Association between Ovarian Endometriomas and Stage of Endometriosis
Abstract
:1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nezhat, F.; Datta, M.S.; Hanson, V.; Pejovic, T.; Nezhat, C.; Nezhat, C. The relationship of endometriosis and ovarian malignancy: A review. Fertil. Steril. 2008, 90, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Veeraswamy, A.; Lewis, M.; Mann, A.; Kotikela, S.; Hajhosseini, B.; Nezhat, C. Extragenital endometriosis. Clin. Obstet. Gynecol. 2010, 53, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Yang, Y.C.; Wang, J.H.; Lin, S.Z.; Ding, D.C. Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. J. Clin. Med. 2021, 10, 4173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melo, A.S.; Rosa-e-Silva, J.C.; Rosa-e-Silva, A.C.; Poli-Neto, O.B.; Ferriani, R.A.; Vieira, C.S. Unfavorable lipid profile in women with endometriosis. Fertil. Steril. 2010, 93, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Okoth, K.; Wang, J.; Zemedikun, D.; Thomas, G.N.; Nirantharakumar, K.; Adderley, N.J. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: A retrospective matched cohort study. BJOG 2021, 128, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu-Zaid, A.; Gari, A.; Tulbah, M.; Alshahrani, M.S.; Khadawardi, K.; Ahmed, A.M.; Baradwan, A.; Bukhari, I.A.; Alyousef, A.; Alomar, O.; et al. Association between endometriosis and obstetric complications: Insight from the National Inpatient Sample. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 292, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Exacoustos, C.; De Felice, G.; Pizzo, A.; Morosetti, G.; Lazzeri, L.; Centini, G.; Piccione, E.; Zupi, E. Isolated Ovarian Endometrioma: A History Between Myth and Reality. J. Minim. Invasive Gynecol. 2018, 25, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef] [PubMed]
- International Working Group of AAGL, ESGE, ESHRE and WES; Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.M.; Missmer, S.; Petrozza, J.; Tomassetti, C.; et al. Endometriosis classification, staging and reporting systems: A review on the road to a universally accepted endometriosis classification. Facts Views Vis. Obgyn. 2021, 13, 305–330. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obstet. Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Abrao, M.S.; Andres, M.P.; Miller, C.E.; Gingold, J.A.; Rius, M.; Neto, J.S.; Carmona, F. AAGL 2021 Endometriosis Classification: An Anatomy-based Surgical Complexity Score. J Minim Invasive Gynecol. 2021, 28, 1941–1950.e1. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Szyllo, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampson, J.A. Perforating hemorrhagic (chocolate) cysts of the ovary: Their importance and especially their relation to pelvic adenomas of endometrial type (“adenomyoma” of the uterus, rectovaginal septum, sigmoid etc.). Arch. Surg. 1921, 3, 245–323. [Google Scholar] [CrossRef]
- Blaustein, A.; Kantius, M.; Kaganowicz, A.; Pervez, N.; Wells, J. Inclusions in ovaries of females aged day 1–30 years. Int. J. Gynecol. Pathol. 1982, 1, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kerner, H.; Gaton, E.; Czernobilsky, B. Unusual ovarian, tubal and pelvic mesothelial inclusions in patients with endometriosis. Histopathology 1981, 5, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Nezhat, F.; Nezhat, C.; Seidman, D.S. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum. Reprod. 1994, 9, 2212–2213. [Google Scholar] [CrossRef] [PubMed]
- Redwine, D.B. Ovarian endometriosis: A marker for more extensive pelvic and intestinal disease. Fertil. Steril. 1999, 72, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.S.D.C.; Maia, S.B.; Lúcio, J.D.; Lima, M.D.; Ribeiro, H.S.A.A.; Ribeiro, P.A.A.G. Mapping of endometriosis in patients with unilateral endometrioma. Medicine 2021, 100, e26979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Fozan, H.; Tulandi, T. Left lateral predisposition of endometriosis and endometrioma. Obstet. Gynecol. 2003, 101, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Crowgey, S.; Nezhat, F. Videolaseroscopy for the treatment of endometriosis associated with infertility. Fertil. Steril. 1989, 51, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Pais, A.S.; Flagothier, C.; Tebache, L.; Almeida Santos, T.; Nisolle, M. Impact of Surgical Management of Endometrioma on AMH Levels and Pregnancy Rates: A Review of Recent Literature. J. Clin. Med. 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chapron, C.; Vercellini, P.; Barakat, H.; Vieira, M.; Dubuisson, J.B. Management of ovarian endometriomas. Hum. Reprod. Update 2002, 8, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, J.; Saridogan, E.; Ulrich, U.A.; Sillem, M.; Oppelt, P.; Schweppe, K.W.; Krentel, H.; Janschek, E.; Exacoustos, C.; Malzoni, M.; et al. The #Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet. Gynecol. Scand. 2021, 100, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Menakaya, U.; Reid, S.; Lu, C.; Gerges, B.; Infante, F.; Condous, G. Performance of ultrasound-based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet. Gynecol. 2016, 48, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Condous, G.; Gerges, B.; Thomassin-Naggara, I.; Becker, C.; Tomassetti, C.; Krentel, H.; van Herendael, B.J.; Malzoni, M.; Abrao, M.S.; Saridogan, E.; et al. Non-invasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An International Consensus Statement. Ultrasound Obstet. Gynecol. 2024, 64, 129–144. [Google Scholar] [CrossRef] [PubMed]
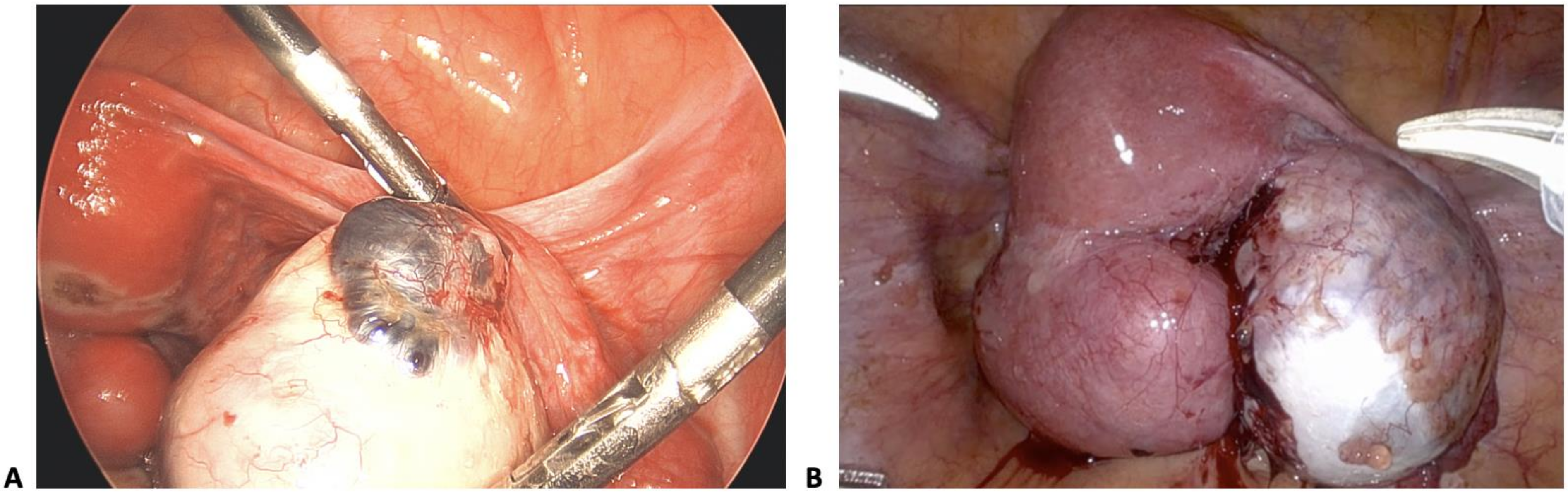

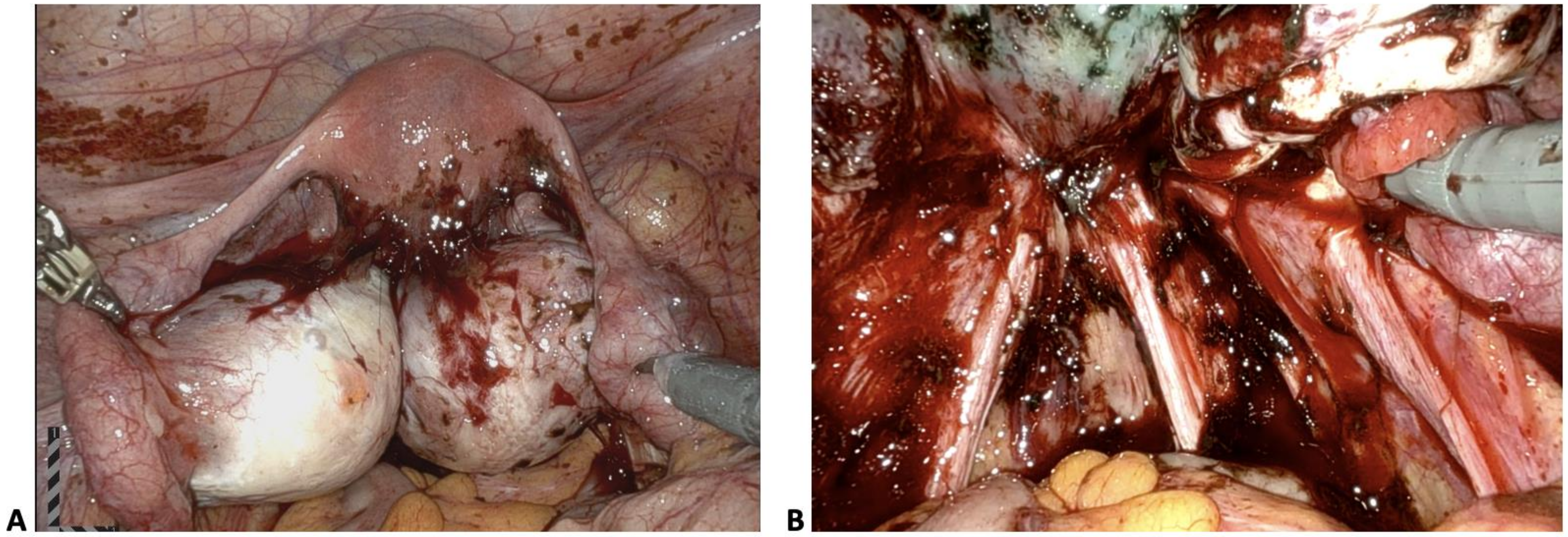
| Stage of Endometriosis | ||||
|---|---|---|---|---|
| 3 (n = 15) | 4 (n = 71) | p-Value | ||
| Laterality of endometrioma | Left | 10 (66.67%) | 18 (25.35%) | 0.0001 |
| Right | 5 (33.33%) | 17 (23.94%) | ||
| Bilateral | 0 (0%) | 36 (50.7%) | ||
| Size of right endometrioma (cm) | ≤3 cm | 1 (20%) | 19 (35.85%) | 0.8741 |
| 3–6 cm | 3 (60%) | 21 (39.62%) | ||
| 6–9 cm | 1 (20%) | 10 (18.87%) | ||
| ≥10 cm | 0 (0%) | 3 (5.66%) | ||
| Size of left endometrioma (cm) | ≤3 cm | 7 (70%) | 21 (38.89%) | 0.3964 |
| 3–6 cm | 2 (20%) | 21 (38.89%) | ||
| 6–9 cm | 1 (10%) | 9 (16.67%) | ||
| ≥10 cm | 0 (0%) | 3 (5.56%) | ||
| Presence of Endometriomas | ||||
|---|---|---|---|---|
| No (n = 136) | Yes (n = 86) | p-Value | ||
| Age | 36.6 ± 8.4 | 37.2 ± 7.6 | 0.5675 | |
| Gravidity | 1.32 ± 1.74 | 1.05 ± 1.45 | 0.2411 | |
| Parity | 0.67 ± 0.95 | 0.52 ± 0.84 | 0.2865 | |
| Medical treatment prior to surgery | 74 (54.41%) | 48 (55.81%) | 0.8379 | |
| Indication for surgery | Pelvic pain | 83 (61.03%) | 47 (54.65%) | 0.3473 |
| Infertility | 31 (22.79%) | 29 (33.72%) | 0.0741 | |
| Dysmenorrhea | 72 (52.94%) | 57 (66.28%) | 0.0497 | |
| Dyspareunia | 47 (34.56%) | 33 (38.37%) | 0.5643 | |
| Abnormal uterine bleeding | 81 (59.56%) | 48 (55.81%) | 0.5817 | |
| Other ovarian cyst | 8 (5.88%) | 8 (9.3%) | 0.3371 | |
| Leiomyoma | 28 (20.59%) | 4 (4.65%) | 0.0010 | |
| History of endometriosis | 41 (30.15%) | 28 (32.56%) | 0.7053 | |
| General pelvic pain (pelvic pain, dysmenorrhea, and dyspareunia) | 118 (86.76%) | 82 (95.35%) | 0.0370 | |
| Prior surgery for endometriosis | 52 (38.24%) | 30 (34.88%) | 0.6142 | |
| Prior abdominal surgery | 60 (44.12%) | 36 (41.86%) | 0.7409 | |
| Pre-operative imaging | MRI | 48 (35.29%) | 49 (56.98%) | 0.0015 |
| Ultrasound | 98 (72.06%) | 47 (54.65%) | 0.0079 | |
| Stage of endometriosis | 1 | 35 (25.74%) | 0 (0%) | <0.0001 |
| 2 | 51 (37.5%) | 0 (0%) | ||
| 3 | 21 (15.44%) | 15 (17.44%) | ||
| 4 | 29 (21.32%) | 71 (82.56%) | ||
| Duration of Medical Management Prior to Surgery | Types of Medical Management | |||||
|---|---|---|---|---|---|---|
| GnRH Agonist | GnRH Antagonist | Combined Oral Contraceptive Pills | Progesterone only | Other | Total | |
| ≤3 mo | 14 | 11 | 11 | 6 | 3 | 45 |
| >3 but ≤6 mo | 5 | 0 | 2 | 3 | 2 | 12 |
| >6 but ≤12 mo | 1 | 0 | 3 | 2 | 0 | 6 |
| >12 mo | 1 | 0 | 32 | 10 | 4 | 47 |
| Total | 21 | 11 | 48 | 21 | 9 | 110 |
| Duration of Medical Management Prior to Surgery | Stage of Endometriosis | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| ≤3 mo | 2 | 5 | 7 | 31 | 45 |
| >3 but ≤6 mo | 2 | 0 | 3 | 7 | 12 |
| >6 but ≤12 mo | 0 | 1 | 1 | 4 | 6 |
| >12 mo | 11 | 18 | 7 | 11 | 47 |
| Total | 15 | 24 | 18 | 53 | 110 |
| Type of Endometrioma | Stageof Endometriosis | ||
|---|---|---|---|
| 3 | 4 | Total | |
| Type I (<3 cm) | 6 | 15 | 21 |
| Type II (≥3 cm) | 7 | 54 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seraji, S.; Ali, A.; Demirel, E.; Akerman, M.; Nezhat, C.; Nezhat, F.R. Association between Ovarian Endometriomas and Stage of Endometriosis. J. Clin. Med. 2024, 13, 4530. https://doi.org/10.3390/jcm13154530
Seraji S, Ali A, Demirel E, Akerman M, Nezhat C, Nezhat FR. Association between Ovarian Endometriomas and Stage of Endometriosis. Journal of Clinical Medicine. 2024; 13(15):4530. https://doi.org/10.3390/jcm13154530
Chicago/Turabian StyleSeraji, Shadi, Aliyah Ali, Esra Demirel, Meredith Akerman, Camran Nezhat, and Farr R. Nezhat. 2024. "Association between Ovarian Endometriomas and Stage of Endometriosis" Journal of Clinical Medicine 13, no. 15: 4530. https://doi.org/10.3390/jcm13154530






