Unveiling the Role of Metal Ion Concentration versus Immune Sensitization in Orthodontic Patients—A Long-Term Prospective Evaluation
Abstract
1. Introduction
- -
- the amount of metal ions released by fixed orthodontic appliances in saliva is not sufficient to cause toxic or allergic reactions; however, prolonged exposure could potentially lead to sensitization in these patients;
- -
- positive sensitization shows a positive correlation to local exposure in saliva.
| Author(s) (Year) | Observational Period (OP) | N (Age) | Dental Material and Immune System Tested | Metal Ions | Procedures Employed | Outcome (Metal Release, Immune System, Intraoral Findings) |
|---|---|---|---|---|---|---|
| Agaoglu et al. (2001) [25] | 1 OP -unique timepoint for each group: before MBA insertion, 1st w, 1st m, 1st y, 2 y later | 100 -5 groups (12–33 y) | -MBA: 4 bd, 20 br and wires: 1. NiTi; 2. SS -Saliva and blood | Ni, Cr | Electrothermal AAS | -Ni, Cr increase in 1st month -Ni, Cr decrease in 2nd year -no toxic levels -no relationship between Ni level in saliva and serum |
| Faccioni et al. (2003) [1] | 1 sample collection | 85: 55 MBA, 30 control (12–35 y) | Buccal mucosa cells | Ni, Co | -ICP-MS -Comet assay | -2.8-fold–3.4-fold higher ion levels -DNA damage |
| Fernandez-Minano et al. (2011) [17] | 30 d -2 timepoints: -before -30 d after orthodontics | 15 (12–16 y) | -SS, Ni, Ti, Ni-free -Buccal mucosa cells | Ti, Cr, Mn, Co, Ni, Mo, Fe | -ICP-MS -Comet assay | -Ni free MBA higher levels of Cr and Fe -Ti alloys induced increased levels of Mn -Ti alloys no toxic effect -SS and Ni-free MBA greater DNA damage |
| Nayak et al. (2015) [26] | Orthodontic treatment -pre-treatment -after aligning -10–12 m after treatment-beginning | 30 (10–25 y) | -Br, bd and wires: 1. NiTi, 2. NiTi (heat activated), 3. SS -saliva (after 30 s rinsing) | Ni, Cr | -ICP-MS | -Ni and Cr increase after aligning phase -after 10–12 m, increased Cr and decreased Ni levels -concern about biocompatibility and allergic reaction frequency |
| Gölz et al. (2016) [27] | 8 w: before treatment, after br and bd placement, before and after archwire insertion -4 and 8 w later | 30 (10–13 y) | -br (self-ligating), bd -wires: NiTi -unstimulated saliva | Ni | -ICP-MS | -significant increase after br/bd insertion -decrease after 4 w -below dietary intake |
| Pazzini et al. (2016) [28] | -before treatment -12 m: every 3 m -1 m after removal | 42 allergic patients (10–45 y) | -21 conventional br -21 nickel-free br | Ni | -patch test -gingival index -blood | -both groups: increased basophils -conventional group: decreased eosinophils and immunoglobin E -Ni levels: increased while treatment; decreased 1 m after -Ni-free br while treatment: gingival health, smaller blood changes |
| Quadras et al. (2019) [29] | 1.5 y -5 timepoints: before archwire insertion; after 1 week, 3 m, 1 y, and 1.5 y | 80 (15–40 y) -50 MBAs -30 controls | -20 br, 4–8 bd: SS -2 wires: NiTi/SS -saliva (after rinsing); blood | Ni, Cr, Zi | AAS | -Increase before and after insertion of the appliance -below toxic levels -after 1.5 y: significant difference between treated and control group |
| Lucarelli et al. (2020) [30] | 1 y 2 tests: before and 1 y after treatment | 60 | -Ti rapid palatal expander and corrector | -Ni (allergy) -Ti | -patch test | -first test: sensitivity in 8 patients (2 males/6 females) -second test: 37 positive nickel sensitizations (25 females) -Ti appliances have high resistance and no allergic reaction |
| Zigante et al. (2022) [31] | 6 w–1 y treatment | 235 (11–45 y) | -orthodontic appliances -oral mucosa, gingiva, tongue, lips | -Ni -Ti | -Patch test -clinical signs | -clinical predictors of metal sensitization: adult age, female sex, exfoliative cheilitis, history of contact hypersensitivity to metals and piercings -patch test alone not conclusive for allergies |
2. Patients, Materials, and Methods
2.1. Participants’ General Characteristics and Inclusion and Exclusion Criteria
2.2. Material and Methods
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
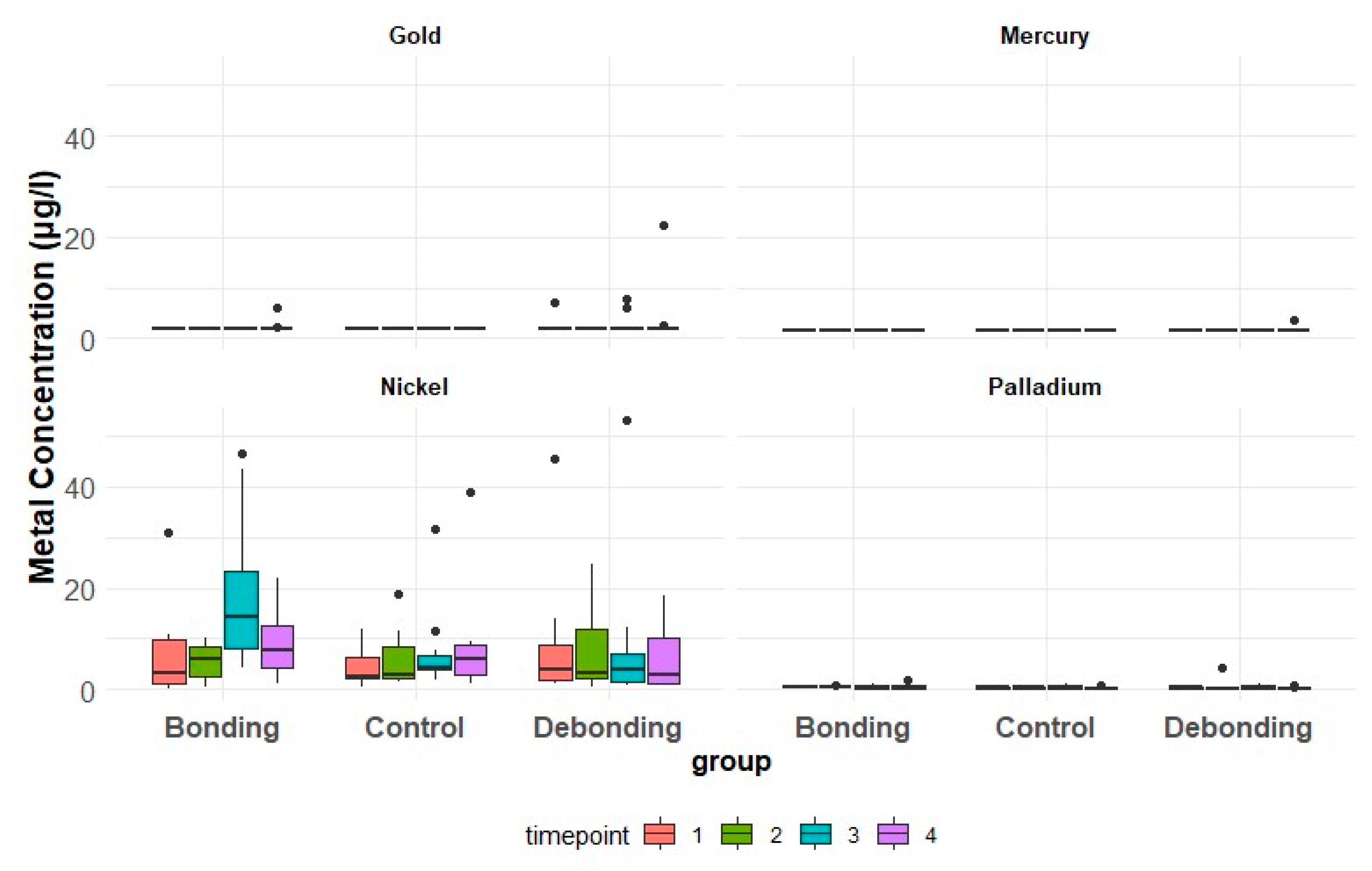
3.1. Metal Ion Concentration in Saliva
3.1.1. Ni
3.1.2. Au
3.1.3. Pd
3.1.4. Hg
3.2. Lymphocyte Transformation Test
3.2.1. Debonding Group (G1)
3.2.2. Bonding Group (G2)
3.2.3. Control Group (G3)
3.3. Correlation between LTT and Metal Ion Concentrations
3.3.1. Ni
3.3.2. Au
3.3.3. Pd
3.3.4. Hg
4. Discussion
4.1. Ni
4.1.1. Debonding Group
4.1.2. Bonding Group
4.1.3. Control Group
4.2. Au
4.3. Pd
4.4. Hg
4.5. Metal Ions
4.6. Sensitization
4.7. Patients, Materials, and Methods
5. Strengths and Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faccioni, F.; Franceschetti, P.; Cerpelloni, M.; Fracasso, M.E. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.; Hanć, A.; Barałkiewicz, D.; Rzymski, P. Metals and Metalloids Release from Orthodontic Elastomeric and Stainless Steel Ligatures: In Vitro Risk Assessment of Human Exposure. Biol. Trace Elem. Res. 2020, 196, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V. Metals as a common trigger of inflammation resulting in non-specific symptoms: Diagnosis and treatment. Isr. Med. Assoc. J. 2014, 16, 753–758. [Google Scholar] [PubMed]
- Stejskal, V.; Reynolds, T.; Bjørklund, G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J. Trace Elem. Med. Biol. 2015, 31, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Martin-Camean, A.; Jos, A.; Mellado-Garcia, P.; Iglesias-Linares, A.; Solano, E.; Camean, A.M. In vitro and in vivo evidence of the cytotoxic and genotoxic effects of metal ions released by orthodontic appliances: A review. Environ. Toxicol. Pharmacol. 2015, 40, 86–113. [Google Scholar] [CrossRef] [PubMed]
- Huesker, V.B.e. Allergologie und Toxikologie kieferorthpädischer Materialien. Umw. Med. Ges. 2015, 28, 194–200. [Google Scholar]
- Downarowicz, P.; Mikulewicz, M. Trace metal ions release from fixed orthodontic appliances and DNA damage in oral mucosa cells by in vivo studies: A literature review. Adv. Clin. Exp. Med. 2017, 26, 1155–1162. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K. Release of metal ions from orthodontic appliances by in vitro studies: A systematic literature review. Biol. Trace Elem. Res. 2011, 139, 241–256. [Google Scholar] [CrossRef]
- Zinelis, S.; Polychronis, G.; Papadopoulos, F.; Kokkinos, C.; Economou, A.; Panayi, N.; Papageorgiou, S.N.; Eliades, T. Mechanical and electrochemical characterization of 3D printed orthodontic metallic appliances after in vivo ageing. Dent. Mater. 2022, 38, 1721–1727. [Google Scholar] [CrossRef]
- Eliades, T.; Pratsinis, H.; Kletsas, D.; Eliades, G.; Makou, M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mikulewicz, M.; Chojnacka, K.; Wolowiec, P. Release of metal ions from fixed orthodontic appliance: An in vitro study in continuous flow system. Angle. Orthod. 2014, 84, 140–148. [Google Scholar] [CrossRef]
- Kuhta, M.; Pavlin, D.; Slaj, M.; Varga, S.; Lapter-Varga, M.; Slaj, M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle. Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Fróis, A.; Mendes, A.R.; Pereira, S.A.; Louro, C.S. Metal Release and Surface Degradation of Fixed Orthodontic Appliances during the Dental Levelling and Aligning Phase: A 12-Week Study. Coatings 2022, 12, 554. [Google Scholar] [CrossRef]
- Jusufi Osmani, Z.; Poljšak, B.; Zelenika, S.; Kamenar, E.; Marković, K.; Perčić, M.; Katić, V. Ion Release and Surface Changes of Nickel-Titanium Archwires Induced by Changes in the pH Value of the Saliva-Significance for Human Health Risk Assessment. Materials 2022, 15, 1994. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K. Trace metal release from orthodontic appliances by in vivo studies: A systematic literature review. Biol. Trace Elem. Res. 2010, 137, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Miñano, E.; Ortiz, C.; Vicente, A.; Calvo Guirado, J.L.; Ortiz, A.J. Metallic ion content and damage to the DNA in oral mucosa cells of children with fixed orthodontic appliances. Biometals 2011, 24, 935. [Google Scholar] [CrossRef]
- Pillai, A.R.; Gangadharan, A.; Gangadharan, J.; Kumar, N.V. Cytotoxic effects of the nickel release from the stainless steel brackets: An in vitro study. J. Pharm. Bioallied Sci. 2013, 5, S1–S4. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of dental casting alloys. Crit. Rev. Oral Biol. Med. 2002, 13, 71–84. [Google Scholar] [CrossRef]
- Schmalz, G.; Arenholt-Bindslev, D. Biokompatibilität Zahnärztlicher Werkstoffe; Elsevier GmbH Urban und Fischer Verlag München: München, Germany, 2005. [Google Scholar]
- Kimber, I.; Basketter, D.A. Allergic Sensitization to Nickel and Implanted Metal Devices: A Perspective. Dermatitis 2022, 33, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Aparicio-Soto, M.; Curato, C.; Thierse, H.J.; Siewert, K.; Luch, A. Immunological Mechanisms of Metal Allergies and the Nickel-Specific TCR-pMHC Interface. Int. J. Environ. Res. Public Health 2021, 18, 10867. [Google Scholar] [CrossRef] [PubMed]
- Gölz, L.; Papageorgiou, S.N.; Jäger, A. Nickel hypersensitivity and orthodontic treatment: A systematic review and meta-analysis. Contact Dermat. 2015, 73, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ağaoğlu, G.; Arun, T.; Izgi, B.; Yarat, A. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances. Angle Orthod. 2001, 71, 375–379. [Google Scholar] [PubMed]
- Nayak, R.S.; Khanna, B.; Pasha, A.; Vinay, K.; Narayan, A.; Chaitra, K. Evaluation of Nickel and Chromium Ion Release During Fixed Orthodontic Treatment Using Inductively Coupled Plasma-Mass Spectrometer: An In Vivo Study. J. Int. Oral Health 2015, 7, 14–20. [Google Scholar]
- Gölz, L.; Knickenberg, A.C.; Keilig, L.; Reimann, S.; Papageorgiou, S.N.; Jäger, A.; Bourauel, C. Nickelionenkonzentration im Speichelvon Patienten mit selbstligierenden festsitzenden Apparaturen: Eine prospektive Kohortenstudie. J. Orofac. Orthop. 2016, 77, 85–93. [Google Scholar] [CrossRef][Green Version]
- Pazzini, C.A.; Pereira, L.J.; Marques, L.S.; Ramos-Jorge, J.; Aparecida da Silva, T.; Paiva, S.M. Nickel-free vs conventional braces for patients allergic to nickel: Gingival and blood parameters during and after treatment. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Quadras, D.D.; Nayak, U.S.K.; Kumari, N.S.; Priyadarshini, H.R.; Gowda, S.; Fernandes, B. In vivo study on the release of nickel, chromium, and zinc in saliva and serum from patients treated with fixed orthodontic appliances. Dent. Res. J. 2019, 16, 209–215. [Google Scholar] [CrossRef]
- Lucarelli, D.; Stabilini, A.; De Filippis, A.; D’Avola, V.; Mainardi, E.; Esposito, L. Orthodontic appliances in patients allergic to nickel. J. Biol. Regul. Homeost. Agents 2020, 34, 2375–2378. [Google Scholar] [CrossRef]
- Zigante, M.; Peternel, S.; Muhvic Urek, M.; Rincic Mlinaric, M.; Pop Acev, D.; Spalj, S. Smell and taste in titanium and nickel allergic sensitization in orthodontic patients. Orthod. Craniofac. Res. 2020, 23, 517–522. [Google Scholar] [CrossRef]
- von Baehr, V.; Mayer, W.; Liebenthal, C.; von Baehr, R.; Bieger, W.; Volk, H.D. Improving the in vitro antigen specific T cell proliferation assay: The use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J. Immunol. Methods 2001, 251, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Muris, J.; Goossens, A.; Goncalo, M.; Bircher, A.J.; Gimenez-Arnau, A.; Foti, C.; Rustemeyer, T.; Feilzer, A.J.; Kleverlaan, C.J. Sensitization to palladium and nickel in Europe and the relationship with oral disease and dental alloys. Contact Dermat. 2015, 72, 286–296. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Counts, A.L.; Miller, M.A.; Khakhria, M.L.; Strange, S. Nickel allergy associated with a transpalatal arch appliance. J. Orofac. Orthop. 2002, 63, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Anderson, S.; Caicedo, M.; Skipor, A.; Campbell, P.; Jacobs, J.J. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J. Arthroplast. 2004, 19, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.B.; Pelletier, J.L.; Jacob, S.E.; Schneider, L.C.; Cohen, B.; Horii, K.A.; Kristal, L.; Maguiness, S.M.; Tollefson, M.M.; Weinstein, M.G.; et al. Nickel Allergic Contact Dermatitis: Identification, Treatment, and Prevention. Pediatrics 2020, 145, e20200628. [Google Scholar] [CrossRef] [PubMed]
- Wennervaldt, M.; Vaher, H.; Ahlström, M.G.; Bischofberger, N.; Menné, T.; Thyssen, J.P.; Johansen, J.D.; Bonefeld, C.M. Subclinical immune responses to nickel in sensitized individuals-a dose-response study. Contact Dermat. 2024, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Banta, E. Allergic Contact Dermatitis and Orthodontics. Pediatr. Allergy Immunol. Pulmonol. 2023, 36, 50–51. [Google Scholar] [CrossRef]
- Janson, G.R.; Dainesi, E.A.; Consolaro, A.; Woodside, D.G.; de Freitas, M.R. Nickel hypersensitivity reaction before, during, and after orthodontic therapy. Am. J Orthod. Dentofac. Orthop. 1998, 113, 655–660. [Google Scholar] [CrossRef]
- Cavani, A.; Nasorri, F.; Ottaviani, C.; Sebastiani, S.; De Pità, O.; Girolomoni, G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 2003, 171, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Di Spirito, F.; Amato, A.; Di Palo, M.P.; Ferraro, R.; Cannatà, D.; Galdi, M.; Sacco, E.; Amato, M. Oral and Extra-Oral Manifestations of Hypersensitivity Reactions in Orthodontics: A Comprehensive Review. J. Funct. Biomater. 2024, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Trapalis, C.; Eliades, G.; Katsavrias, E. Salivary metal levels of orthodontic patients: A novel methodological and analytical approach. Eur. J. Orthod. 2003, 25, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Zigante, M.; Špalj, S. Clinical predictors of metal allergic sensitization in orthodontic patients. Cent. Eur. J. Public Health 2022, 30, 173–178. [Google Scholar] [CrossRef]
- Hwang, C.J.; Shin, J.S.; Cha, J.Y. Metal release from simulated fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Behroozi, Z.; Momeni Danaei, S.; Reza Sardarian, A.; Moshkelghosha, V.; Sardarian, A.R. Evaluation of the Corrosion of Five Different Bracket-Archwire Combination: An In-vitro Analysis Using Inductively Coupled Plasma Mass Spectrometry. J. Dent. Shiraz Univ. Med. Sci. 2016, 17, 262–267. [Google Scholar] [PubMed]
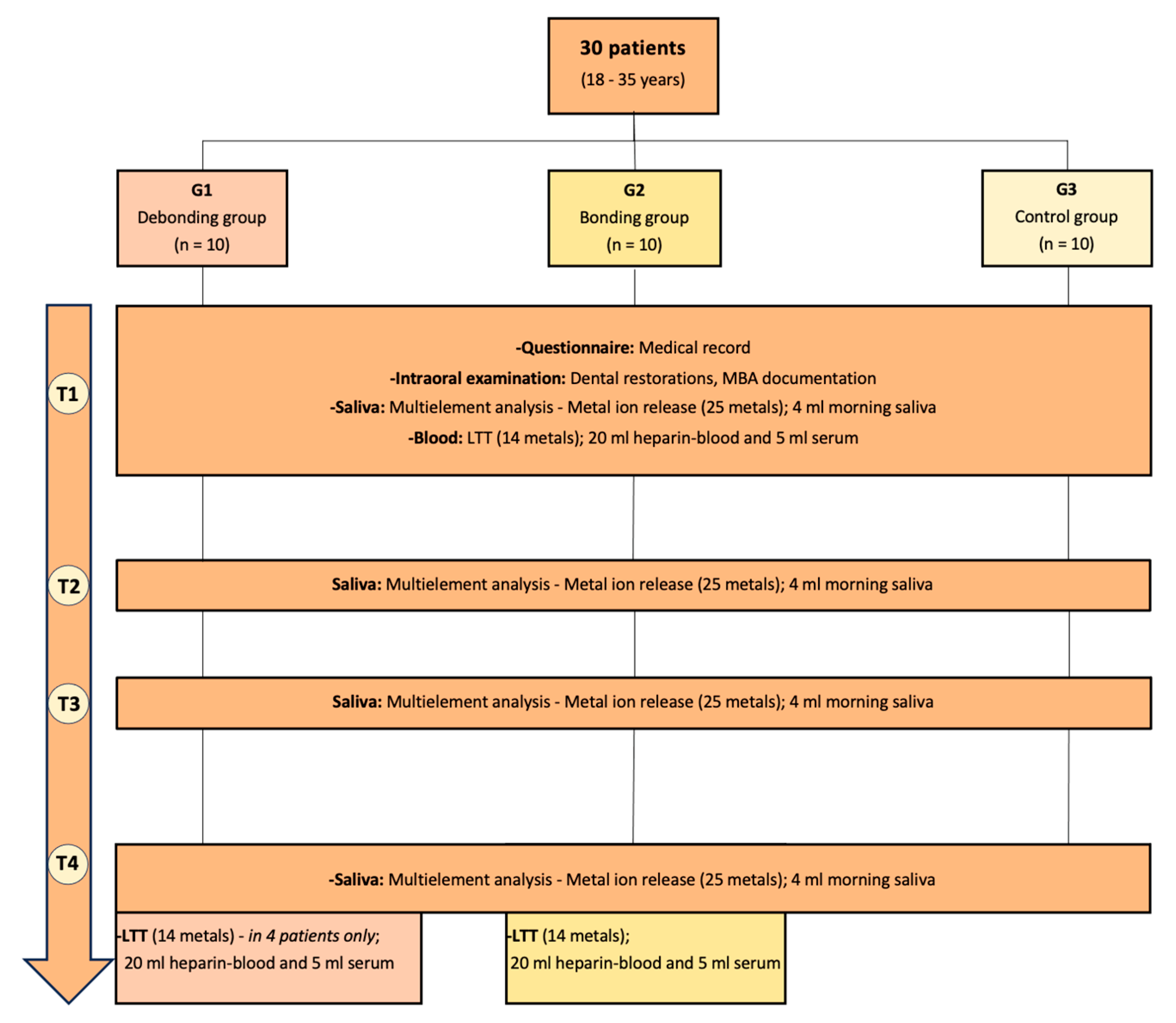

| G1 (N = 10) | G2 (N = 10) | G3 (N = 10) | |
|---|---|---|---|
| Male/female (N) | 6/4 | 5/5 | 5/5 |
| Mean age (years) | 20.6 | 20.9 | 22.8 |
| Inclusion criteria |
|
| No history of orthodontic treatment or metal dental restorations |
| Exclusion criteria |
| ||
| G1 | G2 | G3 | |
|---|---|---|---|
| T1 | 1st day; before MBA removal | 1st day; before MBA placement | 1st day |
| T2 | 1st morning after MBA removal | 1st morning after MBA placement | 2nd morning |
| T3 | 7th morning after MBA removal | 7th morning with MBA | 7th morning |
| T4 | 21st day after MBA removal | 21st day with MBA | 21st day |
| Debonding | Bonding | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time-Point | R [µg/L] | Median | First Q | Third Q | Median | First Q | Third Q | Median | First Q | Third Q | |
| Nickel [µg/L] | T1 | 1.2 | 3.10 | 2.20 | 11.98 | 6.15 | 2.38 | 8.25 | 2.90 | 2.10 | 8.48 |
| T2 | 1.2 | 3.95 | 1.50 | 6.88 | 14.35 | 8.03 | 23.20 | 4.25 | 4.05 | 6.85 | |
| T3 | 1.2 | 2.75 | 1.25 | 10.30 | 7.70 | 4.40 | 12.53 | 5.85 | 2.70 | 8.68 | |
| T4 | 1.2 | 3.90 | 1.93 | 8.65 | 6.25 | 3.23 | 10.53 | 2.50 | 2.23 | 5.70 | |
| Gold [µg/L] | T1 | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| T2 | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| T3 | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| T4 | 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| Palladium [µg/L] | T1 | 1.2 | 0.35 | 0.23 | 0.55 | 0.40 | 0.30 | 0.75 | 0.35 | 0.23 | 0.58 |
| T2 | 1.2 | 0.25 | 0.20 | 0.30 | 0.40 | 0.30 | 0.48 | 0.45 | 0.23 | 0.60 | |
| T3 | 1.2 | 0.40 | 0.20 | 0.65 | 0.25 | 0.20 | 0.70 | 0.50 | 0.23 | 0.70 | |
| T4 | 1.2 | 0.25 | 0.20 | 0.30 | 0.30 | 0.20 | 0.70 | 0.20 | 0.20 | 0.30 | |
| Mercury [µg/L] | T1 | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| T2 | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | |
| T3 | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | |
| T4 | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | |
| Metal | Odds Ratio (95% Confidence Interval) | p-Value | ||
|---|---|---|---|---|
| Nickel | Group | Debonding | 0.47 (0.10, 2.23) | 0.34 |
| Bonding | 0.57 (0.12, 2.82) | 0.49 | ||
| Control | Reference | |||
| Time | 1 | Reference | ||
| 2 | 1.56 (0.25, 9.73) | 0.63 | ||
| 3 | 0.72 (0.15, 3.44) | 0.68 | ||
| 4 | 0.55 (0.12, 2.49) | 0.44 | ||
| Gold | Group | Debonding | 1.89 (0.23, 15.37) | 0.55 |
| Bonding | Not estimable | |||
| Control | Reference | |||
| Time | 1 | Reference | ||
| 2 | 2.12 (0.30, 14.75) | 0.45 | ||
| 3 | 4.81 (0.76, 30.70) | 0.10 | ||
| 4 | Not estimable | |||
| Palladium | Group | Debonding | 0.48 (0.05, 4.92) | 0.54 |
| Bonding | Not estimable | |||
| Control | Reference | |||
| Time | 1 | Reference | ||
| 2 | 1 (0.05, 18.67) | 1 | ||
| 3 | 1 (0.05, 18.67) | 1 | ||
| 4 | Not estimable |
| Timepoint T1 (Day 0) | Timepoint T4 (Day 21) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Patient (sex) | Pd | Hg | Au | Ni | Pd | Hg | Au | Ni |
| G1 | 101 (f) | 1.0 | 1.0 | 1.0 | 1.0 | 3.3 | 1.4 | 1.4 | 2.8 |
| 102 (m) | 1.1 | 2.4 | 1.0 | 1.2 | 1.1 | 1.0 | 1.0 | 1.0 | |
| 111 (f) | 1.4 | 1.8 | 1.7 | 2.5 | 1.2 | 1.0 | 1.0 | 1.0 | |
| G2 | 202 (m) | 1.3 | 1.6 | 1.8 | 4 | 1.1 | 1.0 | 1.2 | 1.5 |
| 204 (m) | 1.7 | 1.4 | 1.4 | 4.4 | 1.1 | 1.3 | 1.5 | 1.4 | |
| 207 (m) | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 | 1.0 | 17.5 | |
| 208 (f) | 1.3 | 1.2 | 1.5 | 1.6 | 1.0 | 1.0 | 1.0 | 4.8 | |
| 211 (m) | 1.0 | 1.5 | 1.5 | 1.9 | 2.3 | 1.0 | 1.0 | 4.1 | |
| G3 | 303 (f) | 1.0 | 1.0 | 2.9 | 46.3 | - | - | - | - |
| 305 (m) | 1.4 | 1.7 | 1.4 | 3.3 | - | - | - | - | |
| 307 (f) | 1.2 | 2.6 | 1.7 | 3.3 | - | - | - | - | |
| 310 (f) | 1.2 | 3.4 | 1.1 | 1.5 | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschaei, N.; Müller, W.-D.; Schmidt, F.; Hüsker, K.; von Baehr, V.; Pandis, N.; Jost-Brinkmann, P.-G.; Bartzela, T. Unveiling the Role of Metal Ion Concentration versus Immune Sensitization in Orthodontic Patients—A Long-Term Prospective Evaluation. J. Clin. Med. 2024, 13, 4545. https://doi.org/10.3390/jcm13154545
Paschaei N, Müller W-D, Schmidt F, Hüsker K, von Baehr V, Pandis N, Jost-Brinkmann P-G, Bartzela T. Unveiling the Role of Metal Ion Concentration versus Immune Sensitization in Orthodontic Patients—A Long-Term Prospective Evaluation. Journal of Clinical Medicine. 2024; 13(15):4545. https://doi.org/10.3390/jcm13154545
Chicago/Turabian StylePaschaei, Nusha, Wolf-Dieter Müller, Franziska Schmidt, Katrin Hüsker, Volker von Baehr, Nikolaos Pandis, Paul-Georg Jost-Brinkmann, and Theodosia Bartzela. 2024. "Unveiling the Role of Metal Ion Concentration versus Immune Sensitization in Orthodontic Patients—A Long-Term Prospective Evaluation" Journal of Clinical Medicine 13, no. 15: 4545. https://doi.org/10.3390/jcm13154545
APA StylePaschaei, N., Müller, W.-D., Schmidt, F., Hüsker, K., von Baehr, V., Pandis, N., Jost-Brinkmann, P.-G., & Bartzela, T. (2024). Unveiling the Role of Metal Ion Concentration versus Immune Sensitization in Orthodontic Patients—A Long-Term Prospective Evaluation. Journal of Clinical Medicine, 13(15), 4545. https://doi.org/10.3390/jcm13154545






