Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
4.1. Myocardial Fibrosis in Athletes and the General Population
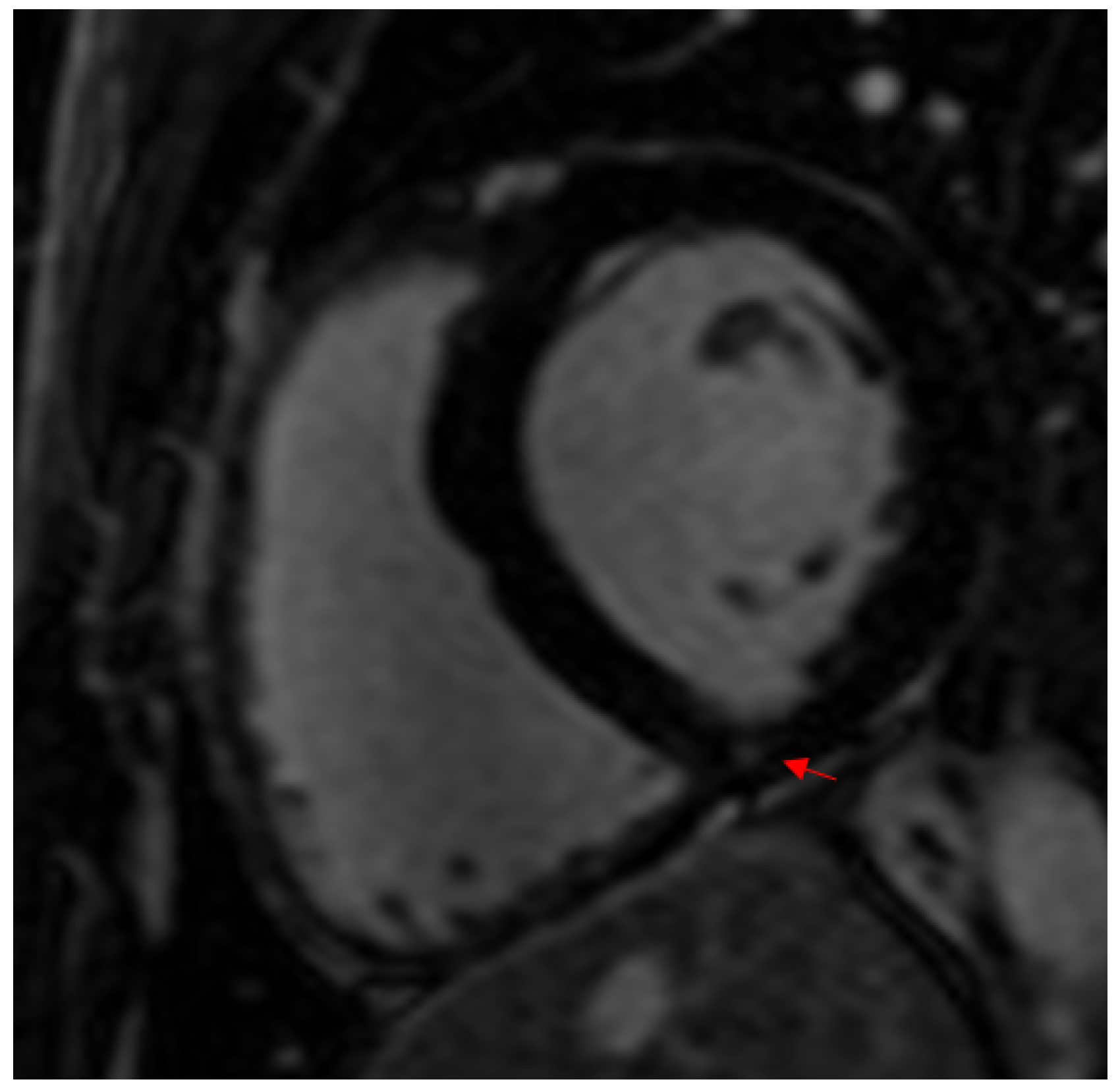
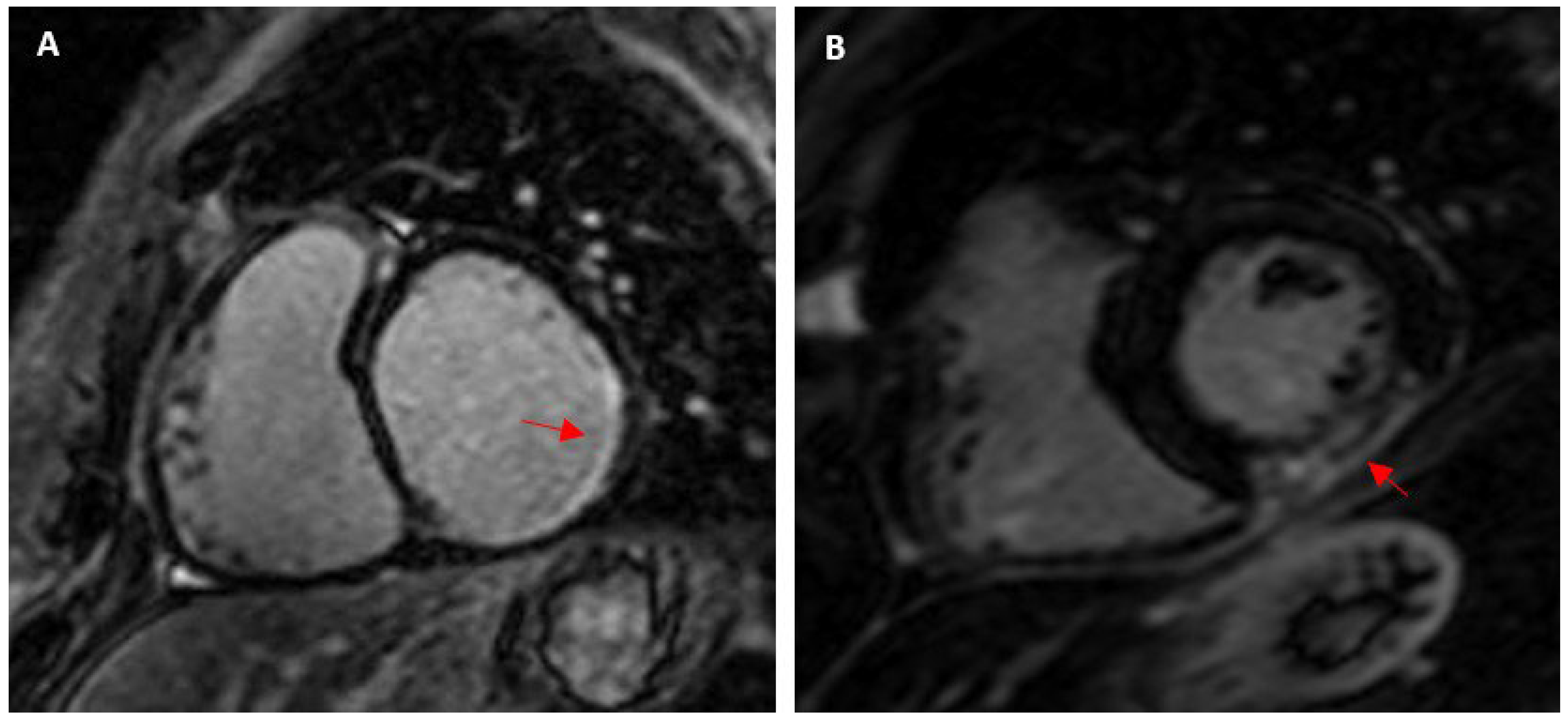
4.2. Factors Associated with Myocardial Fibrosis in Athletes
4.2.1. Exercise Dose
4.2.2. Pressure Overload
4.2.3. Viral Myocarditis
4.2.4. Coronary Artery Calcification
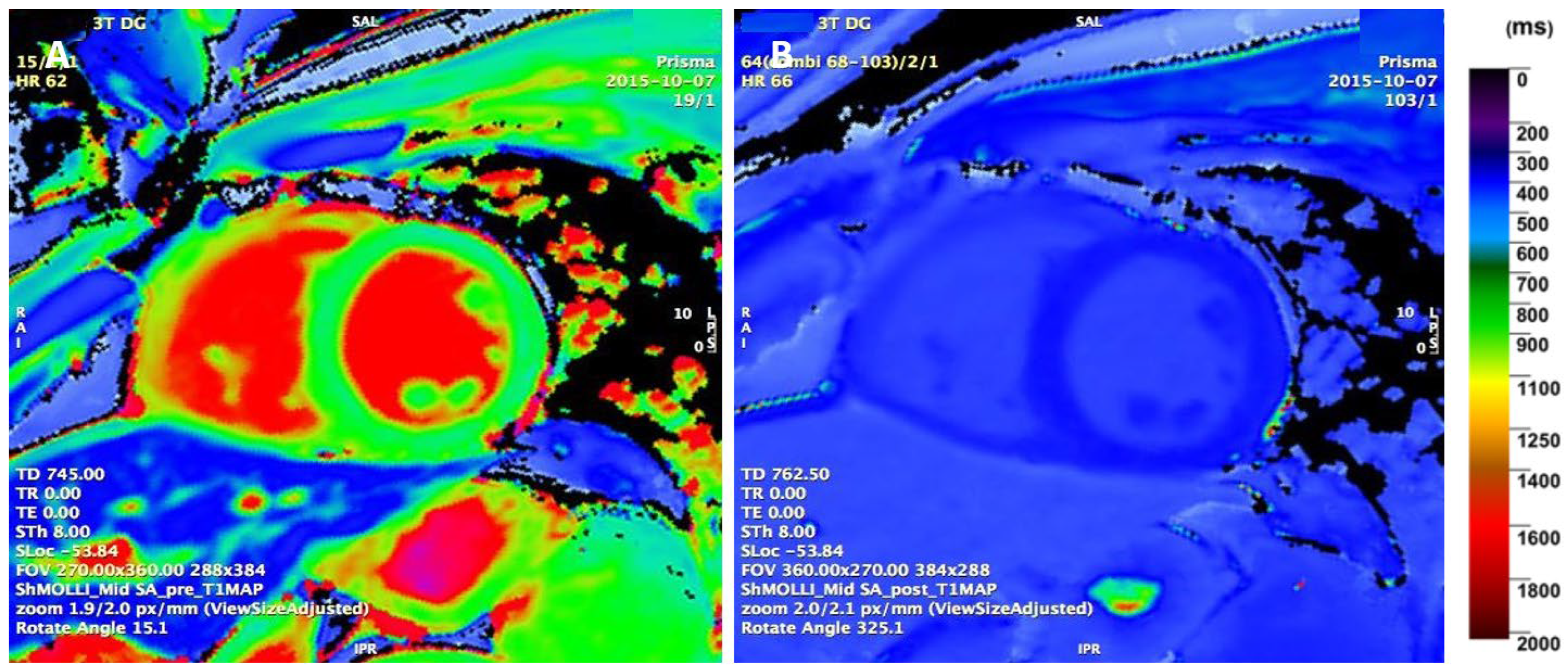
4.3. Parametric T1 Mapping
4.4. Clinical Implications
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Małek, Ł.A.; Bucciarelli-Ducci, C. Myocardial fibrosis in athletes—Current perspective. Clin. Cardiol. 2020, 43, 882–888. [Google Scholar] [CrossRef] [PubMed]
- van de Schoor, F.R.; Aengevaeren, V.L.; Hopman, M.T.; Oxborough, D.L.; George, K.P.; Thompson, P.D.; Eijsvogels, T.M. Myocardial Fibrosis in Athletes. Mayo Clin. Proc. 2016, 91, 1617–1631. [Google Scholar] [CrossRef]
- Bing, R.; Dweck, M.R. Myocardial fibrosis: Why image, how to image and clinical implications. Heart 2019, 105, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Androulakis, E.; Mouselimis, D.; Tsarouchas, A.; Antonopoulos, A.; Bakogiannis, C.; Papagkikas, P.; Vlachopoulos, C. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Myocardial Fibrosis in Young and Veteran Athletes: Insights from a Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 784474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-D.; Xu, S.-L.; Wang, X.-Y.; Tao, L.-Y.; Zhao, W.; Gao, W. Prevalence of Myocardial Fibrosis in Intensive Endurance Training Athletes: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 585692. [Google Scholar] [CrossRef]
- Androulakis, E.; Swoboda, P.P. The Role of Cardiovascular Magnetic Resonance in Sports Cardiology; Current Utility and Future Perspectives. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.W.; Bhalla, S.; Javidan-Nejad, C.; Bierhals, A.J.; Gutierrez, F.R.; Woodard, P.K. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics 2009, 29, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, V.; Merghani, A.; Rosmini, S.; Cox, A.; Bulluck, H.; Culotta, V.; Cheang, M.; Fontana, M.; A Treibel, T.; Abdel-Gadir, A.; et al. CMR findings in high endurance veteran athletes-a 247 subject study. J. Cardiovasc. Magn. Reson. 2016, 18 (Suppl. S1), O38. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tahir, E.; Starekova, J.; Muellerleile, K.; von Stritzky, A.; Münch, J.; Avanesov, M.; Weinrich, J.M.; Stehning, C.; Bohnen, S.; Radunski, U.K.; et al. Myocardial Fibrosis in Competitive Triathletes Detected by Contrast-Enhanced CMR Correlates with Exercise-Induced Hypertension and Competition History. JACC Cardiovasc. Imaging 2018, 11, 1260–1270. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes with a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Merghani, A.; Maestrini, V.; Rosmini, S.; Schofield, R.; Papadakis, M.; Manisty, C.; Moon, J.; Sharma, S. P3990Exercise-induced arrhythmogenic right ventricular remodeling in master endurance athletes. Eur. Heart J. 2017, 38, 831. [Google Scholar] [CrossRef]
- Verwijs, S.M.; Van Hattum, J.C.; Spies, J.L.; Boekholdt, S.; Planken; Groenink, M.; Van Randen, A.; Van Luijk, R.; Berg-Faaij, A.V.D.; Bakermans, A.; et al. Late gadolinium enhancement of the hinge point is a common finding in asymptomatic ELITE athletes. Eur. J. Prev. Cardiol. 2022, 29, zwac056.268. [Google Scholar] [CrossRef]
- Banks, L.; Altaha, M.A.; Yan, A.T.; Dorian, P.; Konieczny, K.; Deva, D.P.; LA Gerche, A.; Akhavein, F.; Bentley, R.F.; Connelly, K.A.; et al. Left Ventricular Fibrosis in Middle-Age Athletes and Physically Active Adults. Med. Sci. Sports Exerc. 2020, 52, 2500–2507. [Google Scholar] [CrossRef]
- Małek, Ł.A.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar] [CrossRef]
- Altaha, M.A.; Connelly, K.; Yan, A.T.; Banks, L.; Dorian, P.; Goodman, J. Prevalence of late gadolinium enhancement in middle-aged, sub-elite athletes. Can. J. Cardiol. 2016, 32, S265–S266. [Google Scholar] [CrossRef]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef]
- Domenech-Ximenos, B.; la Garza, M.S.-D.; Prat-González, S.; Sepúlveda-Martínez, A.; Crispi, F.; Duran-Fernandez, K.; Perea, R.J.; Bijnens, B.; Sitges, M. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J. Cardiovasc. Magn. Reson. 2020, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; O’Hanlon, R.; Prasad, S.; Deighan, A.; MacMillan, P.; Oxborough, D.; Godfrey, R.; Smith, G.; Maceira, A.; Sharma, S.; et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J. Appl. Physiol. 2011, 110, 1622–1626. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; López-Ramón, M.; Alis, R.; Garatachea, N.; Pareja-Galeano, H.; Santos-Lozano, A.; Catalán, P.; Sansoni, V.; Perego, S.; Lombardi, G.; et al. No evidence of adverse cardiac remodeling in former elite endurance athletes. Int. J. Cardiol. 2016, 222, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Andresen, K.; Klaeboe, L.G.; Lie, Ø.H.; Broch, K.; Kvaslerud, A.; Bosse, G.; Hopp, E.; Haugaa, K.; Edvardsen, T. No signs of diffuse myocardial fibrosis by T1 mapping in male elite endurance athletes. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, jeab289.423. [Google Scholar] [CrossRef]
- De Bosscher, R.; Claeys, M.; Dausin, C.; Goetschalckx, K.; Bogaert, J.; Van De Heyning, C.; Ghekiere, O.; Herbots, L.; Claus, P.; Kalman, J.; et al. Hinge point fibrosis in athletes is not associated with structural, functional or electrical consequences: A comparison between young and middle-aged elite endurance athletes. Eur. Heart J. 2020, 41, ehaa946.3126. [Google Scholar] [CrossRef]
- Breuckmann, F.; Möhlenkamp, S.; Nassenstein, K.; Lehmann, N.; Ladd, S.; Schmermund, A.; Sievers, B.; Schlosser, T.; Jöckel, K.-H.; Heusch, G.; et al. Myocardial late gadolinium enhancement: Prevalence, pattern, and prognostic relevance in marathon runners. Radiology 2009, 251, 50–57. [Google Scholar] [CrossRef]
- Pujadas, S.; Doñate, M.; Li, C.-H.; Merchan, S.; Cabanillas, A.; Alomar, X.; Pons-Llado, G.; Serra-Grima, R.; Carreras, F. Myocardial remodelling and tissue characterisation by cardiovascular magnetic resonance (CMR) in endurance athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000422. [Google Scholar] [CrossRef] [PubMed]
- Karlstedt, E.; Chelvanathan, A.; Da Silva, M.; Cleverley, K.; Kumar, K.; Bhullar, N.; Lytwyn, M.; Bohonis, S.; Oomah, S.; Nepomuceno, R.; et al. The impact of repeated marathon running on cardiovascular function in the aging population. J. Cardiovasc. Magn. Reson. 2012, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, P.P.; McDiarmid, A.K.; Erhayiem, B.; Broadbent, D.A.; Dobson, L.E.; Garg, P.; Ferguson, C.; Page, S.P.; Greenwood, J.P.; Plein, S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy from Athlete’s Heart. J. Am. Coll. Cardiol. 2016, 67, 2189–2190. [Google Scholar] [CrossRef]
- McDiarmid, A.K.; Swoboda, P.P.; Erhayiem, B.; Lancaster, R.E.; Lyall, G.K.; Broadbent, D.A.; Dobson, L.E.; Musa, T.A.; Ripley, D.P.; Garg, P.; et al. Athletic Cardiac Adaptation in Males Is a Consequence of Elevated Myocyte Mass. Circ. Cardiovasc. Imaging 2016, 9, e003579. [Google Scholar] [CrossRef] [PubMed]
- Bohm, P.; Schneider, G.; Linneweber, L.; Rentzsch, A.; Krämer, N.; Abdul-Khaliq, H.; Kindermann, W.; Meyer, T.; Scharhag, J. Right and Left Ventricular Function and Mass in Male Elite Master Athletes: A Controlled Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Circulation 2016, 133, 1927–1935. [Google Scholar] [CrossRef]
- Mangold, S.; Kramer, U.; Franzen, E.; Erz, G.; Bretschneider, C.; Seeger, A.; Claussen, C.D.; Niess, A.M.; Burgstahler, C. Detection of cardiovascular disease in elite athletes using cardiac magnetic resonance imaging. Rofo-Fortschritte Geb. Rontgenstrahlen Bild. Verfahr. 2013, 185, 1167–1174. [Google Scholar] [CrossRef]
- Androulakis, E.; Papatheodorou, S.; Merghani, A.; Sharma, S.; Papadakis, M. Patterns and clinical significance of non-specific myocardial fibrosis; Evidence from a cohort of young competitive athletes referred to a tertiary referral centre. Eur. J. Prev. Cardiol. 2022, 29, zwac056.152. [Google Scholar] [CrossRef]
- Franzen, E.; Mangold, S.; Erz, G.; Claussen, C.D.; Niess, A.M.; Kramer, U.; Burgstahler, C. Comparison of morphological and functional adaptations of the heart in highly trained triathletes and long-distance runners using cardiac magnetic resonance imaging. Heart Vessel. 2013, 28, 626–631. [Google Scholar] [CrossRef]
- Abdullah, S.M.; Barkley, K.W.; Bhella, P.S.; Hastings, J.L.; Matulevicius, S.; Fujimoto, N.; Shibata, S.; Carrick-Ranson, G.; Palmer, M.D.; Gandhi, N.; et al. Lifelong Physical Activity Regardless of Dose Is Not Associated with Myocardial Fibrosis. Circ. Cardiovasc. Imaging 2016, 9, e005511. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, E.B.; Nacif, M.S.; Guo, M.; McClelland, R.L.; Teixeira, P.B.R.P.; Bild, D.E.; Barr, R.G.; Shea, S.; Post, W.; Burke, G.; et al. Prevalence and Correlates of Myocardial Scar in a US Cohort. JAMA 2015, 314, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Barbier, C.E.; Bjerner, T.; Johansson, L.; Lind, L.; Ahlström, H. Myocardial scars more frequent than expected: Magnetic resonance imaging detects potential risk group. J. Am. Coll. Cardiol. 2006, 48, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Cao, J.J.; Sigurdsson, S.; Aspelund, T.; Kellman, P.; Aletras, A.H.; Dyke, C.K.; Thorgeirsson, G.; Eiriksdottir, G.; Launer, L.J.; et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012, 308, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L. Focal Fibrosis in the Endurance Athlete’s Heart Running Scarred or Running Scared? JACC: Cardiovasc. Imaging 2018, 11, 1271–1273. [Google Scholar] [CrossRef]
- Benito, B.; Gay-Jordi, G.; Serrano-Mollar, A.; Guasch, E.; Shi, Y.; Tardif, J.-C.; Brugada, J.; Nattel, S.; Mont, L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011, 123, 13–22. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Heidbüchel, H.; Burns, A.T.; Mooney, D.J.; Taylor, A.J.; Pfluger, H.B.; Inder, W.J.; Macisaac, A.I.; Prior, D.L. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med. Sci. Sports Exerc. 2011, 43, 974–981. [Google Scholar] [CrossRef]
- Castelletti, S.; Gati, S. The Female Athlete’s Heart: Overview and Management of Cardiovascular Diseases. Eur. Cardiol. Rev. 2021, 16, e47. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Biella, F.; Lemme, E.; Maestrini, V.; Di Giacinto, B.; Pelliccia, A. Female Athlete’s Heart: Sex Effects on Electrical and Structural Remodeling. Circ. Cardiovasc. Imaging 2020, 13, e011587. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Juhasz, V.; Brunetti, G.; Cipriani, A.; Szabo, L.; Merkely, B.; Corrado, D.; D’ascenzi, F.; Vago, H.; Zorzi, A. May Strenuous Endurance Sports Activity Damage the Cardiovascular System of Healthy Athletes? A Narrative Review. J. Cardiovasc. Dev. Dis. 2022, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschöpe, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.; McNamara, D.M.; Krejčí, J.; et al. Elevated Sera sST2 Is Associated with Heart Failure in Men ≤50 Years Old with Myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef] [PubMed]
- Cabinian, A.E.; Kiel, R.J.; Smith, F.; Ho, K.L.; Khatib, R.; Reyes, M.P. Modification of exercise-aggravated coxsackievirus B3 murine myocarditis by T lymphocyte suppression in an inbred model. J. Lab. Clin. Med. 1990, 115, 454–462. [Google Scholar] [PubMed]
- Möhlenkamp, S.; Lehmann, N.; Breuckmann, F.; Bröcker-Preuss, M.; Nassenstein, K.; Halle, M.; Budde, T.; Mann, K.; Barkhausen, J.; Heusch, G.; et al. Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: The risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur. Heart J. 2008, 29, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.; Tintut, Y.; Demer, L.L.; Hsu, J.J. Potential mechanisms linking high-volume exercise with coronary artery calcification. Heart 2023, 109, 1139–1145. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Sharma, S.; Prakken, N.H.; Möhlenkamp, S.; Thompson, P.D.; Velthuis, B.K.; Eijsvogels, T.M. Exercise and Coronary Atherosclerosis: Observations, Explanations, Relevance, and Clinical Management. Circulation 2020, 141, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.J.; Shah, A.B. Exercise and the Female Heart. Clin. Ther. 2022, 44, 41–49. [Google Scholar] [CrossRef]
- Di Marco, A.; Brown, P.F.; Bradley, J.; Nucifora, G.; Anguera, I.; A Miller, C.; Schmitt, M. Extracellular volume fraction improves risk-stratification for ventricular arrhythmias and sudden death in non-ischaemic cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2022, 24, 512–521. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhao, S.; Lu, M. Detection of myocardial fibrosis: Where we stand. Front. Cardiovasc. Med. 2022, 9, 926378. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Brunetti, G.; Cipriani, A.; Juhasz, V.; Graziano, F.; Hirschberg, K.; Dohy, Z.; Balla, D.; Drobni, Z.; Marra, M.P.; et al. Certainties and Uncertainties of Cardiac Magnetic Resonance Imaging in Athletes. J. Cardiovasc. Dev. Dis. 2022, 9, 361. [Google Scholar] [CrossRef]
- Grigoratos, C.; Pantano, A.; Meschisi, M.; Gaeta, R.; Ait-Ali, L.; Barison, A.; Todiere, G.; Festa, P.; Sinagra, G.; Aquaro, G.D. Clinical importance of late gadolinium enhancement at right ventricular insertion points in otherwise normal hearts. Int. J. Cardiovasc. Imaging 2020, 36, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, C.; Zorzi, A.; Vessella, T.; Martino, A.; Panattoni, G.; Cipriani, A.; De Lazzari, M.; Marra, M.P.; Fusco, A.; Sciarra, L.; et al. Predictors of Left Ventricular Scar Using Cardiac Magnetic Resonance in Athletes with Apparently Idiopathic Ventricular Arrhythmias. J. Am. Heart Assoc. 2021, 10, e018206. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Marra, M.P.; Rigato, I.; De Lazzari, M.; Susana, A.; Niero, A.; Pilichou, K.; Migliore, F.; Rizzo, S.; Giorgi, B.; et al. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ. Arrhythmia Electrophysiol. 2016, 9, e004229. [Google Scholar] [CrossRef]
- Brunetti, G.; Graziano, F.; Cavigli, L.; Cipriani, A.; D’ascenzi, F.; Bauce, B.; Pilichou, K.; Marra, M.P.; Corrado, D.; Zorzi, A. Reproducibility of ventricular arrhythmias at exercise testing for prediction of non-ischemic left ventricular scar in athletes. Eur. J. Prev. Cardiol. 2022, 30, 107–116. [Google Scholar] [CrossRef]
- Schnell, F.; Claessen, G.; La Gerche, A.; Bogaert, J.; Lentz, P.-A.; Claus, P.; Mabo, P.; Carré, F.; Heidbuchel, H. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: Let sleeping dogs lie? Br. J. Sports Med. 2016, 50, 111–117. [Google Scholar] [CrossRef]
- Parry-Williams, G.; Gati, S.; Sharma, S. The heart of the ageing endurance athlete: The role of chronic coronary stress. Eur. Heart J. 2021, 42, 2737–2744. [Google Scholar] [CrossRef]
- Möhlenkamp, S.; Leineweber, K.; Lehmann, N.; Braun, S.; Roggenbuck, U.; Perrey, M.; Broecker-Preuss, M.; Budde, T.; Halle, M.; Mann, K.; et al. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res. Cardiol. 2014, 109, 391. [Google Scholar] [CrossRef]



| Type of Sport | Athletes | CMR Findings | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Athlete Group | Exercise Exposure | Age (y), Mean ± SD | Sex (%) BSA (m2) | LGE | Pattern/Location | T1 (ms) | ECV (%) |
| Zaidi et al. (2017) [13] | 170 Master endurance | - | 54.4 ± 8.5 | M: 71 F: 29 | 69/170 (40.6%) | - | - | - |
| Verwijs et al. (2022) [14] 1.5 T, 3 T | 210 Elite international, national, Olympic: 38 road cycling, 28 field hockey, 27 water polo, 21 soccer, 18 rowing, 13 swimming, 12 track cycling, 10 tennis, 10 sailing | - | 28 ± 7 | M: 66 2 ± 0.2 F: 34 | M: 64/138 (46.4%) F: 20/72 (27.8%) Total: 84/210 (40%) | M: 64 RV insertion points F: 20 RV insertion points | 959 ± 77 LGE+ 956 ± 24 LGE- 960 ± 96 | 24 ± 2 LGE+ 24 ± 2 LGE- 25 ± 2 |
| Domenech Ximenos et al. (2020) [19] 1.5, 3 T | 93 Triathlon | >12 h/wk training during last 5 y | 35.7 ± 5.8 | M: 53 1.91 ± 0.13 F: 47 1.63 ± 0.1 | M: 17/49 (34.7%) F: 18/44 (40.9%) Total: 35/93 (37.6%) | RV insertion points | - | 26 ± 2.3% LGE+ 27.1 ± 2.2 LGE- 25.2 ± 2.1 |
| Banks et al. (2020) [15] 3 T | 72 24 endurance runners, 20 cycling, 28 triathletes | 10 y of competition, 7.6 ± 4.5 h/wk vigorous exercise | 53 ± 5 | M: 74 1.9 ± 0.1 F: 26 1.6 ± 0.2 | M: 18/53 (34%) F: 5/16 (31%) Total: 23/69 (33%) | M: 12 RV insertion points, 2 ischaemic, 4 nonischaemic F: 5 RV insertion points | M: 1164 ± 36 F: 1190 ± 23 Total: 1169 ± 35 | M: 22.1 ± 3.3 F: 24.2 ± 3.9 Total: 22.6 ± 3.5 |
| Malek et al. (2019) [16] 3 T | 30 Active healthy ultramarathon runners | Median 9 y running with frequent competitions | 40.9 ± 6.6 | M: 100 | M: 8/30 (27%) | Nonischaemic: 5 RV insertion point, 3 septum or inferolateral wall | 1200 ± 59 | 26.1 ± 2.9 |
| Wilson et al. (2011) [20] 1.5 T | 29 12 lifelong veteran endurance and 17 young endurance: marathon, ultramarathon, ironman, triathlon | Veteran: 43 ± 6 y of competitive training Young: 18 ± 7 y of competitive training | 57 ± 6 31 ± 5 | M: 100 1.96 ± 0.14 2 ± 0.14 | Veteran: 6/12 (50%) Young: 0/17 Total: 6/29 (20.7%) | 1 CAD pattern: subendocardial septal and lateral wall infarction pattern 5 non-CAD pattern: 1 subepicardial lateral wall (myocarditis), 4 junctional: basal and mid insertion point, inferior insertion point and mid/apical, inferior mid/apical insertion point, inferior insertion point | - | - |
| Sanchis-Gomar et al. (2016) [21] 3 T | 53 Highly trained endurance: 11 elite and 42 sub-elite cyclists, runners | Elite: 29 ± 9 y training, 10.6 ± 3.1 h/wk Sub-elite: 24 ± 9 y training, 10.6 ± 4.2 h/wk | 54 ± 4 (elite) 55 ± 9 (sub-elite) | M: 100 | 2/10 (20%) | Nonischaemic pattern, intra-myocardial LV lateral wall, basal inferolateral LV wall | - | - |
| Andresen et al. (2022) [22] 3 T | 27 Healthy elite endurance athletes | 379 ± 161 h/y exercise duration, 9.2 ± 0.9 MET | 41 ± 9 | M: 100 | 5/27 (18.5%) | - | 1214 ± 24 (septal) LGE+ 1220 ± 4 LGE- 1212 ± 27 | 22.5 ± 3.1 (septal) LGE+ 22 ± 1.2 LGE- 22.7 ± 3.4 |
| Altaha et al. (2016) [17] 3 T | 33 Sub-elite endurance: 10 runners, 12 cyclists, 10 triathletes | >10 y of exercise, 4.8 ± 2.5 h/wk exercise | 55 ± 5.6 | M: 76 F: 24 | M: 4/25 (16%) F: 1/8 (12.5%) Total: 5/33 (15.2%) | Non-specific RV inferior hinge-point | - | - |
| La Gerche et al. (2012) [18] 1.5 T | 40 7 marathon runners, 11 endurance triathletes, 9 alpine cyclists, 13 ultra-triathletes | 10 ± 9 years training, 16.3 ± 5.1 h/wk intense training | 37 ± 8 | M: 90 F: 10 | 5/39 (12.8%) | Interventricular septum, frequently in the RV attachment | - | - |
| Bosscher et al. (2020) [23] 3 T | 231 Elite endurance athletes | - | 18 ± 2 (young) 38 ± 5 (middle-aged) | M: 79 F: 21 | M: 27/187 (14.4%) F: 1/50 (2%) Total: 28 (12.5%) | M: 24 RV insertion points, 3 subepicardial LV lateral wall F: 1 RV insertion points | - | - |
| Breuckmann et al. (2009) [24] 1.5 T | 102 Marathon runners | ≥5 marathons in ≤3 y | 57 ± 6 | M: 100 | 12/102 (11.8%) | 5: subendocardial layer typical myocardial infarction (10 LAD, 1 LCA, 3 RCA segments) 7: mid-myocardial patchy nonischaemic pattern (3 LAD, 5 LCA, 9 RCA segments) | - | - |
| Tahir et al. (2018) [11] 1.5 T | 83 Triathletes | 12.6 y competitions, >10 h/wk training | 43 ± 10 | M: 65 1.98 ± 0.12 F: 35 1.73 ± 0.12 | M: 9/54 (16.7%) F: 0/29 Total: 9/83 (10.8%) | Nonischaemic: 5: subepicardial (myocarditis)–inferolateral LV wall, 2: posterior RV insertion points, 1: transmural | M: 990 ± 28 F: 1015 ± 25 M: LGE+ 1005 ± 32 M: LGE- 987 ± 27 | M: 24.8 ± 2.2 F: 27.8 ± 1.9 M: LGE+ 26.3 ± 1.8 M: LGE- 24.4 ± 2.2 |
| Merghani et al. (2017) [12] 1.5 T | 152 Master cyclists, runners | M: 33.4 ± 12.9 y endurance exercise, 7.5 ± 3.8 h/wk F: 26.1 ± 10.9 y endurance exercise, 7.7 ± 2.9 h/wk | 54.4 ± 8.5 | M: 70 1.9 ± 0.12 F: 30 1.62 ± 0.12 | M: 15/106 (14.2%) F: 1/46 (2.2%) Total: 16/152 (10.5%) | M: 7 subendocardial LGE consistent with myocardial infarction, 5 midmyocardial, 3 epicardial distribution F: 1 subendocardial LGE | - | - |
| Pujadas et al. (2018) [25] 1.5 T | 34 Healthy endurance veterans: marathons | 28.06 ± 10.84 y training, 9.38 ± 3.52 h/wk, still in regular training | 48.17 ± 7.4 | M: 100 1.8 ± 0.11 | 3/34 (8.8%) | Nonischaemic: mesocardial in septal–apical wall, subepicardial inferior apical wall, mesocardial lateral wall | 943.6 ± 53 (septal) | 25 ± 2% (septal) |
| Karlstedt et al. (2012) [26] 1.5 T | 25 Healthy marathon runners | ≥3 marathons in the past 2 y, 47 ± 7 miles/wk training | 55 ± 4 | M: 84 F: 16 | 2/25 (8%) | Subendocardial distribution of LV anterior wall (before running marathon), with evidence of obstructive LAD artery disease | - | - |
| Swoboda et al. (2016) [27] 3 T | 40 Competitive athletes: 11 runners, 13 triathletes, 16 cyclists | >6 h/wk training | <45 y | - | 2/40 (5%) | Subepicardial lateral in a myocarditis pattern | 1182.7 ± 42.4 | 22.7 ± 3.3 |
| McDiarmid et al. (2016) [28] 3 T | 30 Endurance: 7 runners, 11 cyclists, 12 triathletes | Regional, national, or international level >6 h/wk training | 31.7 ± 7.7 | M: 100 | 1/30 (3.3%) | Nonischaemic (post myocarditis pattern) | 1178 ± 32 | 22.5 ± 2.6 |
| Bohm et al. (2016) [29] 1.5 T | 33 16 former elite master endurance athletes: marathon, triathlons, ironman, rowing, cycling | 29 ± 8 y training history, 16.7 ± 4.4 h/wk training | 47 ± 8 | M: 100 1.96 ± 0.1 | 1/33 (3%) | Nonischaemic, subepicardial LV posteroinferior region (most likely due to former pericarditis) | - | - |
| Mangold et al. (2013) [30] 1.5 T | 95 39 long-distance runners, 8 cyclists, 34 triathletes, 13 handball players, 1 speed skater | 13.1 ± 4.2 h/wk for ≥2 y M: 13.1 ± 4.5 h/wk (5–30) F: 12.8 ± 3 h/wk (7–20) | 35.2 ± 11.4 | M: 77 1.91 ± 0.13 F: 23 1.7 ± 0.2 | M: 1/73 (1.3%) F: 1/22 (4.4%) Total: 2/95 (2.1%) | Nonischaemic, post myocarditis pattern (spot-shaped), disseminated and intramural | - | - |
| Androulakis et al. (2022) [31] | 61 Endurance sport: 40 | 12.5 ± 3.3 h/wk | 27.9 ± 6.7 | M: 80 1.99 ± 0.2 F: 20 | 60 | Minor MF: 28 insertion points Major MF: 18 mid-myocarial, 10 subepicardial, 4 subepicardial | - | - |
| Athletes | |||||
|---|---|---|---|---|---|
| CMR Findings | All | Male | Female | Young | Veteran |
| 1.5 T (3 studies) | (n = 145) | (n = 88) | (n = 29) | (n = 28) | (n = 117) |
| T1 (ms) | 990.9 ± 32 * | 966.8 ± 40 | 1015 ± 25 | - | 990.9 ± 32 |
| ECV (%) | 26.2 ± 2.1 | 24.9 ± 2.1 | 27.8 ± 1.9 | 26 ± 2.3 | 26.3 ± 2 |
| LGE + (1.5 T) (2 studies) | (n = 22) | ||||
| T1 (ms) | - | 1005 ± 32 | (n = 9) | - | - |
| ECV (%) | - | 26.7 ± 2 | (n = 22) | - | - |
| LGE − (1.5 T) | (n = 60) | ||||
| T1 (ms) | - | 987 ± 27 | (n = 45) | - | - |
| ECV (%) | - | 24.8 ± 2.1 | (n = 60) | - | - |
| 3 T (5 studies) | (n = 177) | (n = 95) | (n = 12) | (n = 30) | (n = 107) |
| T1 (ms) | 1185.8 ± 34 | 1192.7 ± 40 | 1190 ± 23 | 1178 ± 32 | 1191.3 ± 31 |
| ECV (%) | 23.2 ± 3.3 | 23.6 ± 3.1 | 24.2 ± 3.9 | 22.5 ± 2.6 | 23.9 ± 3.5 |
| LGE + (3 T) (1 study) | (n = 5) | ||||
| T1 (ms) | - | 1220 ± 4 | - | - | - |
| ECV (%) | - | 22 ± 1.2 | - | - | - |
| LGE − (3 T) | (n = 22) | ||||
| T1 (ms) | - | 1212 ± 27 | - | - | - |
| ECV (%) | - | 22.7 ± 3.4 | - | - | - |
| Controls | |||||
| 1.5 T (2 studies) | (n = 48) | (n = 34) | (n = 14) | (n = 48) | |
| T1 (ms) | 1029 ± 27 | 999.1 ± 32 | 1059 ± 22 | - | 1029 ± 27 |
| ECV (%) | 25.95 ± 2.9 | 23 ± 2.5 | 28.9 ± 3.3 | - | 25.95 ± 2.9 |
| 3 T (5 studies) | (n = 97) | (n = 35) | (n = 7) | (n = 20) | (n = 42) |
| T1 (ms) | 1207.5 ± 32 ^ | 1221.7 ± 35 | 1197 ± 22 | 1202 ± 33 | 1209.3 ± 28 |
| ECV (%) | 22.84 ± 2.6 | 23.7 ± 2.6 | 20.4 ± 2.8 | 24.5 ± 2.2 | 22 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allwood, R.P.; Papadakis, M.; Androulakis, E. Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature. J. Clin. Med. 2024, 13, 4536. https://doi.org/10.3390/jcm13154536
Allwood RP, Papadakis M, Androulakis E. Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature. Journal of Clinical Medicine. 2024; 13(15):4536. https://doi.org/10.3390/jcm13154536
Chicago/Turabian StyleAllwood, Richard P., Michael Papadakis, and Emmanuel Androulakis. 2024. "Myocardial Fibrosis in Young and Veteran Athletes: Evidence from a Systematic Review of the Current Literature" Journal of Clinical Medicine 13, no. 15: 4536. https://doi.org/10.3390/jcm13154536





