Investigating the Added Value of Beck’s Depression Inventory in Atherosclerosis Prediction: Lessons from Paracelsus 10,000
Abstract
:1. Introduction
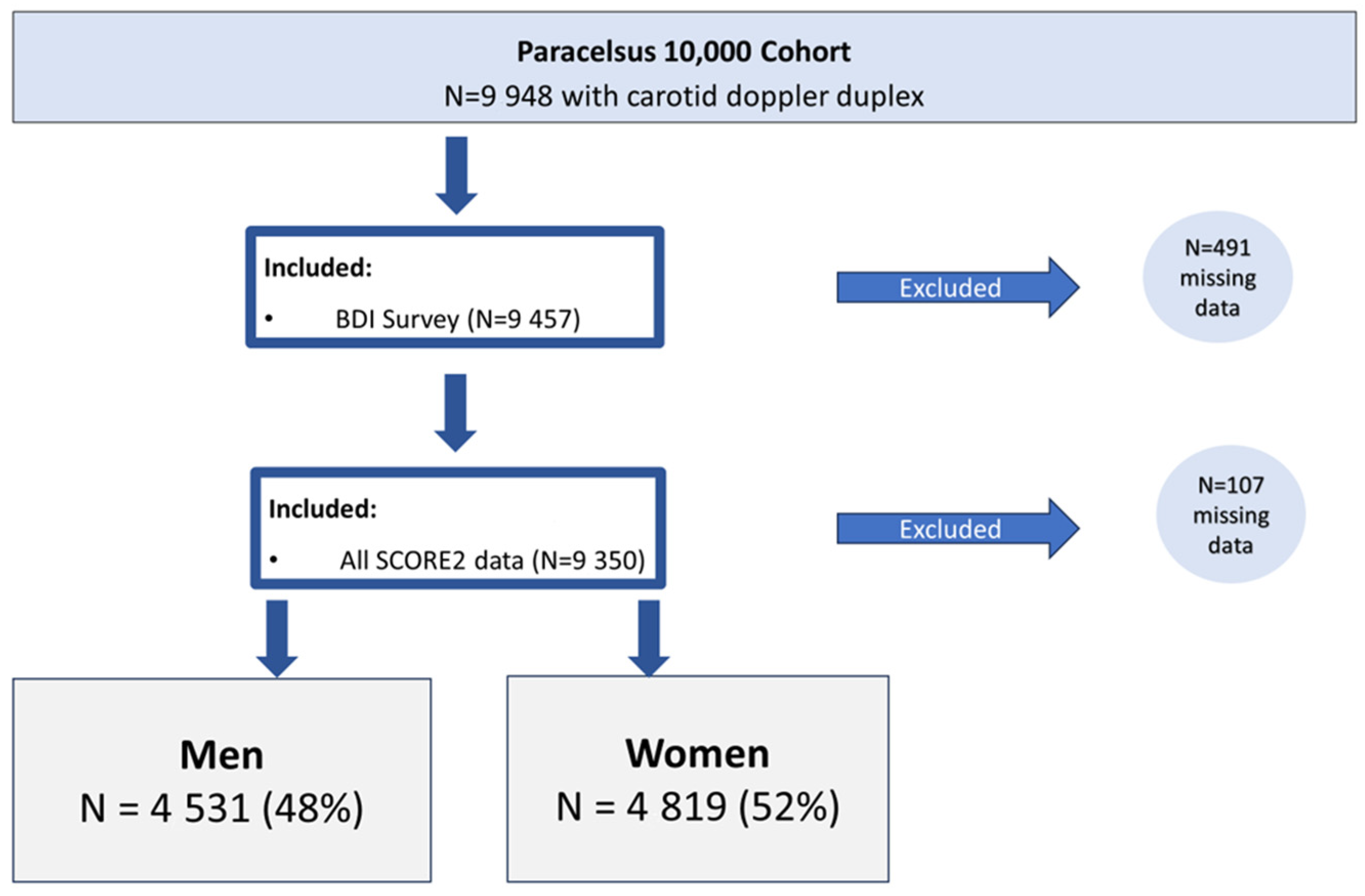
2. Subjects and Methods
2.1. Subjects
2.2. Statistical Analysis
2.3. Ethics
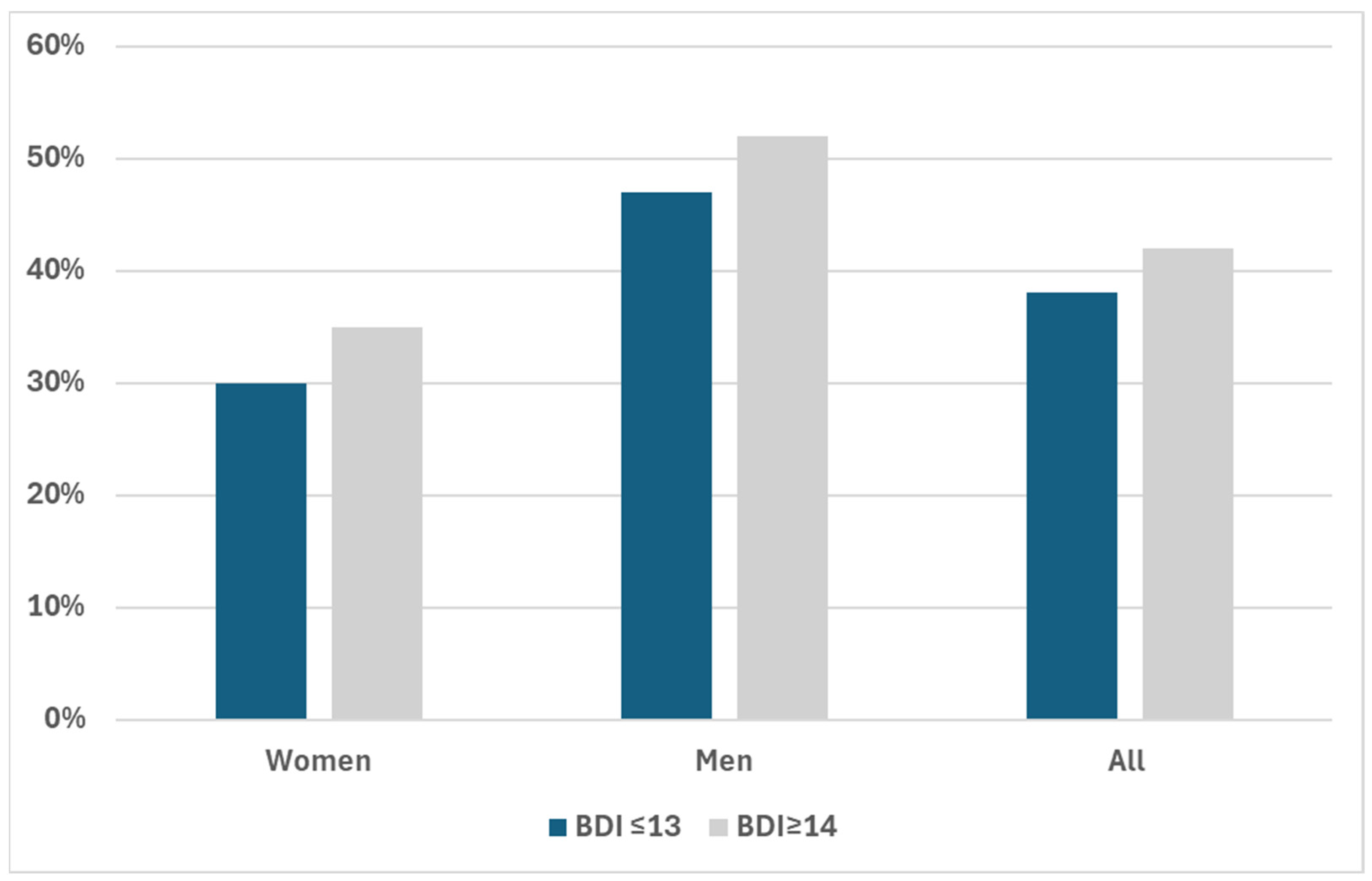
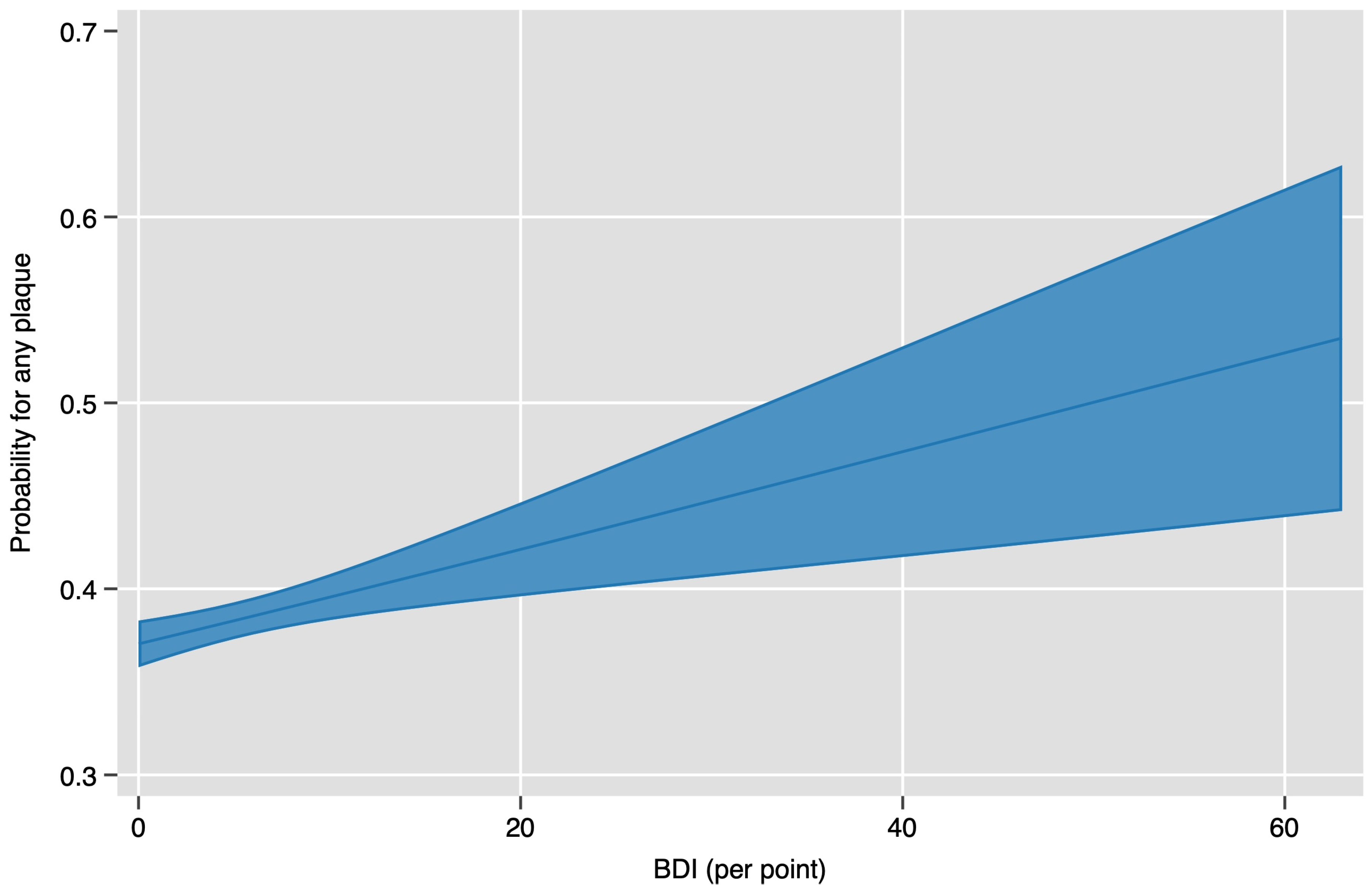
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Comprehensive Mental Health Action Plan 2013–2030. Available online: https://iris.who.int/bitstream/handle/10665/345301/9789240031029-eng.pdf?sequence=1 (accessed on 18 February 2024).
- Arias De La Torre, J.; Vilagut, G.; Ronaldson, A.; Serrano-Blanco, A.; Valderas, J.; Martín, V.; Dregan, A.; Bakolis, I.; Alonso, J. Prevalence of depression in Europe using two different PHQ-8 scoring methods. Eur. Psychiatr. 2022, 65, S299. [Google Scholar] [CrossRef]
- Sobocki, P.; Jönsson, B.; Angst, J.; Rehnberg, C. Cost of depression in Europe. J. Ment. Health Policy Econ. 2006, 9, 87–98. [Google Scholar] [PubMed]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.; Emmet, E.; Wang, Y.; McKevitt, C.; Wolfe, C.D.A. The Burden of Stroke in Europe; King’s College, London, for the Stroke Alliance for Europe: london, UK, 2017; Available online: https://strokeeurope.eu/report-info/introduction/ (accessed on 17 January 2024).
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Beutel, M.E.; Wiltink, J.; Kirschner, Y.; Sinning, C.; Espinola-Klein, C.; Wild, P.S.; Münzel, T.; Blettner, M.; Zwiener, I.; Lackner, K.; et al. History of depression but not current depression is associated with signs of atherosclerosis: Data from the Gutenberg Health Study. Psychol. Med. 2014, 44, 919–925. [Google Scholar] [CrossRef]
- Faramawi, M.F.; Gustat, J.; Wildman, R.P.; Rice, J.; Johnson, E.; Sherwin, R. Relation between Depressive Symptoms and Common Carotid Artery Atherosclerosis in American Persons ≥65 Years of Age. Am. J. Cardiol. 2007, 99, 1610–1613. [Google Scholar] [CrossRef]
- Guan, S.; Fang, X.; Gu, X.; Hua, Y.; Tang, Z.; Liu, B.; Zhang, Z. Bidirectional association between depressive symptoms and carotid atherosclerosis in community-based older adults in China. Arch. Gerontol. Geriatr. 2019, 83, 1–6. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation-Associated Co-morbidity between Depression and Cardiovascular Disease. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Dantzer, R., Capuron, L., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2016; Volume 31, pp. 45–70. ISBN 978-3-319-51151-1. Available online: http://link.springer.com/10.1007/7854_2016_28 (accessed on 18 February 2024).
- Han, L.; Shen, S.; Wu, Y.; Zhong, C.; Zheng, X. Trajectories of depressive symptoms and risk of cardiovascular disease: Evidence from the China Health and Retirement Longitudinal Study. J. Psychiatr. Res. 2022, 145, 137–143. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahzad, U.; Zarak, M.S.; Channa, J.; Khan, I.; Ghani, M.O.A. Association of Depression with Subclinical Coronary Atherosclerosis: A Systematic Review. J. Cardiovasc. Trans. Res. 2021, 14, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a Risk Factor for Poor Prognosis Among Patients with Acute Coronary Syndrome: Systematic Review and Recommendations: A Scientific Statement From the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.; Bots, M.L.; Del Sol, A.I.; Koudstaal, P.J.; Witteman, J.C.M.; Grobbee, D.E.; Hofman, A.; Breteler, M.M.B. Carotid Plaques Increase the Risk of Stroke and Subtypes of Cerebral Infarction in Asymptomatic Elderly: The Rotterdam Study. Circulation 2002, 105, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Marko, M.; Ospel, J.M.; Goyal, M.; Almekhlafi, M. The Risk of Stroke and TIA in Nonstenotic Carotid Plaques: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2020, 41, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Rigo, F.; Facchetti, R.; Carerj, S.; Giannattasio, C.; Moreo, A.; Mureddu, G.F.; Salvetti, M.; Grolla, E.; Faden, G.; et al. Differential incremental value of ultrasound carotid intima–media thickness, carotid plaque, and cardiac calcium to predict angiographic coronary artery disease across Framingham risk score strata in the APRES multicentre study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, Impact, and Predictive Value of Detecting Subclinical Coronary and Carotid Atherosclerosis in Asymptomatic Adults. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Plichart, M.; Celermajer, D.S.; Zureik, M.; Helmer, C.; Jouven, X.; Ritchie, K.; Tzourio, C.; Ducimetière, P.; Empana, J.-P. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis 2011, 219, 917–924. [Google Scholar] [CrossRef]
- Nezu, T.; Hosomi, N. Usefulness of Carotid Ultrasonography for Risk Stratification of Cerebral and Cardiovascular Disease. JAT 2020, 27, 1023–1035. [Google Scholar] [CrossRef]
- Ihle-Hansen, H.; Vigen, T.; Berge, T.; Walle-Hansen, M.M.; Hagberg, G.; Ihle-Hansen, H.; Thommessen, B.; Ariansen, I.; Røsjø, H.; Rønning, O.M.; et al. Carotid Plaque Score for Stroke and Cardiovascular Risk Prediction in a Middle-Aged Cohort From the General Population. JAHA 2023, 12, e030739. [Google Scholar] [CrossRef] [PubMed]
- Dženkevičiūtė, V.; Adomavičius, T.; Tarutytė, G.; Rinkūnienė, E.; Kasiulevičius, V.; Badarienė, J. Carotid Plaques and Hypertension as Risk Factors for Cardiovascular Disease and All-Cause Mortality in Middle-Aged Adults. JCM 2024, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1046–e1081. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 working group and ESC Cardiovascular risk collaboration; Hageman, S.; Pennells, L.; Ojeda, F.; Kaptoge, S.; Kuulasmaa, K.; de Vries, T.; Xu, Z.; Kee, F.; Chung, R.; et al. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Quinn, N.; Knifton, L.; Goldie, I.; van Bortel, T.; Dowds, J.; Lasalvia, A.; Scheerder, G.; Boumans, J.; Svab, V.; Lanfredi, M.; et al. Nature and impact of European anti-stigma depression programmes. Health Promot. Int. 2014, 29, 403–413. [Google Scholar] [CrossRef]
- Paracelsus 10,000: An Observational Cohort Study About the Health Status of the Population of Salzburg, Austria. Rationale, Objectives and Study Design. PPExMed 2023, 1, 1–17. [CrossRef]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- Morris, D.R.; Ayabe, K.; Inoue, T.; Sakai, N.; Bulbulia, R.; Halliday, A.; Goto, S. Evidence-Based Carotid Interventions for Stroke Prevention: State-of-the-art Review. J. Atheroscler. Thromb. 2017, 24, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Guth, D.; Steer, R.A.; Ball, R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav. Res. Ther. 1997, 35, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Dozois, D.J.A.; Dobson, K.S.; Ahnberg, J.L. A psychometric evaluation of the Beck Depression Inventory–II. Psychol. Assess. 1998, 10, 83–89. [Google Scholar] [CrossRef]
- Schneider, S.L. The classification of education in surveys: A generalized framework for ex-post harmonization. Qual Quant. 2022, 56, 1829–1866. [Google Scholar] [CrossRef]
- Dienhart, C.; Paulweber, B.; Frey, V.N.; Iglseder, B.; Trinka, E.; Langthaler, P.; Aigner, E.; Granitz, M.; Wernly, B. Inverse Association between Educational Status and Coronary CT Calcium Scores: Should We Reflect This in Our ASCVD Risk Assumptions? Int. J. Environ. Res. Public Health 2023, 20, 6065. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, M.; Ramspek, C.L.; Jager, K.J.; Zoccali, C.; Dekker, F.W. Prediction versus aetiology: Common pitfalls and how to avoid them. Nephrol. Dial. Transplant. 2017, 32, ii1–ii5. [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Beil, M.; Guidet, B.; Flaatten, H.; Jung, C. Odds ratios versus risk ratios in intensive care research: Using frailty in intensive care as a case study. Acad. Emerg. Med. 2024, 31, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.C.; Miller, M.M.; Kleinman, L.C. Computing Adjusted Risk Ratios and Risk Differences in Stata. Stata J. 2013, 13, 492–509. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Łaszewska, A.; Österle, A.; Wancata, J.; Simon, J. Prevalence of mental diseases in Austria: Systematic review of the published evidence. Wien Klin Wochenschr. 2018, 130, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Yun, J.-S.; Ko, S.-H.; Ahn, Y.-B.; Yoo, K.-D.; Her, S.-H.; Moon, D.; Jung, S.-H.; Won, H.-H.; Kim, D. Impacts of gender and lifestyle on the association between depressive symptoms and cardiovascular disease risk in the UK Biobank. Sci. Rep. 2023, 13, 10758. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.W.; Freedland, K.E. Effect of DRGs on three-month readmission rate of geriatric patients with congestive heart failure. Am. J. Public Health 1988, 78, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; Tribouilloy, C.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Evans, J.M.; Bailey, K.R.; Redfield, M.M. Congestive Heart Failure in the Community: A Study of All Incident Cases in Olmsted County, Minnesota, in 1991. Circulation 1998, 98, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- De Torbal, A.; Boersma, E.; Kors, J.A.; Van Herpen, G.; Deckers, J.W.; Van Der Kuip, D.A.M.; Stricker, B.H.; Hofman, A.; Witteman, J.C.M. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: The Rotterdam Study. Eur. Heart J. 2006, 27, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Mittelmark, M.; Psaty, B.M.; Rautaharju, P.M.; Fried, L.P.; Borhani, N.O.; Tracy, R.P.; Gardin, J.M.; O’Leary, D.H.; Kronmal, R. Prevalence of Cardiovascular Diseases among Older Adults. Am. J. Epidemiol. 1993, 137, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Faragher, E.B. The relationship between job satisfaction and health: A meta-analysis. Occup. Environ. Med. 2005, 62, 105–112. [Google Scholar] [CrossRef]
- Clays, E.; De Bacquer, D.; Leynen, F.; Kornitzer, M.; Kittel, F.; De Backer, G. Job stress and depression symptoms in middle-aged workers—Prospective results from the Belstress study. Scand. J. Work Environ. Health 2007, 33, 252–259. [Google Scholar] [CrossRef]
- World Health Organization. Optimizing Brain Health across the Life Course: WHO Position Paper; World Health Organization: Geneva, Switzerland, 2022; Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo/ (accessed on 18 February 2024).
- Bus, B.A.A.; Marijnissen, R.M.; Holewijn, S.; Franke, B.; Purandare, N.; De Graaf, J.; Den Heijer, M.; Buitelaar, J.K.; Voshaar, R.C.O. Depressive symptom clusters are differentially associated with atherosclerotic disease. Psychol. Med. 2011, 41, 1419–1428. [Google Scholar] [CrossRef]
- Elovainio, M.; Keltikangas-Järvinen, L.; Kivimäki, M.; Pulkki, L.; Puttonen, S.; Heponiemi, T.; Juonala, M.; Viikari, J.S.A.; Raitakari, O.T. Depressive Symptoms and Carotid Artery Intima-Media Thickness in Young Adults: The Cardiovascular Risk in Young Finns Study. Psychosom. Med. 2005, 67, 561–567. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Fisher, A.J.; Kibbey, K.J.; Jacka, F.N.; Kotowicz, M.A.; Williams, L.J.; Stuart, A.L.; Berk, M.; Lewandowski, P.A.; Taylor, C.B.; et al. Depression is a risk factor for incident coronary heart disease in women: An 18-year longitudinal study. J. Affect. Disord. 2016, 196, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Seldenrijk, A.; Vogelzangs, N.; Van Hout, H.P.J.; Van Marwijk, H.W.J.; Diamant, M.; Penninx, B.W.J.H. Depressive and anxiety disorders and risk of subclinical atherosclerosis. J. Psychosom. Res. 2010, 69, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Kollia, N.; Tousoulis, D. The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas 2018, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Häfner, S.; Schüle, C.; Eser, D.; Zill, P.; Manook, A.; Weigl, J.; Jooyandeh, S.; et al. Classical Risk Factors and Inflammatory Biomarkers: One of the Missing Biological Links between Cardiovascular Disease and Major Depressive Disorder. IJMS 2018, 19, 1740. [Google Scholar] [CrossRef] [PubMed]
- 2020 ESC-EHN Blueprint Digital Edition. Available online: https://www.escardio.org/static-file/Escardio/Advocacy/Documents/2020%20ESC-EHN-blueprint_digital%20edition.pdf (accessed on 1 March 2024).
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Patten, S.B.; Vos, T.; Whiteford, H.A. The Epidemiological Modelling of Major Depressive Disorder: Application for the Global Burden of Disease Study 2010. PLoS ONE 2013, 8, e69637. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Somerville, A.J.; Baxter, A.J.; Norman, R.; Patten, S.B.; Vos, T.; Whiteford, H.A. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Pieh, C.; Budimir, S.; Probst, T. The effect of age, gender, income, work, and physical activity on mental health during coronavirus disease (COVID-19) lockdown in Austria. J. Psychosom. Res. 2020, 136, 110186. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Harris, M.G.; McKeon, G.; Baxter, A.; Pennell, C.; Barendregt, J.J.; Wang, J. Estimating remission from untreated major depression: A systematic review and meta-analysis. Psychol. Med. 2013, 43, 1569–1585. [Google Scholar] [CrossRef]
- de Kruif, M.; Spijker, A.T.; Molendijk, M.L. Depression during the perimenopause: A meta-analysis. J. Affect. Disord. 2016, 206, 174–180. [Google Scholar] [CrossRef]
- Krajewska-Ferishah, K.; Kułak-Bejda, A.; Szyszko-Perłowska, A.; Shpakou, A.; Van Damme-Ostapowicz, K.; Chatzopulu, A. Risk of Depression during Menopause in Women from Poland, Belarus, Belgium, and Greece. J. Clin. Med. 2022, 11, 3371. [Google Scholar] [CrossRef] [PubMed]
| Men (N = 4531) | Total | BDI ≤ 13 | BDI ≥ 14 | p-Value |
|---|---|---|---|---|
| N = 4531 | N = 4200 (93%) | N = 331 (7%) | ||
| Age (Median) | 55 (50–62) | 55 (50–62) | 55 (50–61) | 0.90 |
| Age by decade | 0.40 | |||
| Age 40–49 yrs | 24% (1073) | 24% (1000) | 22% (73) | |
| Age 50–59 yrs | 44% (1974) | 43% (1815) | 48% (159) | |
| Age 60–69 yrs | 28% (1281) | 28% (1195) | 26% (86) | |
| Age ≥ 70 yrs | 4% (203) | 5% (190) | 4% (13) | |
| Total cholesterol mg/dL | 206 (181–231) | 206 (181–231) | 206 (177–237) | 0.93 |
| Triglycerides mg/dL | 111 (80–157) | 110 (79–155) | 131 (88–193) | <0.001 |
| HDL cholesterol mg/dL | 54 (45–64) | 54 (46–64) | 50 (42–61) | <0.001 |
| LDL cholesterol mg/dL | 142 (118–166) | 142 (118–166) | 138 (117–167) | 0.51 |
| Leucocytes | 5.8 (4.9–6.9) | 5.8 (4.9–6.9) | 6.1 (5.0–7.7) | <0.001 |
| hsCRP mg/dL | 0.12 (0.07–0.23) | 0.12 (0.06–0.22) | 0.14 (0.07–0.28) | <0.001 |
| Height cm | 177 (173–182) | 177 (173–182) | 177 (172–181) | 0.051 |
| Weight kg | 84 (77–94) | 84 (76–93) | 86 (78–97) | 0.002 |
| BMI kg/m2 | 27 (24–29) | 27 (24–29) | 28 (25–30) | <0.001 |
| Obesity vs. Non-obese | <0.001 | |||
| BMI < 30 | 79% (3573) | 80% (3338) | 71% (235) | |
| BMI ≥ 30 | 21% (951) | 20% (856) | 29% (95) | |
| Abdom. circumference cm | 98 (91–105) | 97 (91–105) | 100 (93–110) | <0.001 |
| Self-reported | ||||
| Dyslipidemia | 14% (637) | 14% (569) | 21% (68) | <0.001 |
| Diabetes Mellitus type 2 | 5% (219) | 5% (189) | 9% (30) | <0.001 |
| Hypertension | 27% (1193) | 25% (1064) | 40% (129) | <0.001 |
| Coronary artery disease | 3% (144) | 3% (130) | 4% (14) | 0.24 |
| Chronic heart failure | 1% (34) | 1% (29) | 2% (5) | 0.092 |
| Peripheral vascular disease | 1% (24) | 0% (20) | 1% (4) | 0.074 |
| COPD | 2% (102) | 2% (87) | 5% (15) | 0.003 |
| Chronic kidney disease | 1% (24) | 0% (20) | 1% (4) | 0.075 |
| Metabolic syndrome 1 | 21% (929) | 20% (832) | 30% (97) | <0.001 |
| Diabetes Mellitus type 2 | 8% (357) | 8% (316) | 12% (41) | 0.002 |
| SCORE2 10-yr CVD risk (%) | 6 (4–9) | 6 (4–9) | 6 (4–9) | 0.008 |
| HbA1c level (%) | 0.003 | |||
| HbA1c < 6.5% | 96% (4216) | 97% (3920) | 93% (296) | |
| HbA1c ≥ 6.5% | 4% (158) | 3% (137) | 7% (21) | |
| Glucose levels | 0.014 | |||
| Glucose < 126 mg/dL | 94% (4250) | 95% (3951) | 91% (299) | |
| Glucose ≥ 126 mg/dL | 6% (250) | 5% (222) | 9% (28) | |
| Alcohol g/week | 63 (53–74) | 63 (53–74) | 65 (55–77) | 0.022 |
| Excessive alcohol intake 2 | 7% (255) | 6% (225) | 11% (30) | 0.002 |
| Smoking history | <0.001 | |||
| Never smoker | 42% (1905) | 42% (1785) | 36% (120) | |
| Previous smoking | 40% (1825) | 40% (1701) | 37% (124) | |
| Current smoker | 18% (801) | 17% (714) | 26% (87) | |
| Monthly household income | <0.001 | |||
| ≤EUR 1000 | 4% (161) | 3% (126) | 11% (35) | |
| EUR 1001–2000 | 22% (1001) | 21% (896) | 32% (105) | |
| EUR 2001–3000 | 30% (1346) | 30% (1250) | 29% (96) | |
| EUR 3001–4000 | 18% (824) | 19% (785) | 12% (39) | |
| EUR 4001–5000 | 11% (477) | 11% (459) | 5% (18) | |
| >EUR 5000 | 8% (370) | 8% (353) | 5% (17) | |
| Did not disclose | 8% (352) | 8% (331) | 6% (21) | |
| GISCED educational status | <0.001 | |||
| Low | 6% (277) | 6% (241) | 11% (36) | |
| Medium | 70% (3127) | 70% (2901) | 69% (226) | |
| High | 24% (1079) | 24% (1015) | 20% (64) |
| Women (N = 4819) | Total | BDI ≤ 13 | BDI ≥ 14 | p-Value |
|---|---|---|---|---|
| N = 4819 | N = 4288 (89%) | N = 531 (11%) | ||
| Age (Median) | 54 (49–61) | 55 (49–61) | 54 (50–60) | 0.18 |
| Age by decade | 0.005 | |||
| Age 40–49 | 25% (1224) | 26% (1095) | 24% (129) | |
| Age 50–59 | 43% (2085) | 42% (1822) | 50% (263) | |
| Age 60–69 | 28% (1340) | 28% (1222) | 22% (118) | |
| Age ≥ 70 | 4% (170) | 3% (149) | 4% (21) | |
| Total cholesterol mg/dL | 212 (188–238) | 212 (188–238) | 213 (188–238) | 0.54 |
| Triglycerides mg/dL | 87 (65–120) | 86 (65–118) | 99 (75–138) | <0.001 |
| HDL cholesterol mg/dL | 70 (59–82) | 70 (59–82) | 65 (55–78) | <0.001 |
| LDL cholesterol mg/dL | 138 (114–163) | 138 (114–163) | 140 (117–166) | 0.17 |
| Leucocytes | 5.7 (4.8–6.8) | 5.7 (4.8–6.8) | 5.9 (5.0–7.2) | <0.001 |
| hsCRP mg/dL | 0.11 (0.06–0.25) | 0.11 (0.06–0.24) | 0.14 (0.07–0.30) | <0.001 |
| Height cm | 165 (161–169) | 165 (161–169) | 164 (160–169) | 0.043 |
| Weight kg | 67 (60–76) | 66 (60–76) | 70 (60–82) | <0.001 |
| BMI kg/m2 | 25 (22–28) | 24 (22–28) | 26 (23–30) | <0.001 |
| Obesity vs. Non-obese | <0.001 | |||
| BMI < 30 | 83% (4003) | 85% (3623) | 72% (380) | |
| BMI ≥ 30 | 17% (812) | 15% (661) | 28% (151) | |
| Abdom. circumference cm | 87 (79–96) | 86 (79–95) | 90 (80–101) | <0.001 |
| Self-reported | ||||
| Dyslipidemia | 10% (481) | 9% (392) | 17% (89) | <0.001 |
| Diabetes Mellitus type 2 | 2% (105) | 2% (87) | 3% (18) | 0.042 |
| Hypertension | 18% (866) | 17% (729) | 26% (137) | <0.001 |
| Coronary artery disease | 1% (40) | 1% (32) | 2% (8) | 0.068 |
| Chronic heart failure | 0% (16) | 0% (16) | 0% (0) | 0.16 |
| Peripheral vascular disease | 0% (13) | 0% (12) | 0% (1) | 0.70 |
| COPD | 1% (67) | 1% (53) | 3% (14) | 0.009 |
| Metabolic syndrome 1 | 13% (602) | 12% (501) | 19% (101) | <0.001 |
| SCORE2 10-yr CVD risk (%) | 3 (1–5) | 3 (1–5) | 3 (2–5) | 0.003 |
| HbA1c in DM range | 0.66 | |||
| HbA1c < 6.5 | 99% (4526) | 99% (4022) | 99% (504) | |
| HbA1c ≥ 6.5 | 1% (64) | 1% (58) | 1% (6) | |
| Fasting glucose | 0.71 | |||
| Glucose < 126 mg/dL | 98% (4703) | 98% (4190) | 98% (513) | |
| Glucose ≥ 126/dL | 2% (82) | 2% (72) | 2% (10) | |
| Alcohol g/week | 63 (52–76) | 62 (51–75) | 65 (54–78) | <0.001 |
| Excessive alcohol intake 2 | 3% (139) | 3% (120) | 4% (19) | 0.24 |
| Smoking history | <0.001 | |||
| Never smoker | 48% (2297) | 48% (2070) | 43% (227) | |
| Previous smoking | 33% (1597) | 34% (1439) | 30% (158) | |
| Current smoker | 19% (925) | 18% (779) | 27% (146) | |
| Monthly household income | <0.001 | |||
| ≤EUR 1000 | 11% (509) | 10% (421) | 17% (88) | |
| EUR 1001–2000 | 40% (1906) | 39% (1693) | 40% (213) | |
| EUR 2001–3000 | 21% (995) | 21% (899) | 18% (96) | |
| EUR 3001–4000 | 9% (444) | 9% (396) | 9% (48) | |
| EUR 4001–5000 | 6% (273) | 6% (264) | 2% (9) | |
| >EUR 5000 | 4% (173) | 4% (158) | 3% (15) | |
| Did not disclose | 11% (519) | 11% (457) | 12% (62) | |
| GISCED educational status | <0.001 | |||
| Low | 9% (434) | 8% (356) | 15% (78) | |
| Medium | 69% (3264) | 69% (2917) | 66% (347) | |
| High | 22% (1024) | 22% (927) | 19% (97) |
| Odds Ratio (OR), adj. Relative Risk (ARR) and 95% Confidence Interval | p-Value | Description | |
|---|---|---|---|
| Model 1 | OR 1.16 (1.00–1.34) | 0.043 | Baseline (BDI ≥ 14) |
| ARR 1.09 (1.00–1.19) | 0.046 | ||
| Model 2 | OR 1.43 (1.22–1.69) | <0.001 | Age and sex adjusted |
| ARR 1.18 (1.10–1.26) | <0.001 | ||
| Model 3 | OR 1.32 (1.11–1.56) | <0.001 | Age, sex, MS, and GISCED adjusted 1 |
| ARR 1.13 (1.05–1.21) | 0.001 | ||
| Model 4 | OR 1.21 (1.03–1.43) | 0.023 | Adjusted for SCORE2 components |
| ARR 1.09 (1.01–1.18) | 0.021 | ||
| Model 5 | OR 1.25 (1.06–1.49) | 0.009 | Age, sex, MS, GISCED, and Med adjusted 2 |
| ARR 1.10 (1.03–1.19) | 0.009 | ||
| Sensitivity Analysis 1 | 1.28 (1.06–1.54) | 0.012 | Baseline, women only |
| 1.25 (1.00–1.56) | 0.051 | Baseline, men only | |
| Sensitivity Analysis 2 | 1.46 (1.17–1.81) | 0.001 | Baseline, age ≤ 55 years |
| 1.10 (0.89–1.36) | 0.385 | Baseline, age > 55 years | |
| Odds Ratio (95% Confidence Interval) | p-Value | Description | |
|---|---|---|---|
| Model 1 | 1.01 (1.00–1.02) | 0.001 | Baseline (BDI ≥ 14) |
| Model 2 | 1.02 (1.01–1.03) | <0.001 | Age and sex adjusted |
| Model 3 | 1.02 (1.01–1.02) | <0.001 | Age, sex, MS, and GISCED adjusted 1 |
| Model 4 | 1.01 (1.00–1.02) | 0.001 | Adjusted for SCORE2 components |
| Men (N = 4531) | Total | BDI ≤ 13 | BDI ≥ 14 | p-Value |
| Plaque (Binomial) | 0.050 | |||
| No Plaques | 53% (2397) | 53% (2239) | 48% (158) | |
| Plaques | 47% (2134) | 47% (1961) | 52% (173) | |
| Plaque diameter (cm2) | 0.00 (0.00–18.66) | 0.00 (0.00–18.57) | 4.77 (0.00–20.82) | 0.087 |
| Stenosis by category | 0.025 | |||
| No stenosis | 67% (3035) | 68% (2832) | 62% (203) | |
| ECST < 50% | 32% (1448) | 32% (1329) | 36% (119) | |
| ECST 50–69% | 1% (27) | 1% (22) | 2% (5) | |
| ECST 70–80% | 0% (6) | 0% (5) | 0% (1) | |
| ECST > 80% | 0% (4) | 0% (3) | 0% (1) | |
| Women (N = 4819) | Total | BDI ≤ 13 | BDI ≥ 14 | p-value |
| Plaque (Binomial) | 0.012 | |||
| No Plaques | 70% (3368) | 70% (3022) | 65% (346) | |
| Plaques | 30% (1451) | 30% (1266) | 35% (185) | |
| Plaque diameter (cm2) | 0.00 (0.00–5.24) | 0.00 (0.00–4.98) | 0.00 (0.00–6.80) | 0.023 |
| Stenosis by category | 0.54 | |||
| No stenosis | 81% (3907) | 81% (3492) | 78% (415) | |
| ECST < 50% | 19% (899) | 18% (786) | 21% (113) | |
| ECST 50–69% | 0% (9) | 0% (8) | 0% (1) | |
| ECST 70–80% | 0% (1) | 0% (1) | 0% (0) | |
| ECST > 80% | 0% (1) | 0% (1) | 0% (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dienhart, C.; Aigner, E.; Iglseder, B.; Frey, V.; Gostner, I.; Langthaler, P.; Paulweber, B.; Trinka, E.; Wernly, B. Investigating the Added Value of Beck’s Depression Inventory in Atherosclerosis Prediction: Lessons from Paracelsus 10,000. J. Clin. Med. 2024, 13, 4492. https://doi.org/10.3390/jcm13154492
Dienhart C, Aigner E, Iglseder B, Frey V, Gostner I, Langthaler P, Paulweber B, Trinka E, Wernly B. Investigating the Added Value of Beck’s Depression Inventory in Atherosclerosis Prediction: Lessons from Paracelsus 10,000. Journal of Clinical Medicine. 2024; 13(15):4492. https://doi.org/10.3390/jcm13154492
Chicago/Turabian StyleDienhart, Christiane, Elmar Aigner, Bernhard Iglseder, Vanessa Frey, Isabella Gostner, Patrick Langthaler, Bernhard Paulweber, Eugen Trinka, and Bernhard Wernly. 2024. "Investigating the Added Value of Beck’s Depression Inventory in Atherosclerosis Prediction: Lessons from Paracelsus 10,000" Journal of Clinical Medicine 13, no. 15: 4492. https://doi.org/10.3390/jcm13154492





