Toll-like Receptors: Key Players in Squamous Cell Carcinoma Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Patients and Research Material
2.2. Immunophenotyping
2.3. Quantification of Soluble Forms of TLR Forms
2.4. Statistical Analysis
3. Results
3.1. Characteristics of SCC Patients Included in the Study at the Time of Recruitment, with Particular Emphasis on the Percentage of TLRs Tested on Selected T and B Cell Subpopulations
3.2. TLR Analysis during the 3-Year Follow-Up Period
3.3. Spearman’s Rank Correlation Analysis of Results Obtained from Analyses of the Percentage of Occurrence of Selected TLRs on Individual Lymphocyte Subpopulations and the Concentration of Their Soluble Forms in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Facts & Figures 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 15 April 2024).
- Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 15 April 2024).
- Types of Lung Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/lung-cancer/stages-types-grades/types (accessed on 15 April 2024).
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Lau, S.C.M.; Pan, Y.; Velcheti, V.; Wong, K.K. Squamous Cell Lung Cancer: Current Landscape and Future Therapeutic Options. Cancer Cell 2022, 40, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Bebb, D.G.; Murray, C.; Giannopoulou, A.; Felip, E. Symptoms and Experiences with Small Cell Lung Cancer: A Mixed Methods Study of Patients and Caregivers. Pulm. Ther. 2023, 9, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Sabbula, B.R.; Gasalberti, D.P.; Anjum, F. Squamous Cell Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sun, S.; Yang, Y.; Chen, M.; Wang, L.; Pan, H.; Zhang, X.; Wagnieres, G.; Mohammad, Y.; Barreiro, E.; Pirozzolo, G.; et al. Comparison of Autofluorescence and White-Light Bronchoscopies Performed with the Evis Lucera Spectrum for the Detection of Bronchial Cancers: A Meta-Analysis. Transl. Lung Cancer Res. 2020, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Survival for Lung Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/lung-cancer/survival (accessed on 15 April 2024).
- Jeon, D.S.; Kim, H.C.; Kim, S.H.; Kim, T.-J.; Kim, H.K.; Moon, M.H.; Beck, K.S.; Suh, Y.-G.; Song, C.; Ahn, J.S.; et al. Five-Year Overall Survival and Prognostic Factors in Patients with Lung Cancer: Results from the Korean Association of Lung Cancer Registry (KALC-R) 2015. Cancer Res. Treat. 2023, 55, 103–111. [Google Scholar] [CrossRef]
- Cancer Survival by Stage at Diagnosis for England (Experimental Statistics)—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalbystageatdiagnosisforenglandexperimentalstatistics/adultsdiagnosed20122013and2014andfollowedupto2015 (accessed on 15 April 2024).
- Tang, Y.; Qiao, G.; Xu, E.; Xuan, Y.; Liao, M.; Yin, G. Biomarkers for Early Diagnosis, Prognosis, Prediction, and Recurrence Monitoring of Non-Small Cell Lung Cancer. Onco Targets Ther. 2017, 10, 4527–4534. [Google Scholar] [CrossRef]
- Balata, H.; Fong, K.M.; Hendriks, L.E.; Lam, S.; Ostroff, J.S.; Peled, N.; Wu, N.; Aggarwal, C. Prevention and Early Detection for NSCLC: Advances in Thoracic Oncology 2018. J. Thorac. Oncol. 2019, 14, 1513–1527. [Google Scholar] [CrossRef]
- Shcheblyakov, D.V.; Logunov, D.Y.; Tukhvatulin, A.I.; Shmarov, M.M.; Naroditsky, B.S.; Gintsburg, A.L. Toll-Like Receptors (TLRs): The Role in Tumor Progression. Acta Naturae 2010, 2, 21–29. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Fu, Y. The Critical Role of Toll-like Receptor-Mediated Signaling in Cancer Immunotherapy. Med. Drug Discov. 2022, 14, 100122. [Google Scholar] [CrossRef]
- Farooq, M.; Batool, M.; Kim, M.S.; Choi, S. Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies. Front. Cell Dev. Biol. 2021, 9, 756315. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern Recognition Receptors and the Innate Immune Response to Viral Infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, C.; Liu, J. The Role of Toll-Like Receptors in Oncotherapy. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Patidar, A.; Sarma, U.; Chauhan, P.; Pandey, S.P.; Chandel, H.S.; Bodhale, N.; Ghosh, S.K.; Guzman, C.A.; Ebensen, T.; et al. Interdependencies between Toll-like Receptors in Leishmania Infection. Immunology 2021, 164, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Patidar, A.; Shukla, D.; Bodhale, N.; Saha, B. TLR2, TLR3, TLR4, TLR9 and TLR11 Expression on Effector CD4+ T-Cell Subsets in Leishmania Donovani Infection. Exp. Parasitol. 2023, 255, 108645. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, M.; Zheng, Q.C. Emerging Role of Toll-like Receptor 4 in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2015, 2, 11–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giurini, E.F.; Madonna, M.B.; Zloza, A.; Gupta, K.H. Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression. Cancers 2022, 14, 2923. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Du, B.; Jiang, Q.-L.; Cleveland, J.; Liu, B.-R.; Zhang, D. Targeting Toll-like Receptors against Cancer. J. Cancer Metastasis Treat. 2016, 2, 463–470. [Google Scholar] [CrossRef]
- Rolfo, C.; Giovannetti, E.; Martinez, P.; McCue, S.; Naing, A. Applications and Clinical Trial Landscape Using Toll-like Receptor Agonists to Reduce the Toll of Cancer. npj Precis. Onc. 2023, 7, 1–11. [Google Scholar] [CrossRef]
- Velasco, W.V.; Khosravi, N.; Castro-Pando, S.; Torres-Garza, N.; Grimaldo, M.T.; Krishna, A.; Clowers, M.J.; Umer, M.; Tariq Amir, S.; Del Bosque, D.; et al. Toll-like Receptors 2, 4, and 9 Modulate Promoting Effect of COPD-like Airway Inflammation on K-Ras-Driven Lung Cancer through Activation of the MyD88/NF-ĸB Pathway in the Airway Epithelium. Front. Immunol. 2023, 14, 1118721. [Google Scholar] [CrossRef]
- Arora, S.; Ahmad, S.; Irshad, R.; Goyal, Y.; Rafat, S.; Siddiqui, N.; Dev, K.; Husain, M.; Ali, S.; Mohan, A.; et al. TLRs in Pulmonary Diseases. Life Sci. 2019, 233, 116671. [Google Scholar] [CrossRef] [PubMed]
- Hoden, B.; DeRubeis, D.; Martinez-Moczygemba, M.; Ramos, K.S.; Zhang, D. Understanding the Role of Toll-like Receptors in Lung Cancer Immunity and Immunotherapy. Front. Immunol. 2022, 13, 1033483. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Cremer, I. Dual Roles of TLR7 in the Lung Cancer Microenvironment. Oncoimmunology 2015, 4, e991615. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Petitprez, F.; Marmier, S.; Lupo, A.; Gillard, M.; Ouakrim, H.; Victor, N.; Vincenzo, D.B.; Joubert, P.E.; et al. Toll like Receptor 7 Expressed by Malignant Cells Promotes Tumor Progression and Metastasis through the Recruitment of Myeloid Derived Suppressor Cells. OncoImmunology 2019, 8, e1505174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, J.; Hu, J.; Luo, Y.; Li, F.; Xiao, L.; Zhong, M. High Expression of Toll-like Receptor 5 Correlates with Better Prognosis in Non-Small-Cell Lung Cancer: An Anti-Tumor Effect of TLR5 Signaling in Non-Small Cell Lung Cancer. J. Cancer Res. Clin Oncol. 2014, 140, 633–643. [Google Scholar] [CrossRef]
- Fan, S.; Liao, Y.; Qiu, W.; Li, L.; Li, D.; Cao, X.; Ai, B. Targeting Toll-like Receptor 4 with CLI-095 (TAK-242) Enhances the Antimetastatic Effect of the Estrogen Receptor Antagonist Fulvestrant on Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2020, 22, 2074–2086. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Wei, F.; Ren, X. The Role of Toll-like Receptor 4 in Tumor Microenvironment. Oncotarget 2017, 8, 66656–66667. [Google Scholar] [CrossRef]
- Sato, Y.; Goto, Y.; Narita, N.; Hoon, D.S.B. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009, 2 (Suppl. S1), 205–214. [Google Scholar] [CrossRef] [PubMed]
- Martín-Medina, A.; Cerón-Pisa, N.; Martinez-Font, E.; Shafiek, H.; Obrador-Hevia, A.; Sauleda, J.; Iglesias, A. TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer. Int. J. Mol. Sci. 2022, 23, 6539. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef] [PubMed]
- Oft, M. Immune Regulation and Cytotoxic T Cell Activation of IL-10 Agonists—Preclinical and Clinical Experience. Semin. Immunol. 2019, 44, 101325. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. TLR4 Signaling Promotes Immune Escape of Human Lung Cancer Cells by Inducing Immunosuppressive Cytokines and Apoptosis Resistance. Mol. Immunol. 2007, 44, 2850–2859. [Google Scholar] [CrossRef]
- Zhu, J.; Mohan, C. Toll-like Receptor Signaling Pathways--Therapeutic Opportunities. Mediat. Inflamm. 2010, 2010, 781235. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Sánchez, A.; Suárez-Martínez, E.; Sánchez-Díaz, L.; Carnero, A. Therapeutic Targeting of Signaling Pathways Related to Cancer Stemness. Front. Oncol. 2020, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Y.; Zhang, P.; Xing, H.; Zhao, S.; Song, Y.; Wan, D.; Yu, J. Targeting Toll-like Receptor 7/8 for Immunotherapy: Recent Advances and Prospectives. Biomark. Res. 2022, 10, 89. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Y.; Xie, B.; Ye, P.; Huang, J.; Lu, Z. Roles of Toll-like Receptors: From Inflammation to Lung Cancer Progression (Review). Biomed. Rep. 2018, 8, 126–132. [Google Scholar] [CrossRef]
- Horvath, L.; Thienpont, B.; Zhao, L.; Wolf, D.; Pircher, A. Overcoming Immunotherapy Resistance in Non-Small Cell Lung Cancer (NSCLC)—Novel Approaches and Future Outlook. Mol. Cancer 2020, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Ran, S. The Role of TLR4 in Chemotherapy-Driven Metastasis. Cancer Res. 2015, 75, 2405–2410. [Google Scholar] [CrossRef]
- Sun, Z.; Luo, Q.; Ye, D.; Chen, W.; Chen, F. Role of Toll-like Receptor 4 on the Immune Escape of Human Oral Squamous Cell Carcinoma and Resistance of Cisplatin-Induced Apoptosis. Mol. Cancer 2012, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Crozet, L.; Damotte, D.; Iribarren, K.; Schramm, C.; Alifano, M.; Lupo, A.; Cherfils-Vicini, J.; Goc, J.; Katsahian, S.; et al. TLR7 Promotes Tumor Progression, Chemotherapy Resistance, and Poor Clinical Outcomes in Non–Small Cell Lung Cancer. Cancer Res. 2014, 74, 5008–5018. [Google Scholar] [CrossRef]
- Haroun, R.; Naasri, S.; Oweida, A.J. Toll-Like Receptors and the Response to Radiotherapy in Solid Tumors: Challenges and Opportunities. Vaccines 2023, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Muresan, X.M.; Bouchal, J.; Culig, Z.; Souček, K. Toll-Like Receptor 3 in Solid Cancer and Therapy Resistance. Cancers 2020, 12, 3227. [Google Scholar] [CrossRef]
- Millar, F.R.; Pennycuick, A.; Muir, M.; Quintanilla, A.; Hari, P.; Freyer, E.; Gautier, P.; Meynert, A.; Grimes, G.; Coll, C.S.; et al. Toll-like Receptor 2 Orchestrates a Tumor Suppressor Response in Non-Small Cell Lung Cancer. Cell Rep. 2022, 41, 111596. [Google Scholar] [CrossRef]
- Wei, F.; Yang, F.; Li, J.; Zheng, Y.; Yu, W.; Yang, L.; Ren, X. Soluble Toll-like Receptor 4 Is a Potential Serum Biomarker in Non-Small Cell Lung Cancer. Oncotarget 2016, 7, 40106–40114. [Google Scholar] [CrossRef]

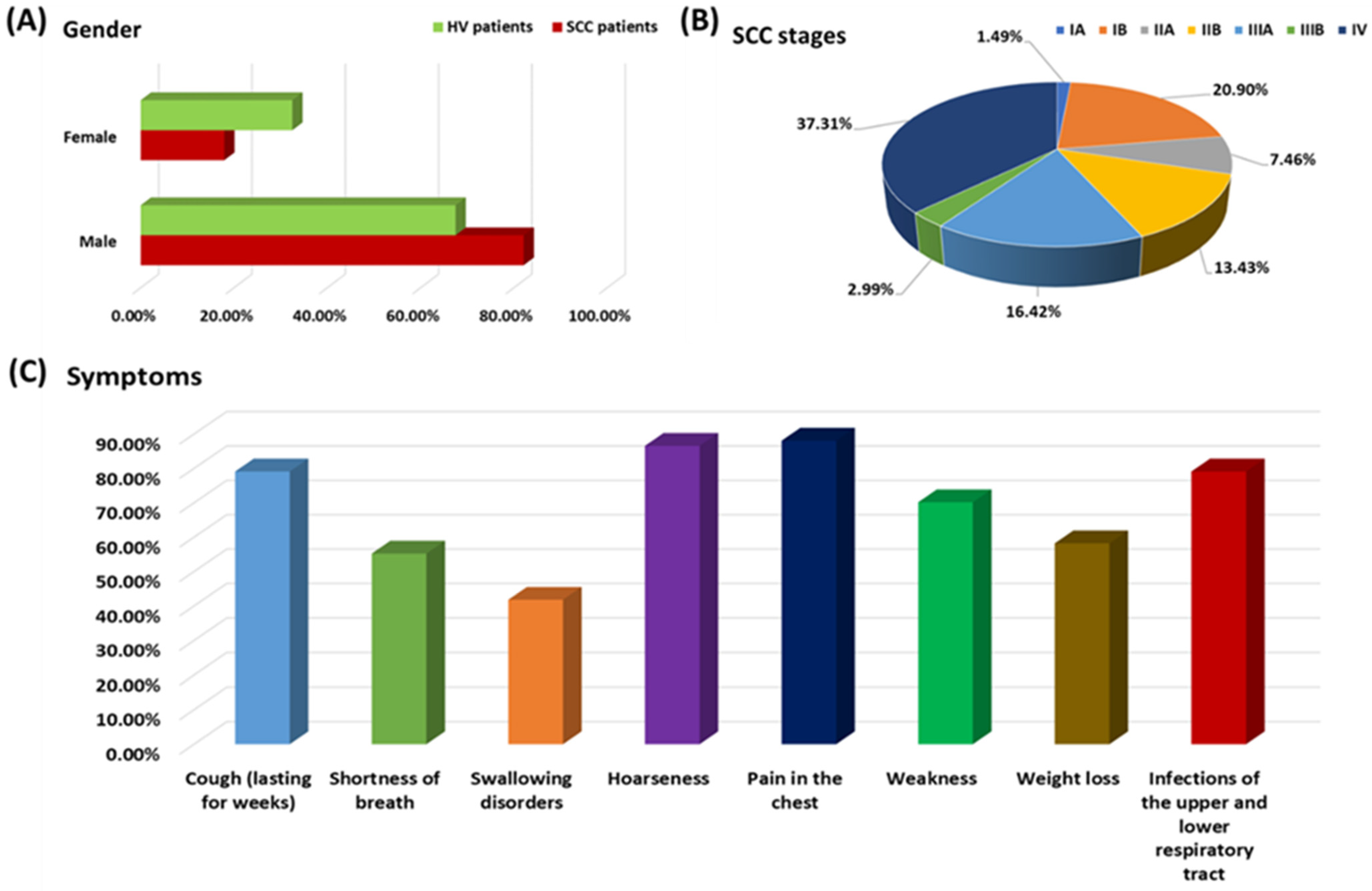
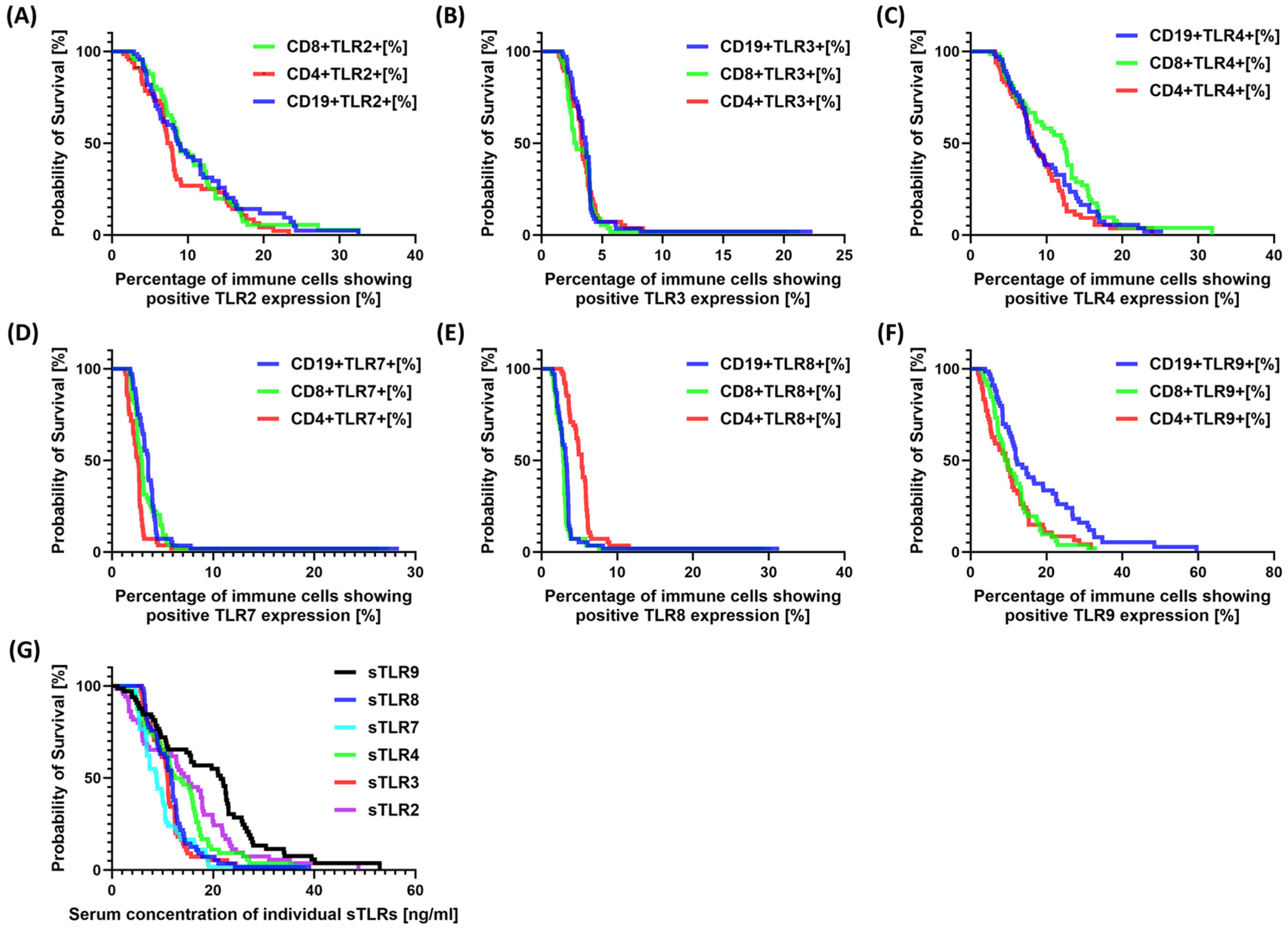
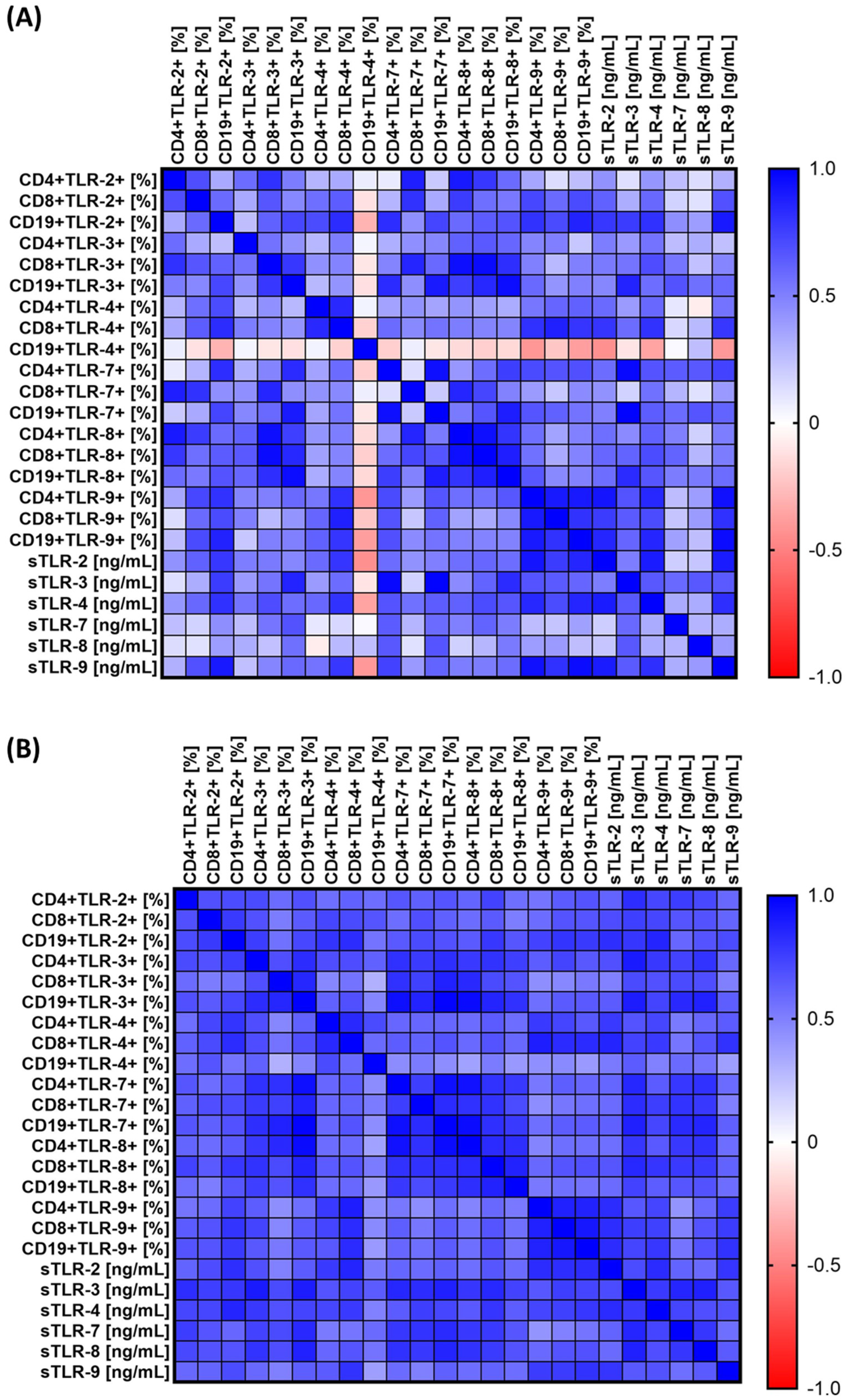
| Parameters | SCC (n = 67) | HV (n = 40) | p-Value |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| WBC [103/mm3] | 6.24 (3.58–16.82) | 6.22 (1.00–7.82) | 0.895 |
| LYM [103/mm3] | 1.10 (0.30–3.13) | 1.85 (1.23–3.10) | 0.000 * |
| MON [103/mm3] | 0.51 (0.28–1.18) | 0.38 (0.22–0.72) | 0.0001 * |
| NEU [103/mm3] | 4.35 (2.37–14.76) | 3.45 (0.22–7.82) | 0.0005 * |
| RBC [106/mm3] | 3.18 (2.45–3.64) | 4.55 (1.78–5.19) | 0.000 * |
| HGB [g/dL] | 9.04 (6.26–11.36) | 6.49 (3.95–8.40) | 0.0004 |
| PLT [103/mm3] | 219.64 (84.32–458.32) | 175.11 (4.63–290.35) | 0.0000 * |
| CRP [mg/L] | 14.65 (0.50–107.63) | 0.16 (0.00–1.95) | 0.0000 * |
| T CD3+ [%] | 47.44 (28.73–61.81) | 71.94 (65.09–96.34) | 0.0000 * |
| T CD3+CD8+ [%] | 20.18 (6.62–39.93) | 26.91 (20.18–31.07) | 0.0000 * |
| T CD3+CD4+ [%] | 27.27 (10.87–38.76) | 46.76 (20.18–65.51) | 0.0000 * |
| Ratio T CD3+CD4+ do T CD3+CD8+ | 1.41 (0.46–5.58) | 1.78 (1.53–2.23) | 0.0000 * |
| B CD19+ [%] | 5.23 (1.31–50.62) | 12.46 (7.13–16.83) | 0.0000 * |
| Parameters | SCC (n = 67) | HV (n = 40) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 55) (Group 1) | Female (n = 12) (Group 2) | Male (n = 27) (Group 3) | Female (n = 13) (Group 4) | 1 vs. 2 | 1 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | |||||
| WBC [103/mm3] | 6.25 (3.58–16.82) | 6.36 (4.74–7.51) | 6.27 (4.95–7.78) | 6.11 (4.75–7.82) | 0.916 | 0.753 | 0.538 | 0.685 |
| LYM [103/mm3] | 1.15 (0.52–3.13) | 0.95 (0.30–1.63) | 1.85 (1.23–2.85) | 1.81 (1.32–3.10) | 0.149 | 0.000 * | 0.000 * | 0.478 |
| MON [103/mm3] | 0.50 (0.28–1.18) | 0.56 (0.33–0.65) | 0.41 (0.22–0.72) | 0.37 (0.24–0.67) | 1.00 | 0.003 * | 0.045 * | 0.080 * |
| NEU [103/mm3] | 4.22 (2.37–14.76) | 4.50 (3.16–6.41) | 3.48 (2.34–7.82) | 3.18 (1.78–4.87) | 0.577 | 0.039 * | 0.002 * | 0.024 * |
| RBC [106/mm3] | 3.15 (2.52–3.64) | 3.24 (2.45–3.59) | 4.58 (3.95–5.19) | 4.54 (4.08–5.10) | 0.656 | 0.000 * | 0.000 * | 0.000 * |
| HGB [g/dL] | 9.04 (6.26–11.36) | 9.01 (6.39–0.27) | 6.49 (4.63–8.40) | 6.69 (6.19–8.10) | 0.980 | 0.000 * | 0.000 * | 0.063 |
| PLT [103/mm3] | 220.32 (84.32–458.32) | 218.62 (150.28–392.36) | 177.13 (104.67–290.35) | 174.61 (104.67–249.59) | 0.827 | 0.011 * | 0.018 * | 0.123 |
| CRP [mg/L] | 15.01 0.50–107.63 | 13.47 (3.62–68.50) | 0.15 (0.00–1.95) | 0.23 (0.03–1.53) | 0.599 | 0.000 * | 0.000 * | 0.000 * |
| T CD3+ [%] | 47.56 (13.87–61.81) | 48.75 (33.20–96.44) | 70.98 (65.09–96.34) | 72.41 (68.05–94.58) | 0.649 | 0.000 * | 0.000 * | 0.000 * |
| T CD3+CD8+ [%] | 17.79 (6.09–39.93) | 20.98 (14.53–30.11) | 26.43 (20.18–30.83) | 27.52 (22.25–31.07) | 0.335 | 0.001 * | 0.000 * | 0.000 * |
| T CD3+CD4+ [%] | 27.27 (7.05–38.76) | 25.08 (17.52–29.08) | 46.04 (42.25–65.51) | 47.69 (42.19–64.31) | 0.993 | 0.000 * | 0.000 * | 0.000 * |
| Ratio T CD3+CD4+ do T CD3+CD8+ | 0.98 (0.31–3.80) | 0.82 (0.47–8.37) | 1.78 (1.53–2.23) | 1.78 (1.53–2.13) | 0.765 | 0.000 * | 0.001 * | 0.001 * |
| B CD19+ [%] | 5.11 (1.31–50.62) | 6.86 (2.46–50.62) | 12.19 (7.81–16.53) | 12.77 (11.03–16.83) | 0.360 | 0.000 * | 0.000 * | 0.000 * |
| Parameters | I (n = 15) | II (n = 14) | III (n = 13) | IV (n = 25) | p-Value |
|---|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | ||
| WBC [103/mm3] | 5.64 (3.58–7.51) | 6.22 (4.75–17.08) | 6.47 (5.08–16.82) | 6.43 (3.70–11.72) | 0.5161 |
| LYM [103/mm3] | 1.08 (0.30–1.73) | 1.27 (0.55–2.65) | 1.02 (0.48–3.13) | 0.94 (0.52–2.22) | 0.2871 |
| MON [103/mm3] | 0.48 (0.28–1.18) | 0.50 (0.29–0.88) | 0.57 (0.33–1.12) | 0.51 (0.33–0.95) | 0.5042 |
| NEU [103/mm3] | 3.47 (2.37–6.41) | 4.59 (2.62–10.57) | 4.35 93.35–14.76) | 4.63 (2.41–9.01) | 0.4404 |
| RBC [106/mm3] | 3.25 (2.45–3.64) | 3.31 (2.53–3.56) | 3.08 (2.52–3.59) | 3.03 (2.54–3.64) | 0.7720 |
| HGB [g/dl] | 9.25 (6.39–10.74) | 9.49 (6.87–11.36) | 8.91 (6.46–10.27) | 8.70 (6.26–10.95) | 0.1379 |
| PLT [103/mm3] | 219.64 (84.32–392.36) | 193.12 (111.52–316.88) | 239.36 (146.88–458.32) | 236.64 (114.92–392.36) | 0.500 |
| CRP [mg/L] | 11.27 (3.08–26.90) | 11.96 (1.86–107.63) | 23.17 (6.00–91.41) | 11.75 (0.50–62.37) | 0.3624 |
| T CD3+ [%] | 47.05 (33.20–59.34) | 47.46 (30.73–60.55) | 47.72 (39.29–54.350 | 47.44 (28.73–61.81) | 0.9441 |
| T CD3+CD8+ [%] | 17.16 (6.62–39.94) | 18.95 (11.10–37.99) | 20.98 (8.80–30.11) | 18.90 (7.44–38.92) | 0.9441 |
| T CD3+CD4+ [%] | 29.82 (15.64–36.94) | 26.76 (17.06–33.03) | 27.27 (20.75–37.73) | 27.53 (10.87–38.76) | 0.8699 |
| Ratio T CD3+CD4+ do T CD3+CD8+ | 1.49 (0.46–5.58) | 1.38 (0.51–2.79) | 1.40 (0.70–3.96) | 1.42 (0.51–4.20) | 0.9441 |
| B CD19+ [%] | 6.79 (2.46–13.93) | 5.97 (2.01–50.62) | 3.58 (1.65–42.58) | 4.37 (1.31–14.56) | 0.0842 |
| Parameters | SCC (n = 67) | HV (n = 40) | p-Value |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| T CD4+TLR-2+ [%] | 3.79 (1.43–14.29) | 0.83 (0.00–2.15) | 0.000 * |
| T CD8+TLR-2+ [%] | 5.52 (2.16–14.91) | 0.56 (0.10–2.91) | 0.000 * |
| B CD19+TLR-2+ [%] | 4.77 (2.69–12.29) | 1.32 (0.10–2.61) | 0.000 * |
| T CD4+TLR-3+ [%] | 1.88 (1.33–3.34) | 1.06 (0.03–2.13) | 0.000 * |
| T CD8+TLR-3+ [%] | 1.84 (1.12–2.71) | 0.67 (0.03–1.83) | 0.000 * |
| B CD19+TLR-3+ [%] | 2.17 (1.44–3.00) | 0.52 (0.12–1.12) | 0.000 * |
| T CD4+TLR-4+ [%] | 4.58 (2.46–11.83) | 1.08 (0.12–2.01) | 0.000 * |
| T CD8+TLR-4+ [%] | 5.48 (2.47–12.78) | 0.99 (0.01–1.79) | 0.000 * |
| B CD19+TLR-4+ [%] | 4.64 (2.58–17.09) | 0.79 (0.01–1.82) | 0.000 * |
| T CD4+TLR-7+ [%] | 1.49 (0.97–2.37) | 0.55 (0.02–1.20) | 0.000 * |
| T CD8+TLR-7+ [%] | 1.90 (0.83–3.04) | 0.32 (0.02–1.26) | 0.000 * |
| B CD19+TLR-7+ [%] | 2.18 (1.52–2.73) | 0.44 (0.02–0.74) | 0.000 * |
| T CD4+TLR-8+ [%] | 3.22 (2.01–4.37) | 0.76 (0.08–1.77) | 0.000 * |
| T CD8+TLR-8+ [%] | 1.63 (0.88–2.77) | 0.33 (0.06–0.93) | 0.000 * |
| B CD19+TLR-8+ [%] | 1.87 (0.82–2.91) | 0.56 (0.06–1.33) | 0.000 * |
| T CD4+TLR-9+ [%] | 4.92 (1.77–14.48) | 0.87 (0.14–2.28) | 0.000 * |
| T CD8+TLR-9+ [%] | 5.74 (2.58–14.00) | 1.41 (0.15–2.31) | 0.000 * |
| B CD19+TLR-9+ [%] | 7.58 (3.75–25.96) | 1.59 (0.15–3.68) | 0.000 * |
| sTLR-2 | 5.79 (0.80–19.35) | 2.63 (0.16–3.96) | 0.000 * |
| sTLR-3 | 5.98 (5.09–11.89) | 1.47 (0.04–2.97) | 0.000 * |
| sTLR-4 | 5.87 (2.74–16.02) | 3.26 (0.04–3.94) | 0.000 * |
| sTLR-7 | 4.90 (3.88–10.10) | 1.07 (0.06–1.93) | 0.000 * |
| sTLR-8 | 6.44 (4.07–12.14) | 1.01 (0.01–1.98) | 0.000 * |
| sTLR-9 | 9.55 (0.88–21.56) | 3.21 (0.01–3.92) | 0.000 * |
| Parameters | SCC (n = 67) | HV (n = 40) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 55) (Group 1) | Female (n = 12) (Group 2) | Male (n = 27) (Group 3) | Female (n = 13) (Group 4) | |||||
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | 1 vs.2 | 1 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| T CD4+TLR-2+ [%] | 3.82 (1.43–14.29) | 3.69 (1.80–8.64) | 1.04 (0.11–2.15) | 0.59 (0.21–1.89) | 0.432 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-2+ [%] | 5.59 (2.16–14.91) | 4.89 (2.69–8.67) | 0.41 (0.10–2.91) | 1.06 (0.19–2.20) | 0.442 | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-2+ [%] | 4.98 (2.69–12.29) | 4.06 (2.81–10.68) | 1.43 (0.50–2.61) | 1.23 (0.45–1.85) | 0.144 | 0.000 * | 0.000 * | 0.000 * |
| T CD4+TLR-3+ [%] | 1.99 (1.35–3.34) | 1.75 (1.33–2.93) | 1.12 (0.03–2.13) | 0.96 (0.20–1.47) | 0.159 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-3+ [%] | 1.86 (1.12–2.71) | 1.84 (1.37–2.60) | 0.54 (0.12–1.64) | 0.77 (0.12–1.83) | 0.840 | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-3+ [%] | 2.19 (1.44–3.00) | 2.02 (1.50–2.67) | 0.52 (0.12–1.12) | 0.52 (0.12–0.74) | 0.135 | 0.000 * | 0.000 * | 0.000 * |
| T CD4+TLR-4+ [%] | 4.66 (2.46–11.83) | 4.26 (3.03–7.46) | 1.20 (0.15–2.01) | 1.02 (0.14–1.50) | 0.481 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-4+ [%] | 5.69 (2.47–12.78) | 4.85 (3.021–0.36) | 1.06 (0.01–1.78) | 0.80 (0.08–1.79) | 0.432 | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-4+ [%] | 4.41 (2.58–17.09) | 5.06 (3.83–9.19) | 0.79 (0.17–1.82) | 0.78 (0.17–1.60) | 0.266 | 0.000 * | 0.000 * | 0.000 * |
| T CD4+TLR-7+ [%] | 1.51 (0.97–2.37) | 1.39 (1.14–1.96) | 0.55 (0.02–1.20) | 0.55 (0.11–0.75) | 0.213 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-7+ [%] | 1.92 (0.83–3.04) | 1.71 (1.40–2.42) | 0.32 (0.02–1.21) | 0.31 (0.08–1.26) | 0.395 | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-7+ [%] | 2.20 (1.52–2.73) | 1.97 (1.61–2.56) | 0.38 (0.08–0.74) | 0.54 (0.41–0.74) | 0.144 | 0.000 * | 0.000 * | 0.000 * |
| T CD4+TLR-8+ [%] | 3.32 (2.01–4.37) | 3.02 (2.13–3.84) | 0.77 (0.09–1.47) | 0.61 (0.22–1.77) | 0.185 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-8+ [%] | 1.74 (0.88–2.77) | 1.50 (1.05–2.58) | 0.30 (0.06–0.90) | 0.41 (0.06–0.93) | 0.140 | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-8+ [%] | 1.98 (0.82–2.91) | 1.69 (1.25–2.67) | 0.59 (0.14–1.33) | 0.52 (0.14–0.84) | 0.144 | 0.000 * | 0.000 * | 0.000 * |
| T CD4+TLR-9+ [%] | 4.99 (1.77–14.48) | 3.21 (1.85–8.40) | 0.82 (0.19–1.88) | 1.03 (0.49–2.28) | 0.081 | 0.000 * | 0.000 * | 0.000 * |
| T CD8+TLR-9+ [%] | 6.37 (2.58–14.00) | 4.57 (3.35–9.59) | 1.26 (0.15–2.08) | 1.48 (0.57–2.31) | 0.040 * | 0.000 * | 0.000 * | 0.000 * |
| B CD19+TLR-9+ [%] | 7.67 (3.75–25.96) | 5.87 (3.92–17.81) | 1.58 (0.16–3.60) | 1.77 (1.01–3.68) | 0.164 | 0.000 * | 0.000 * | 0.000 * |
| sTLR-2 | 6.21 (0.80–19.35) | 3.61 (1.86–12.05) | 2.55 (0.45–3.83) | 3.03 (0.92–3.96) | 0.097 | 0.000 * | 0.122 | 0.000 * |
| sTLR-3 | 6.09 (5.09–11.89) | 5.75 (5.22–9.17) | 1.43 (0.04–2.93) | 1.50 (0.86–2.97) | 0.135 | 0.000 * | 0.000 * | 0.000 * |
| sTLR-4 | 5.97 (2.74–16.02) | 5.31 (2.86–8.46) | 3.24 (0.91–3.94) | 3.33 (1.76–3.89) | 00.073 | 0.000 * | 0.000 * | 0.000 * |
| sTLR-7 | 4.99 (3.88–10.10) | 4.76 (3.98–7.19) | 1.06 (0.09–1.93) | 1.08 (0.06–1.91) | 0.266 | 0.000 * | 0.000 * | 0.000 * |
| sTLR-8 | 6.53 (4.07–12.14) | 6.13 (4.96–7.34) | 1.01 (0.01–1.98) | 0.73 (0.34–1.92) | 0.062 | 0.000 * | 0.000 * | 0.000 * |
| sTLR-9 | 10.01 (0.88–21.56) | 6.10 (2.28–15.90) | 3.20 (2.03–3.92) | 3.22 (2.05–3.92) | 0.032 * | 0.000 * | 0.000 * | 0.000 * |
| Parameters | I (n = 15) | II (n = 14) | III (n = 13) | IV (n = 25) | p-Value |
|---|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | ||
| T CD4+TLR-2+ [%] | 2.47 (11.51–3.58) | 3.17 (1.47–4.56) | 3.85 (1.43–6.69) | 5.95 (1.46–14.29) | 0.000 * |
| T CD8+TLR-2+ [%] | 3.89 (2.67–7.93) | 4.75 (2.92–6.68) | 5.88 (2.45–14.91) | 7.77 (2.16–13.09) | 0.006 * |
| B CD19+TLR-2+ [%] | 4.06 (3.07–10.64) | 4.00 (2.79–12.29) | 4.47 (2.81–9.33) | 9.82 (2.69–12.21) | 0.123 |
| T CD4+TLR-3+ [%] | 1.58 (1.33–2.05) | 1.73 (1.40–2.21) | 1.79 (1.37–2.35) | 2.67 (1.80–3.34) | 0.002 * |
| T CD8+TLR-3+ [%] | 1.58 (1.41–1.96) | 1.77 (1.12–2.10) | 2.11 (1.37–2.67) | 2.06 (1.48–2.71) | 0.007 * |
| B CD19+TLR-3+ [%] | 1.67 (1.46–1.92) | 1.93 (1.44–2.48) | 2.17 (1.76–2.49) | 2.60 (2.22–3.00) | 0.000 * |
| T CD4+TLR-4+ [%] | 4.33 (2.46–6.43) | 3.77 (3.13–5.39) | 4.53 (3.18–8.26) | 7.46 (2.83–11.83) | 0.010 * |
| T CD8+TLR-4+ [%] | 4.98 (3.02–9.38) | 4.57 (2.47–10.64) | 4.76 (3.16–12.06) | 8.29 (3.64–12.78) | 0.128 |
| B CD19+TLR-4+ [%] | 4.60 (2.58–7.42) | 4.26 (2.69–6.83) | 4.60 (2.95–7.02) | 7.99 (2.59–17.09) | 0.606 |
| T CD4+TLR-7+ [%] | 1.22 (0.97–1.35) | 1.35 (1.06–1.62) | 1.49 (1.29–1.65) | 1.86 (1.40–2.37) | 0.000 * |
| T CD8+TLR-7+ [%] | 1.56 (0.90–2.37) | 1.91 (0.83–2.43) | 1.84 (1.63–2.43) | 2.02 (1.75–3.04) | 0.193 |
| B CD19+TLR-7+ [%] | 11.73 (1.54–1.94) | 1.93 (1.52–2.36) | 2.15 (1.77–2.37) | 2.49 (2.21–2.73) | 0.000 * |
| T CD4+TLR-8+ [%] | 2.34 (2.03–2.88) | 2.83 (2.01–3.52) | 3.22 (2.65–3.56) | 3.69 (2.82–4.37) | 0.000 * |
| T CD8+TLR-8+ [%] | 1.2 (0.92–11.44) | 1.45 (0.88–2.31) | 1.61 (1.25–2.14) | 2.33 (1.77–2.77) | 0.000 * |
| B CD19+TLR-8+ [%] | 1.39 (0.99–2.18) | 1.63 (0.92–2.31) | 1.87 (1.48–2.33) | 2.47 (0.82–2.91) | 0.000 * |
| T CD4+TLR-9+ [%] | 4.17 (1.81–12.71) | 3.54 (1.77–14.26) | 2.87 (1.85–9.54) | 6.63 (2.24–14.48) | 0.009 * |
| T CD8+TLR-9+ [%] | 4.95 (3.44–8.32) | 4.64 (2.58–9.72) | 4.57 (3.29–12.44) | 8.29 (4.57–4.00) | 0.029 * |
| B CD19+TLR-9+ [%] | 6.59 (4.42–14.75) | 6.73 (4.67–21.50) | 6.05 (3.75–22.00) | 10.22 (4.74–25.96) | 0.057 |
| sTLR-2 | 5.10 (1.93–12.05) | 3.70 (0.80–15.61) | 4.47 (3.07–17.13) | 11.27 (1.51–19.35) | 0.014 * |
| sTLR-3 | 5.39 (5.09–5.60) | 5.64 (5.09–6.47) | 5.95 (5.49–6.64) | 7.82 (6.15–11.89) | 0.000 * |
| sTLR-4 | 5.25 (2.86–9.38) | 5.09 (2.74–12.35) | 5.61 (4.23–14.59) | 11.38 (4.53–16.02) | 0.002 * |
| sTLR-7 | 4.27 (3.91–7.19) | 4.72 (3.88–7.18) | 4.86 (4.36–5.09) | 5.19 (5.00–10.10) | 0.000 * |
| sTLR-8 | 5.79 (4.07–9.00) | 6.33 (5.72–7.91) | 6.39 (5.99–6.98) | 7.36 (5.94–12.14) | 0.000 * |
| sTLR-9 | 8.55 (2.99–17.50) | 8.90 (2.26–16.26) | 4.69 (2.28–18.92) | 14.83 (0.88–21.56) | 0.016 * |
| Parameters | Dead SCC Patients after 3 Years | Alive SCC Patients after 3 Years | p-Value |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| T CD4+TLR-2+ [%] | 7.31 (1.49–23.31) | 3.40 (2.41–17.37) | 0.0001 * |
| T CD8+TLR-2+ [%] | 8.65 (2.55–32.48) | 4.93 (3.49–18.37) | 0.005 * |
| B CD19+TLR-2+ [%] | 8.40 (2.91–32.48) | 5.18 (3.73–19.37) | 0.264 |
| T CD4+TLR-3+ [%] | 3.28 (1.43–8.36) | 1.99 (1.78–20.37) | 0.003 * |
| T CD8+TLR-3+ [%] | 2.71 (1.56–5.67) | 1.95 (1.83–21.37) | 0.002 * |
| B CD19+TLR-3+ [%] | 3.63 (1.75–8.19) | 2.05 (1.93–22.37) | 0.000 * |
| T CD4+TLR-4+ [%] | 8.12 (3.28–23.92) | 6.03 (3.11–23.37) | 0.052 |
| T CD8+TLR-4+ [%] | 11.47 (2.57–31.84) | 6.29 (3.20–24.37) | 0.118 |
| B CD19+TLR-4+ [%] | 7.87 (3.07–22.90) | 5.11 (3.26–25.37) | 0.008 * |
| T CD4+TLR-7+ [%] | 2.48 (1.29–5.86) | 1.54 (1.02–26.37) | 0.001 * |
| T CD8+TLR-7+ [%] | 2.87 (1.65–6.33) | 2.02 (1.88–27.37) | 0.028 * |
| B CD19+TLR-7+ [%] | 3.56 (1.82–7.79) | 2.17 (2.03–28.37) | 0.0004 * |
| T CD4+TLR-8+ [%] | 5.30 (2.58–11.53) | 3.00 (2.71–29.37) | 0.001 * |
| T CD8+TLR-8+ [%] | 2.89 (1.29–7.60) | 1.50 (1.37–30.37) | 0.000 * |
| B CD19+TLR-8+ [%] | 3.23 (1.46–8.11) | 1.73 (1.61–31.37) | 0.000 * |
| T CD4+TLR-9+ [%] | 8.02 (1.94–31.70) | 5.27 (3.04–32.37) | 0.646 |
| T CD8+TLR-9+ [%] | 8.64 (2.68–30.99) | 7.25 (3.65–33.37) | 0.310 |
| B CD19+TLR-9+ [%] | 11.88 (3.90–59.54) | 8.33 (4.71–34.37) | 0.229 |
| sTLR-2 | 13.43 (0.83–48.75) | 6.48 (2.44–35.37) | 0.095 |
| sTLR-3 | 10.94 (5.64–24.52) | 6.77 (5.87–36.37) | 0.005 * |
| sTLR-4 | 11.81 (2.85–35.51) | 6.43 (3.62–37.37) | 0.024 * |
| sTLR-7 | 8.65 (4.48–19.10) | 5.37 (4.76–38.37) | 0.020 * |
| sTLR-8 | 11.67 (5.95–24.28) | 6.84 (6.13–39.37) | 0.002 * |
| sTLR-9 | 20.31 (1.18–52.94) | 13.35 (3.17–40.37) | 0.170 |
| Parameters | SCC Patients in IV Stage in Recrutation Time | Patients with Stage IV SCC Died after 3 Years of Follow-Up | p-Value |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
| T CD4+TLR-2+ [%] | 5.95 (1.46–14.29) | 7.98 (1.96–21.30) | 0.0143 * |
| T CD8+TLR-2+ [%] | 7.77 (2.16–13.09) | 8.83 (2.89–32.48) | 0.0389 * |
| B CD19+TLR-2+ [%] | 9.82 (2.69–12.21) | 11.64 (3.61–32.48) | 0.0189 * |
| T CD4+TLR-3+ [%] | 2.67 (1.80–3.34) | 3.36 (2.41–8.36) | 0.000 * |
| T CD8+TLR-3+ [%] | 2.06 (1.48–2.71) | 2.71 (2.08–5.67) | 0.000 * |
| B CD19+TLR-3+ [%] | 2.60 (2.22–3.00) | 3.43 (2.67–8.19) | 0.000 * |
| T CD4+TLR-4+ [%] | 7.46 (2.83–11.83) | 10.74 (3.79–23.92) | 0.0045 * |
| T CD8+TLR-4+ [%] | 8.29 (3.64–12.78) | 12.67 (4.88–31.84) | 0.0008 * |
| B CD19+TLR-4+ [%] | 7.99 (2.59–17.09) | 12.34 (4.23–22.90) | 0.0107 * |
| T CD4+TLR-7+ [%] | 1.86 (1.40–2.37) | 2.41 (1.46–5.86) | 0.000 * |
| T CD8+TLR-7+ [%] | 2.02 (1.75–3.04) | 2.86 (2.05–6.33) | 0.000 * |
| B CD19+TLR-7+ [%] | 2.49 (2.21–2.73) | 3.25 (2.55–7.79) | 0.000 * |
| T CD4+TLR-8+ [%] | 3.69 (2.82–4.37) | 4.91 (2.93–11.53) | 0.000 * |
| T CD8+TLR-8+ [%] | 2.33 (1.77–2.77) | 2.99 (2.19–7.60) | 0.000 * |
| B CD19+TLR-8+ [%] | 2.47 (0.82–2.91) | 3.26 (1.84–8.11) | 0.000 * |
| T CD4+TLR-9+ [%] | 6.63 (2.24–14.48) | 9.72 (3.01–31.70) | 0.0079 * |
| T CD8+TLR-9+ [%] | 8.29 (4.57–14.00) | 9.78 (5.87–30.99) | 0.016 * |
| B CD19+TLR-9+ [%] | 10.22 (4.74–25.96) | 13.68 (6.35–59.54) | 0.0084 * |
| sTLR-2 | 11.27 (1.51–19.35) | 17.59 (2.02–48.75) | 0.0070 * |
| sTLR-3 | 7.82 (6.15–11.89) | 10.98 (7.69–24.52) | 0.000 * |
| sTLR-4 | 11.38 (4.53–16.02) | 15.91 (6.07–35.51) | 0.002 * |
| sTLR-7 | 5.19 (5.00–10.10) | 7.43 (5.35–18.54) | 0.000 * |
| sTLR-8 | 7.36 (5.94–12.14) | 11.67 (7.04–24.28) | 0.00 * |
| sTLR-9 | 14.83 (0.88–21.56) | 21.97 (1.18–52.94) | 0.018 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smok-Kalwat, J.; Mertowska, P.; Mertowski, S.; Góźdź, S.; Grywalska, E. Toll-like Receptors: Key Players in Squamous Cell Carcinoma Progression. J. Clin. Med. 2024, 13, 4531. https://doi.org/10.3390/jcm13154531
Smok-Kalwat J, Mertowska P, Mertowski S, Góźdź S, Grywalska E. Toll-like Receptors: Key Players in Squamous Cell Carcinoma Progression. Journal of Clinical Medicine. 2024; 13(15):4531. https://doi.org/10.3390/jcm13154531
Chicago/Turabian StyleSmok-Kalwat, Jolanta, Paulina Mertowska, Sebastian Mertowski, Stanisław Góźdź, and Ewelina Grywalska. 2024. "Toll-like Receptors: Key Players in Squamous Cell Carcinoma Progression" Journal of Clinical Medicine 13, no. 15: 4531. https://doi.org/10.3390/jcm13154531
APA StyleSmok-Kalwat, J., Mertowska, P., Mertowski, S., Góźdź, S., & Grywalska, E. (2024). Toll-like Receptors: Key Players in Squamous Cell Carcinoma Progression. Journal of Clinical Medicine, 13(15), 4531. https://doi.org/10.3390/jcm13154531






