; Triantafyllou, A.; Robinson, M.; et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: Sensitivity, specificity, and prognostic discrimination. Clin. Cancer Res. 2011, 17, 6262–6271. [Google Scholar] [CrossRef] [PubMed]
- Kantang, W.; Chunsrivirot, S.; Muangsin, N.; Poovorawan, Y.; Krusong, K. Design of peptides as inhibitors of human papillomavirus 16 transcriptional regulator E1-E2. Chem. Biol. Drug Des. 2016, 88, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Austin, D.; Guo, D.; Srimatkandada, S.; Wang, T.; Kubushiro, K.; Masumoto, N.; Tsukazaki, K.; Nozawa, S.; Deisseroth, A.B. Peptides inhibitory for the transcriptional regulatory function of human papillomavirus E2. Clin. Cancer Res. 2003, 9, 5423–5428. [Google Scholar]
- Cooper, C.S.; Upmeyer, S.N.; Winokur, P.L. Identification of single amino acids in the human papillomavirus 11 E2 protein critical for the transactivation or replication functions. Virology 1998, 241, 312–322. [Google Scholar] [CrossRef]
- DiGiuseppe, S.; Bienkowska-Haba, M.; Hilbig, L.; Sapp, M. The nuclear retention signal of HPV16 L2 protein is essential for incoming viral genome to transverse the trans-Golgi network. Virology 2014, 458–459, 93–105. [Google Scholar] [CrossRef]
- Lesseur, C.; Diergaarde, B.; Olshan, A.F.; Wunsch-Filho, V.; Ness, A.R.; Liu, G.; Lacko, M.; Eluf-Neto, J.; Franceschi, S.; Lagiou, P.; et al. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat. Genet. 2016, 48, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wurdemann, N.; Wagner, S.; Sharma, S.J.; Prigge, E.S.; Reuschenbach, M.; Gattenlohner, S.; Klussmann, J.P.; Wittekindt, C. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2017, 7, 129. [Google Scholar] [CrossRef]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Jones, O.S.; Breeze, C.E.; Gilson, R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 2019, 19, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Argirion, I.; Zarins, K.R.; McHugh, J.; Cantley, R.L.; Teeramatwanich, W.; Laohasiriwong, S.; Kasemsiri, P.; Naruikon, J.; Srimanta, P.; Chinn, S.B.; et al. Increasing prevalence of HPV in oropharyngeal carcinoma suggests adaptation of p16 screening in Southeast Asia. J. Clin. Virol. 2020, 132, 104637. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.Z.; Hsiao, J.R.; Tsai, C.R.; Chang, J.S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int. J. Cancer 2015, 137, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, C.; Wagner, S.; Bushnak, A.; Prigge, E.S.; von Knebel Doeberitz, M.; Wurdemann, N.; Bernhardt, K.; Pons-Kuhnemann, J.; Maulbecker-Armstrong, C.; Klussmann, J.P. Increasing Incidence rates of Oropharyngeal Squamous Cell Carcinoma in Germany and Significance of Disease Burden Attributed to Human Papillomavirus. Cancer Prev. Res. 2019, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Cramer, J.D.; Hicks, K.E.; Rademaker, A.W.; Patel, U.A.; Samant, S. Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head Neck 2018, 40, 457–466. [Google Scholar] [CrossRef]
- Geltzeiler, M.; Bertolet, M.; Albergotti, W.; Gleysteen, J.; Olson, B.; Persky, M.; Gross, N.; Li, R.; Andersen, P.; Kim, S.; et al. Staging HPV-related oropharyngeal cancer: Validation of AJCC-8 in a surgical cohort. Oral Oncol. 2018, 84, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Sethia, R.; Yumusakhuylu, A.C.; Ozbay, I.; Diavolitsis, V.; Brown, N.V.; Zhao, S.; Wei, L.; Old, M.; Agrawal, A.; Teknos, T.N.; et al. Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 2018, 128, 403–411. [Google Scholar] [CrossRef]
- Chau, N.G.; Rabinowits, G.; Haddad, R.I. Human papillomavirus-associated oropharynx cancer (HPV-OPC): Treatment options. Curr. Treat. Options Oncol. 2014, 15, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991, 10, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Huh, K.; Zhou, X.; Hayakawa, H.; Cho, J.Y.; Libermann, T.A.; Jin, J.; Harper, J.W.; Munger, K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 2007, 81, 9737–9747. [Google Scholar] [CrossRef]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Dyson, N.; Howley, P.M.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.; Chakravarthy, A.; Su, X.; Boshoff, C.; Fenton, T.R. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014, 7, 1833–1841. [Google Scholar] [CrossRef]
- Beaty, B.T.; Moon, D.H.; Shen, C.J.; Amdur, R.J.; Weiss, J.; Grilley-Olson, J.; Patel, S.; Zanation, A.; Hackman, T.G.; Thorp, B.; et al. PIK3CA Mutation in HPV-Associated OPSCC Patients Receiving Deintensified Chemoradiation. J. Natl. Cancer Inst. 2020, 112, 855–858. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, X.Y.; Stenzel, D.J.; Frazer, I.H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991, 185, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Schellenbacher, C.; Shafti-Keramat, S.; Jindra, C.; Christensen, N.; Kirnbauer, R. Chimeric L2-Based Virus-Like Particle (VLP) Vaccines Targeting Cutaneous Human Papillomaviruses (HPV). PLoS ONE 2017, 12, e0169533. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall (n = 265) | TORS Group (n = 130) | Non-TORS Group (n = 135) | p-Value |
|---|---|---|---|---|
| Age at diagnosis | 0.357 | |||
| ≤45 | 35 | 14 | 21 | |
| >45 | 230 | 116 | 114 | |
| Gender | 0.019 | |||
| Male | 222 | 108 | 114 | |
| Female | 43 | 22 | 21 | |
| Smoking history | 0.013 | |||
| No | 118 | 65 | 53 | |
| Yes | 147 | 65 | 82 | |
| Drinking history | <0.001 | |||
| No | 77 | 46 | 31 | |
| Yes | 188 | 84 | 104 | |
| HPV status | <0.001 | |||
| Negative | 86 | 62 | 24 | |
| Positive | 85 | 68 | 17 | |
| Unknown | 94 | 0 | 94 | |
| Primary tumor (T) classification | 0.009 | |||
| 1 | 65 | 31 | 34 | |
| 2 | 147 | 81 | 66 | |
| 3 | 21 | 6 | 15 | |
| 4 | 32 | 12 | 20 | |
| Regional lymph node (N) classification | 0.001 | |||
| 0 | 109 | 55 | 54 | |
| 1 | 43 | 23 | 20 | |
| 2 | 110 | 52 | 58 | |
| 3 | 3 | 0 | 3 | |
| Distant metastasis (M) classification | 0.234 | |||
| 0 | 262 | 130 | 132 | |
| 1 | 3 | 0 | 3 | |
| Stage | 0.001 | |||
| I | 57 | 36 | 21 | |
| II | 89 | 65 | 24 | |
| III | 26 | 3 | 23 | |
| IV | 93 | 26 | 67 | |
| Postoperative adjuvant treatment | 0.232 | |||
| Yes | 146 | 69 | 77 | |
| No | 119 | 61 | 58 |
| Disease | SNV | MAF | Amino Acid Variant | Gene | Overall Survival | |||
|---|---|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio | Lower 95% CI | Upper 95% CI | |||||
| HPV+ OPC | SNV1339A>G | 10.3% | Arg447Trp | E1 | <1.0 × 10−6 | 6.08 × 103 | 2.82 × 102 | 1.31 × 105 |
| SNV1950A>C | 5.9% | Glu20Ala | E2 | <1.0 × 10−6 | 3.10 × 104 | 6.79 × 102 | 1.41 × 106 | |
| SNV4298A>G | 10.3% | Thr311Arg | L2 | <1.0 × 10−6 | 5.23 × 102 | 2.43 × 101 | 1.13 × 104 | |
| ≥1 high-risk SNVs | NA | NA | NA | <1.0 × 10−6 | 1.29 × 103 | 7.67 × 101 | 2.16 × 104 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, X.; Yan, S.; Li, X.; Li, M.; Zhang, Y.; Zhang, S.; Li, F.; Song, M. Transoral Robotic Surgery and Human Papillomavirus Infection: Impact on Oropharyngeal Cancer Prognosis. J. Clin. Med. 2024, 13, 4455. https://doi.org/10.3390/jcm13154455
Chen J, Zhang X, Yan S, Li X, Li M, Zhang Y, Zhang S, Li F, Song M. Transoral Robotic Surgery and Human Papillomavirus Infection: Impact on Oropharyngeal Cancer Prognosis. Journal of Clinical Medicine. 2024; 13(15):4455. https://doi.org/10.3390/jcm13154455
Chicago/Turabian StyleChen, Jingtao, Xing Zhang, Shida Yan, Xiyuan Li, Menghua Li, Ying Zhang, Shiting Zhang, Fengjiao Li, and Ming Song. 2024. "Transoral Robotic Surgery and Human Papillomavirus Infection: Impact on Oropharyngeal Cancer Prognosis" Journal of Clinical Medicine 13, no. 15: 4455. https://doi.org/10.3390/jcm13154455




