Evaluation of Anatomical and Tomographic Biomarkers as Predictive Visual Acuity Factors in Eyes with Retinal Vein Occlusion Treated with Dexamethasone Implant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
3. Results
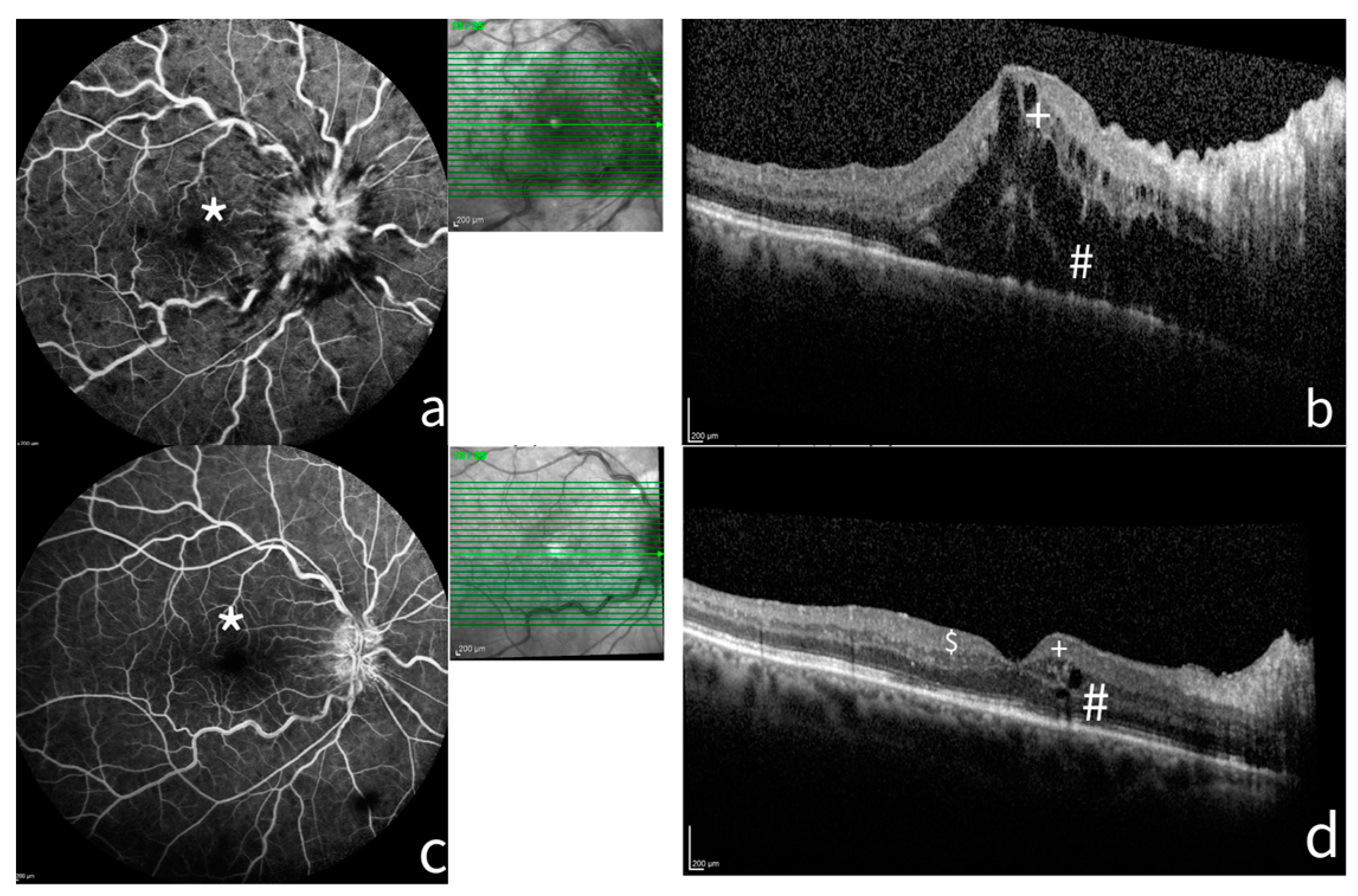
3.1. Role of Macular Ischemia
3.2. Predictive Factors of Visual Acuity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, T.Y.; Scott, I.U. Retinal-Vein Occlusion. N. Engl. J. Med. 2010, 363, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y. The Prevalence of Retinal Vein Occlusion: Pooled Data from Population Studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319.e1. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.J.; Han, D.H.; Kim, I.T.; Oh, I.K.; Kim, K.H.; Lee, D.Y.; Nam, D.H. Changes in Aqueous Concentrations of Various Cytokines After Intravitreal Triamcinolone Versus Bevacizumab for Diabetic Macular Edema. Arch. Ophthalmol. 2011, 152, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Bandello, F.; Belfort, R.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.-H.; Jacques, M.-L.; Jiao, J.; et al. Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Macular Edema Due to Retinal Vein Occlusion. Ophthalmology 2010, 117, 1134–1146.e3. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.A.; Bandello, F.; Belfort, R.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jiao, J.; Li, X.-Y.; et al. Dexamethasone Intravitreal Implant in Patients with Macular Edema Related to Branch or Central Retinal Vein Occlusion. Ophthalmology 2011, 118, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Korobelnik, J.F.; Holz, F.G.; Roider, J.; Ogura, Y.; Simader, C.; Schmidt-Erfurth, U.; Lorenz, K.; Honda, M.; Vitti, R.; Berliner, A.J.; et al. Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion. Ophthalmology 2014, 121, 202–208. [Google Scholar] [CrossRef]
- Brown, D.M.; Heier, J.S.; Clark, W.L.; Boyer, D.S.; Vitti, R.; Berliner, A.J.; Zeitz, O.; Sandbrink, R.; Zhu, X.; Haller, J.A. Intravitreal Aflibercept Injection for Macular Edema Secondary to Central Retinal Vein Occlusion: 1-Year Results From the Phase 3 COPERNICUS Study. Arch. Ophthalmol. 2013, 155, 429–437.e7. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Monés, J.; Tadayoni, R.; Priglinger, S.; Wenzel, A.; Barnes, E.; Pilz, S.; et al. Individualized Ranibizumab Regimen Driven by Stabilization Criteria for Central Retinal Vein Occlusion. Ophthalmology 2016, 123, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Tsujikawa, A.; Kita, M.; Miyamoto, K.; Sakamoto, A.; Yamaike, N.; Kotera, Y.; Yoshimura, N. Integrity of Foveal Photoreceptor Layer in Central Retinal Vein Occlusion. Retina 2008, 28, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kwon, O.W.; Byeon, S.H. Optical Coherence Tomography Predicts Visual Outcome in Acute Central Retinal Vein Occlusion. Retina 2014, 34, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Monés, J.; Glacet-Bernard, A.; Coscas, G. Retinal Vein Occlusions. Macular Edema 2017, 58, 139–167. [Google Scholar]
- Bin, M.; Hai-Ying, Z.; Xuan, J.; Feng, Z. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int. J. Ophthalmol. 2017, 10, 605. [Google Scholar]
- Moon, B.G.; Cho, A.R.; Kim, Y.N.; Kim, J.G. Predictors of Refractory Macular Edema After Branch Retinal Vein Occlusion Following Intravitreal Bevacizumab. Retina 2018, 38, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Banaee, T.; Singh, R.P.; Champ, K.; Conti, F.F.; Wai, K.; Bena, J.; Beven, L.; Ehlers, J.P. Ellipsoid Zone Mapping Parameters in Retinal Venous Occlusive Disease with Associated Macular Edema. Ophthalmol. Retin. 2018, 2, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Babiuch, A.S.; Han, M.; Conti, F.F.; Wai, K.; Silva, F.Q.; Singh, R.P. Association of Disorganization of Retinal Inner Layers with Visual Acuity Response to Anti–Vascular Endothelial Growth Factor Therapy for Macular Edema Secondary to Retinal Vein Occlusion. JAMA Ophthalmol. 2019, 137, 38. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Monferrer-Adsuara, C.; Navarro-Palop, C.; Montero-Hernández, J.; Cervera-Taulet, E. Effect of Dexamethasone Intravitreal Implant on Visual Acuity and Foveal Photoreceptor Integrity in Macular Edema Secondary to Retinal Vascular Disease. Ophthalmologica 2021, 244, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Chung, H.; Kim, H.C. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011, 89, e35–e40. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Fujihara-Mino, A.; Inomoto, N.; Sano, H.; Akaiwa, K.; Semba, K. Optical coherence tomography parameters predictive of visual outcome after anti-VEGF therapy for retinal vein occlusion. Clin. Ophthalmol. 2016, 10, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.H.; Soliman, A.Z.; Tokarev, J.; Zhang, L.; van Kuijk, F.J.; Koozekanani, D.D. Association of Disorganization of Retinal Inner Layers With Vision After Resolution of Center-Involved Diabetic Macular Edema. JAMA Ophthalmol. 2015, 133, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the Retinal Inner Layers as a Predictor of Visual Acuity in Eyes With Center-Involved Diabetic Macular Edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Castro-Navarro, V.; Monferrer-Adsuara, C.; Navarro-Palop, C.; Montero-Hernández, J.; Cervera-Taulet, E. Optical coherence tomography biomarkers in patients with macular edema secondary to retinal vein occlusion treated with dexamethasone implant. BMC Ophthalmol. 2022, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Phadnis, M.A. Sample size calculation for small sample single-arm trials for time-to-event data: Logrank test with normal approximation or test statistic based on exact chi-square distribution? Contemp. Clin. Trials Commun. 2019, 15, 100360. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Zhang, Q.; Tian, M.; Xing, Y. Comparison of dexamethasone intravitreal implant with intravitreal anti-VEGF injections for the treatment of macular edema secondary to branch retinal vein occlusion. Medicine 2019, 98, e15798. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; China Ozurdex in RVO Study Group; Wang, N.; Liang, X.; Xu, G.; Li, X.-Y.; Jiao, J.; Lou, J.; Hashad, Y. Safety and efficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: Randomized, sham-controlled, multicenter study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Torresin, T.; Schiavon, S.; Danieli, L.; Polo, C.; Pilotto, E.; Midena, G.; Frizziero, L. The Disorganization of Retinal Inner Layers Is Correlated to Müller Cells Impairment in Diabetic Macular Edema: An Imaging and Omics Study. Int. J. Mol. Sci. 2023, 24, 9607. [Google Scholar] [CrossRef] [PubMed]
- Luís, M.E.; Sampaio, F.; Costa, J.; Cabral, D.; Teixeira, C.; Ferreira, J.T. Dril Influences Short-term Visual Outcome after Intravitreal Corticosteroid Injection for Refractory Diabetic Macular Edema. Curr. Eye Res. 2021, 46, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Abdolrahimzadeh, S.; Dolz-Marco, R.; Sakurada, Y.; Gal-Or, O.; Scuderi, G. Significance of Hyperreflective Foci as an Optical Coherence Tomography Biomarker in Retinal Diseases: Characterization and Clinical Implications. J. Ophthalmol. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-E.; Ibrahim, F.; Chandrasekaran, P.R.; Teo, K.Y.C. Clinical utility of ultra-widefield fluorescein angiography and optical coherence tomography angiography for retinal vein occlusions. Front. Med. 2023, 10, 1110166. [Google Scholar] [CrossRef] [PubMed]
- Wons, J.; Pfau, M.; Wirth, M.A.; Freiberg, F.J.; Becker, M.D.; Michels, S. Optical Coherence Tomography Angiography of the Foveal Avascular Zone in Retinal Vein Occlusion. Ophthalmologica 2016, 235, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.C.; Kvanta, A.; Amrén, U.; Epstein, D. Optical Coherence Tomography Angiography in Central Retinal Vein Occlusion: Correlation Between the Foveal Avascular Zone and Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT242–OCT246. [Google Scholar] [CrossRef] [PubMed]
- Antropoli, A.; Bianco, L.; Arrigo, A.; Bandello, F.; Parodi, M.B. Non-perfusion severity correlates with central macular thickness and microvascular impairment in branch retinal vein occlusions. Eur. J. Ophthalmol. 2023, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Saxena, S.; Kaur, A.; Mahdi, A.A.; Misra, A.; Singh, M.; Meyer, C.H.; Akduman, L. Sequential restoration of external limiting membrane and ellipsoid zone after intravitreal anti-VEGF therapy in diabetic macular oedema. Eye 2021, 35, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Dobson, E.T.A.; Wiedenmann, M.; Oden, N.; VanVeldhuisen, P.; Scott, I.U.; Ip, M.S.; Eliceiri, K.W.; Blodi, B.A.; Domalpally, A. Ellipsoid Zone Defects in Retinal Vein Occlusion Correlates with Visual Acuity Prognosis: SCORE2 Report 14. Transl. Vis. Sci. Technol. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, S.; Zhang, Z.; Shen, J. Predicting the visual acuity for retinal vein occlusion after ranibizumab therapy with an original ranking for macular microstructure. Exp. Ther. Med. 2017, 15, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.B.; Arrigo, A.; Antropoli, A.; Bianco, L.; Saladino, A.; Bandello, F.; Vilela, M.; Mansour, A. Deep Capillary Plexus as Biomarker of Peripheral Capillary Nonperfusion in Central Retinal Vein Occlusion. Ophthalmol. Sci. 2023, 3, 100267. [Google Scholar] [CrossRef]
- Šínová, I.; Řehák, J.; Nekolová, J.; Jirásková, N.; Haluzová, P.; Řeháková, T.; Bábková, B.; Hejsek, L.; Šín, M. Correlation Between Ischemic Index of Retinal Vein Occlusion and Oxygen Saturation in Retinal Vessels. Arch. Ophthalmol. 2018, 188, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; Vazquez-Alfageme, C.; Hykin, P.G.; Bainbridge, J.W.; Sivaprasad, S. The Relationship Between Retinal Vessel Oxygenation and Spatial Distribution of Retinal Nonperfusion in Retinal Vascular Diseases. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2083. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Toto, L.; Frizziero, L.; Covello, G.; Torresin, T.; Midena, G.; Danieli, L.; Pilotto, E.; Figus, M.; Mariotti, C.; et al. Validation of an Automated Artificial Intelligence Algorithm for the Quantification of Major OCT Parameters in Diabetic Macular Edema. J. Clin. Med. 2023, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
| Baseline (n = 46) | |
|---|---|
| Age (years) mean ± SD 95% CI | 74.69 ± 12.49 68.92 to 77.28 |
| Gender, female n (%) | 24 (52.2%) |
| Gender, male n (%) | 22 (47.8%) |
| Right eye n (%) | 22 (47.8%) |
| Left eye n (%) | 24 (52.2%) |
| Type-BRVO n (%) | 28 (60.8%) |
| Type-CRVO n (%) | 18 (39.2%) |
| Macular ischemia, n (%) | 28 (60.8%) |
| Pseudophakia, n (%) | 22 (47.8%) |
| Second implant, n (%) | 28 (60.8%) |
| IOP, mmHg ± SD, 95% CI | 16.4 ± 1.4 15.9 to 16.8 |
| BCVA, mean ± SD, 95% CI | 0.817 ± 0.220 0.4 to 1 |
| CRT, µm ± SD, 95% CI | 666.2 ± 212.2 596.1 to 735.7 |
| Baseline (n = 46) | 6 Months (n = 44) | 12 Months (n = 40) | |||
|---|---|---|---|---|---|
| BCVA, mean ± SD, 95% CI | 0.817 ± 0.220 0.4 to 1 | 0.663 ± 0.267 0.3 to 1 | p < 0.05 a | 0.639 ± 0.321 0.2 to 1 | p < 0.05 a |
| CRT, µm ± SD 95% CI | 666.2 ± 212.2 596.1 to 735.7 | 471.1 ± 215.6 399.8 to 542.5 | p < 0.05 a | 467 ± 175.7 409.9 to 525.4 | p < 0.05 a |
| DRIL (yes %) | 65.2% | 60.9% | p = 0.5 b | 56.5% | p = 0.3 b |
| ELM (yes %) | 47.8% | 47.8% | p > 0.9 b | 43.5% | p = 0.7 b |
| HRF (yes %) | 69.6% | 78.3% | p = 0.3 b | 56.5% | p = 0.1 b |
| EZD (yes %) | 52.2% | 47.8% | p = 0.7 b | 47.8% | p = 0.7 b |
| Baseline Anatomic and OCT Biomarkers | p for Any BCVA Improvement at 6 Months | p for Improved BCVA ≤ 0.3 at 6 Months | p for Any BCVA Improvement at 12 Months | p for Improved BCVA ≤ 0.3 at 12 Months |
|---|---|---|---|---|
| DRIL OR, 95% CI | 0.021 0.14, 0.03 to 0.76 | 0.008 0.13, 0.03 to 0.59 | 0.143 | 0.127 |
| ELM OR, 95% CI | <0.001 0.04, 0.01 to 0.02 | 0.032 0.17, 0.06 to 0.80 | <0.001 0.32, 0.01 to 0.2 | 0.101 |
| HRF OR, 95% CI | 0.506 | 0.739 | 0.698 | 0.684 |
| EZD OR, 95% CI | <0.001 0.05, 0.01 to 0.3 | 0.012 0.07, 0.03 to 0.64 | <0.001 0.05, 0.01 to 0.31 | 0.042 0.2, 0.5 to 0.84 |
| MI OR, 95% CI | 0.005 0.1, 0.02 to 0.56 | 0.027 0.22, 0.06 to 0.79 | 0.002 0.07, 0.11 to 0.41 | 0.002 0.07, 0.11 to 0.41 |
| CRT | 0.006 a | 0.854 a | 0.012 a | 0.191 a |
| BRVO OR, 95% CI | 0.054 | 0.210 | 0.008 0.13, 0.03 to 0.59 | <0.001 0.42, 0.26 to 0.67 |
| Baseline Anatomic and OCT Biomarkers | p Value for Any BCVA Improvement at 6 Months | p Value for Improved BCVA ≤ 0.3 at 6 Months | p Value for Any BCVA Improvement at 12 Months | p Value for Improved BCVA ≤ 0.3 at 12 Months |
|---|---|---|---|---|
| DRIL OR, 95% CI | 0.492 | >0.99 | 0.492 | >0.99 |
| ELM OR, 95% CI | <0.001 0.03, 0.003 to 0.24 | 0.354 | <0.001 0.2, 0.03 to 0.2 | 0.354 |
| HRF OR, 95% CI | 0.673 | 0.289 | 0.673 | 0.289 |
| EZD OR, 95% CI | 0.005 0.07, 0.01 to 0.48 | 0.147 | 0.005 0.07, 0.01 to 0.48 | 0.147 |
| BRVO OR, 95% CI | 0.023 0.12, 0.02 to 0.74 | 0.024 0.62, 0.43 to 0.91 | 0.023 0.12, 0.19 to 0.74 | 0.024 0.62, 0.4 to 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covello, G.; Maglionico, M.N.; Figus, M.; Busoni, C.; Sartini, M.S.; Lupidi, M.; Posarelli, C. Evaluation of Anatomical and Tomographic Biomarkers as Predictive Visual Acuity Factors in Eyes with Retinal Vein Occlusion Treated with Dexamethasone Implant. J. Clin. Med. 2024, 13, 4533. https://doi.org/10.3390/jcm13154533
Covello G, Maglionico MN, Figus M, Busoni C, Sartini MS, Lupidi M, Posarelli C. Evaluation of Anatomical and Tomographic Biomarkers as Predictive Visual Acuity Factors in Eyes with Retinal Vein Occlusion Treated with Dexamethasone Implant. Journal of Clinical Medicine. 2024; 13(15):4533. https://doi.org/10.3390/jcm13154533
Chicago/Turabian StyleCovello, Giuseppe, Maria Novella Maglionico, Michele Figus, Chiara Busoni, Maria Sole Sartini, Marco Lupidi, and Chiara Posarelli. 2024. "Evaluation of Anatomical and Tomographic Biomarkers as Predictive Visual Acuity Factors in Eyes with Retinal Vein Occlusion Treated with Dexamethasone Implant" Journal of Clinical Medicine 13, no. 15: 4533. https://doi.org/10.3390/jcm13154533





