Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study
Abstract
1. Introduction
2. Methods
2.1. Relatives’ Cohort
2.2. Lp(a) Determinations
2.3. Variables’ Definitions
2.4. Statistical Analysis
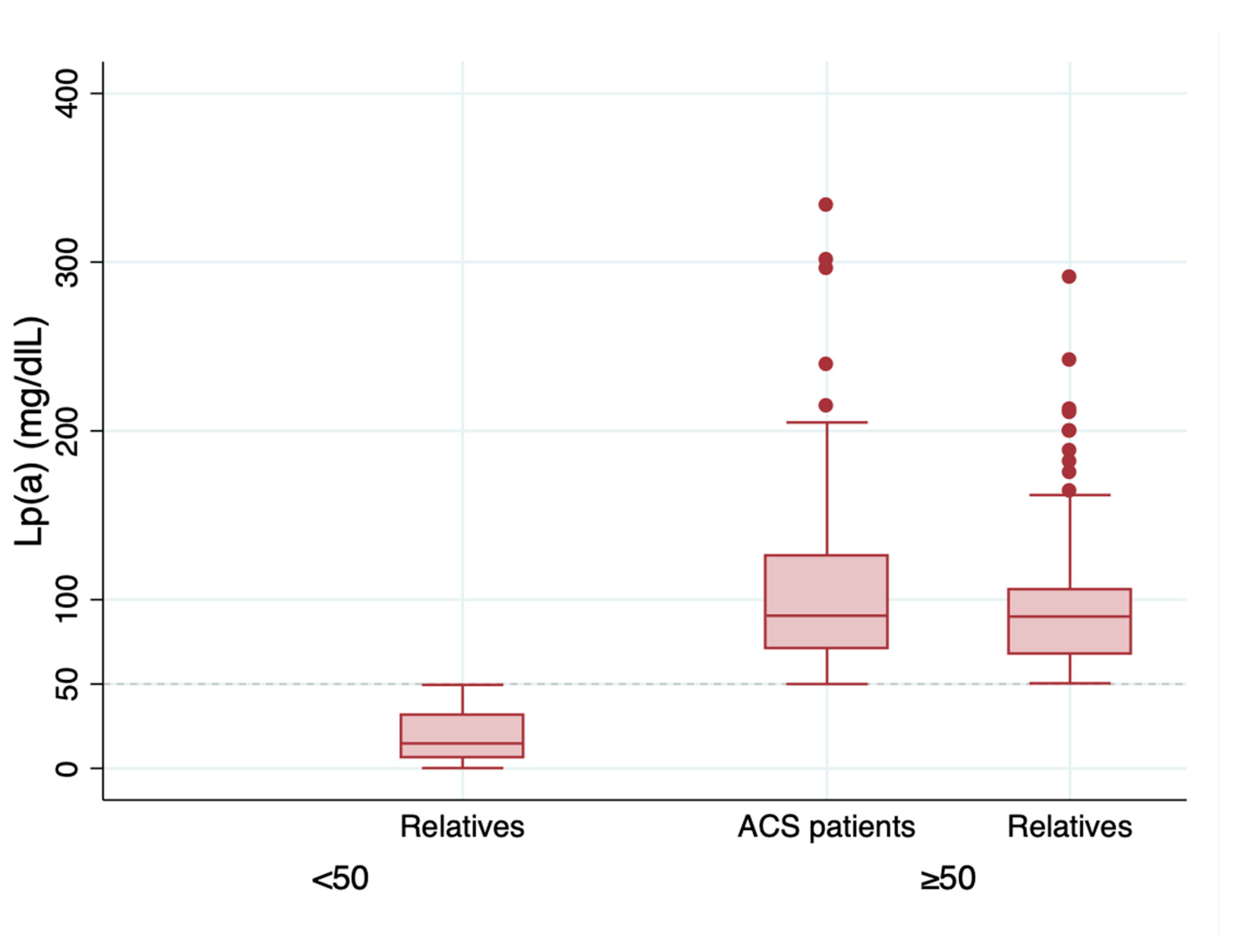
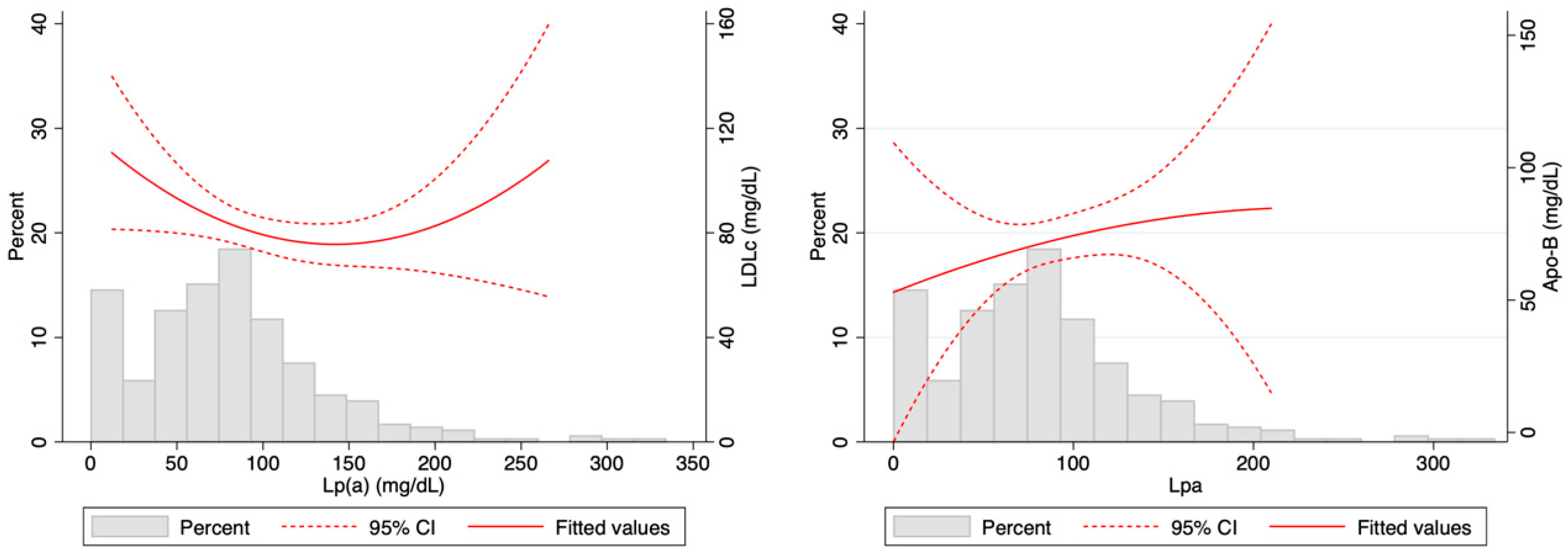
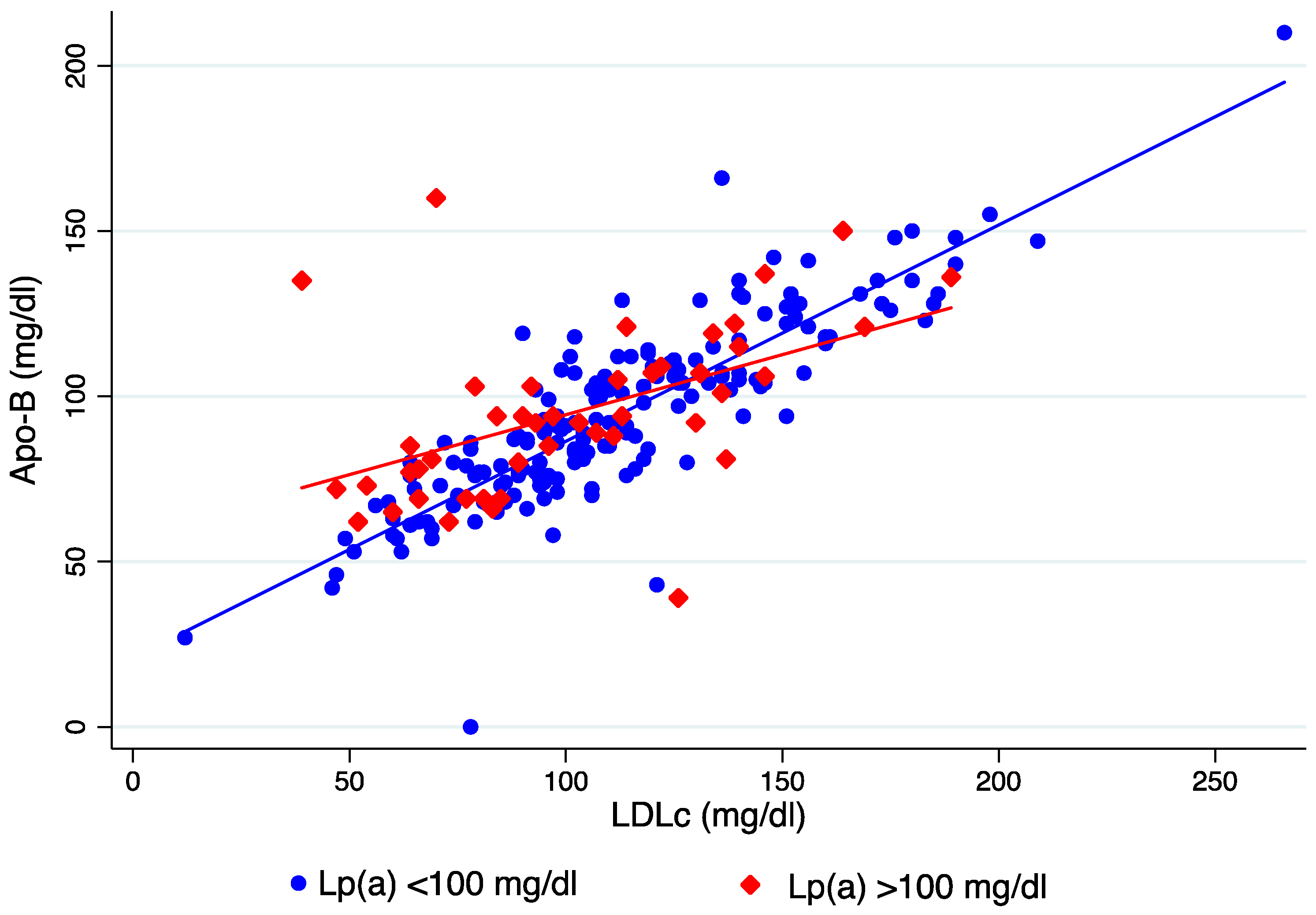
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawi, M.M.; Frohlich, J.; Chan, S.Y. Lipoprotein(a) the Insurgent: A New Insight into the Structure, Function, Metabolism, Pathogenicity, and Medications Affecting Lipoprotein(a) Molecule. J. Lipids 2020, 2020, 3491764. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Albers, J.J. Lipoprotein(a) measurements for clinical application. J. Lipid Res. 2016, 57, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Gill, D.; Mason, A.M.; Jiang, T.; Bäck, M.; Butterworth, A.S.; Burgess, S. Lipoprotein(a) in Alzheimer, Atherosclerotic, Cerebrovascular, Thrombotic, and Valvular Disease: Mendelian Randomization Investigation. Circulation 2020, 141, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Sabatine, M.S. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Szarek, M.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Jukema, J.W.; Landmesser, U.; López-Jaramillo, P.; Manvelian, G.; Pordy, R.; et al. Lipoprotein(a) and Benefit of PCSK9 Inhibition in Patients with Nominally Controlled LDL Cholesterol. J. Am. Coll. Cardiol. 2021, 78, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Paruchuri, K.; Haidermota, S.; Bernardo, R.; Zekavat, S.M.; Gilliland, T.; Januzzi, J.; Natarajan, P. Repeat Measures of Lipoprotein(a) Molar Concentration and Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 79, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Moriarty, P.M.; Stroes, E.S. Emerging RNA Therapeutics to Lower Blood Levels of Lp(a): JACC Focus Seminar 2/4. J. Am. Coll. Cardiol. 2021, 77, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K.; Cho, L.; Nicholls, S.J.; Kastelein, J.; Leitersdorf, E.; Landmesser, U.; Blaha, M.; Lincoff, A.M.; Morishita, R.; et al. Lipoprotein(a) levels in a global population with established atherosclerotic cardiovascular disease. Open Heart 2022, 9, e002060. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Pang, J.; Chan, D.C.; Ellis, K.L.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Moses, E.K.; Watts, G.F. Cascade testing for elevated lipoprotein(a) in relatives of probands with familial hypercholesterolaemia and elevated lipoprotein(a). Atherosclerosis 2021, 349, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Boizel, R.; Benhamou, P.Y.; Lardy, B.; Laporte, F.; Foulon, T.; Halimi, S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care 2000, 23, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.L.; de Isla, L.P.; Alonso, R.; Fuentes, F.; Watts, G.F.; Mata, P. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J. Am. Coll. Cardiol. 2019, 73, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Tokgözoğlu, L.; Libby, P. The dawn of a new era of targeted lipid-lowering therapies. Eur. Heart J. 2022, 43, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–189. [Google Scholar] [CrossRef] [PubMed]
- Stürzebecher, P.; Schorr, J.; Klebs, S.; Lauf, U. Trend and consequences of lipoprotein(a) testig: Cross-selectional and longitudinal health insurance claims database. Atherosclerosis 2023, 367, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Witztum, J.L. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef] [PubMed]
- O’donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, S.N.; Afanasieva, O.I.; Ezhov, M.V. Therapeutic Apheresis for Management of Lp(a) Hyperlipoproteinemia. Curr. Atheroscler. Rep. 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
| Total | ACS Patients | Relatives | p | |
|---|---|---|---|---|
| N | 413 | 180 | 233 | |
| Age | 47.3 (1–89) | 59.1 (35–89) | 37.5 (1–89) | <0.001 |
| Women (%) | 40.4 | 23.9 | 53.2 | <0.001 |
| Hypertension (%) | 30.3 | 54.7 | 12 | <0.001 |
| Diabetes Mellitus (%) | 16.2 | 30.7 | 5.3 | <0.001 |
| Current smokers (%) | 24.9 | 42.2 | 10.6 | <0.001 |
| Dislipidemia (%) | 37 | 61.7 | 18.7 | <0.001 |
| Previous CHD (%) | 2.4 | 22.3 | 4 | <0.001 |
| Peripheral arterial disease (%) | 4.1 | 7.8 | 1.3 | <0.001 |
| Stroke (%) | 2.4 | 4.5 | 0.9 | <0.001 |
| Thromboembolic disease (%) | 1.5 | 2.8 | 0.4 | <0.001 |
| Valvulopathy (%) | 3.4 | 6.7 | 0.9 | <0.001 |
| Family history | ||||
| CHD FA (%) | 73.4 | 45.9 | 100 | <0.001 |
| Premature CHD FA (%) | 57.5 | 56.6 | 57.8 | NS |
| Dislipidemia FA (%) | 4.6 | 4.7 | 4.9 | NS |
| Lipid-lowering therapies | ||||
| Statins (%) | 27.4 | 48 | 12.1 | <0.001 |
| Ezetimibe (%) | 5.1 | 20.1 | 3.9 | <0.001 |
| Biochemical determinations | ||||
| Lp(a) (mg/dL) | 82.1 (0.2–334) | 103.4 (39–334) | 64.9 (0.2–291) | <0.001 |
| Total cholesterol (mg/dL) | 178.8 (68–298) | 172.3(68–293) | 184.1 (80–298) | <0.001 |
| HDLc (mg/dL) | 49.1 (21–96) | 41.2 (21–85) | 55.5 (24–96) | <0.001 |
| LDLc (mg/dL) | 107.6 (12–266) | 103.3(22–266) | 110.9 (12–259) | 0.05 |
| Triglycerides (mg/dL) | 127 (38–626) | 150 (64–626) | 108.5 (38–388) | <0.001 |
| ApoB (mg/dL) | 93.5 (27–210) | 101.8 (30–210) | 91.2 (27–150) | 0.01 |
| Non-HDL cholesterol (mg/dL) | 129.9 (32–258) | 131.6 (35–258) | 128.6 (32–240) | NS |
| TC/HDLc | 3.9 (1.67–8.9) | 4.4 (1.7–8.9) | 3.5 (1.67–8.57) | <0.001 |
| Tg/HDLc | 3 (0.52–16.91) | 4 (0.96–16.92) | 2.2 (0.52–12.1) | <0.001 |
| LDLc/ApoB | 1.1 (0.29–3.23) | 1.1 (0.29–3.23) | 1.1 (0.4–1.6) | NS |
| GFR (mL/min/1.72 m2) | 94.6 (5.03–224) | 83.5 (5.03–117.5) | 103.6(14.8–224) | <0.001 |
| HbA1c (%) | 5.7 (2–12.5) | 6.1 (2.5–12.5) | 5.5 (4.4–9) | <0.001 |
| Lp(a) mg/dL | p | ||
|---|---|---|---|
| <50 | ≥50 | ||
| N | 94 | 139 | |
| Edad | 36.2 | 38.5 | NS |
| Women (%) | 52.2 | 55.3 | NS |
| Hypertension (%) | 13.0 | 11.5 | NS |
| Diabetes Mellitus (%) | 5.5 | 5.3 | NS |
| Current smokers (%) | 7.02 | 12.6 | NS |
| Dislipidemia (%) | 14.4 | 21.5 | NS |
| FA of premature CHD (%) | 60 | 55.8 | NS |
| FA of dislipidemia (%) | 4.4 | 5.3 | NS |
| Lp(a) (mg/dL) | 18.8 | 96.8 | <0.001 |
| Total cholesterol (mg/dL) | 178.6 | 188.1 | NS |
| HDLc (mg/dL) | 54.3 | 56.3 | NS |
| LDLc (mg/dL) | 105.2 | 115.1 | 0.05 |
| Triglycerides (mg/dL) | 117.6 | 102.3 | NS |
| ApoB (mg/dL) | 87.1 | 94.2 | NS |
| Non-HDL cholesterol (mg/dL) | 124.2 | 131.8 | NS |
| TC/HDLc | 3.4 | 3.5 | NS |
| TG/HDLc | 2.5 | 2.03 | NS |
| LDLc/ApoB | 1.1 | 1.1 | NS |
| GFR (mL/min/1.72 m2) | 107.5 | 100.6 | NS |
| HbA1c (%) | 5.5 | 5.5 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Olmo, M.R.; Bailen, M.C.; Martínez Quesada, M.; Rus Mansilla, C.; Martin Toro, M.; López Suarez, A.; Lucas García, M.; Cortez Quiroga, G.; Calvo Bernal, B.; Ortiz Cruces, S.; et al. Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study. J. Clin. Med. 2024, 13, 2256. https://doi.org/10.3390/jcm13082256
Fernández-Olmo MR, Bailen MC, Martínez Quesada M, Rus Mansilla C, Martin Toro M, López Suarez A, Lucas García M, Cortez Quiroga G, Calvo Bernal B, Ortiz Cruces S, et al. Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study. Journal of Clinical Medicine. 2024; 13(8):2256. https://doi.org/10.3390/jcm13082256
Chicago/Turabian StyleFernández-Olmo, M. Rosa, Magdalena Carrillo Bailen, Mar Martínez Quesada, Carmen Rus Mansilla, Miriam Martin Toro, Ana López Suarez, Marta Lucas García, Gustavo Cortez Quiroga, Beatriz Calvo Bernal, Samuel Ortiz Cruces, and et al. 2024. "Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study" Journal of Clinical Medicine 13, no. 8: 2256. https://doi.org/10.3390/jcm13082256
APA StyleFernández-Olmo, M. R., Bailen, M. C., Martínez Quesada, M., Rus Mansilla, C., Martin Toro, M., López Suarez, A., Lucas García, M., Cortez Quiroga, G., Calvo Bernal, B., Ortiz Cruces, S., Torres Llergo, J., García Ruano, A., Fernández Anguita, M., Franco, D., & Cordero, A. (2024). Lp(a) Levels in Relatives of Patients with Acute Coronary Syndrome and Elevated Lp(a): HER(a) Study. Journal of Clinical Medicine, 13(8), 2256. https://doi.org/10.3390/jcm13082256







