Utilization of a Cortical Xenogeneic Membrane for Guided Bone Regeneration: A Retrospective Case Series
Abstract
:1. Introduction
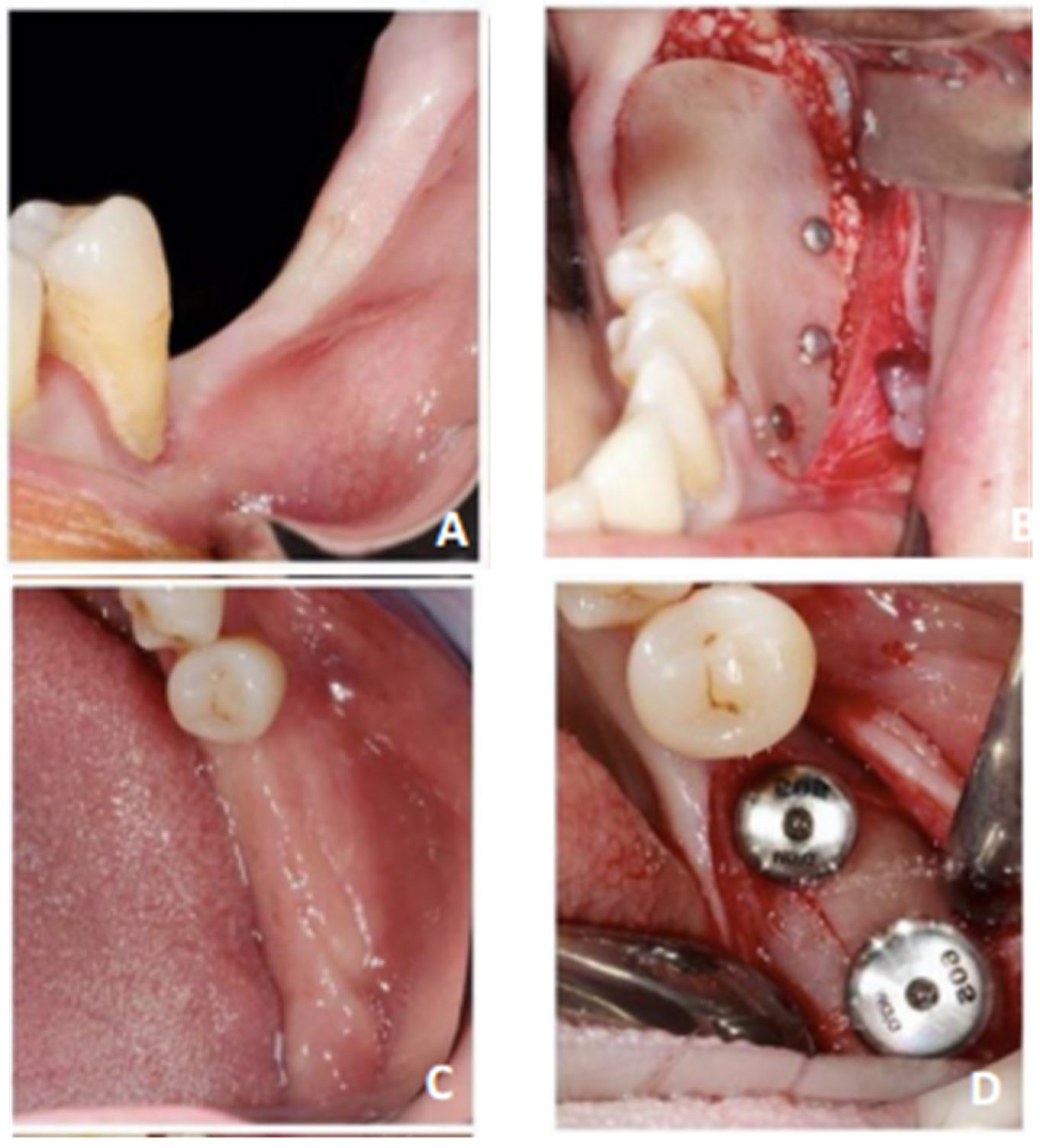
2. Materials and Methods
- ▪
- A line perpendicular to the top of the preoperative ridge (red, named D1);
- ▪
- A line passing through the most coronal and apical points of the preoperative ridge (orange, named D2);
- ▪
- Lines D1 and D2 intersect at a point named A;
- ▪
- Point B is defined as equidistant from the most coronal and apical points of the preoperative ridge on line D2;
- ▪
- Horizontal measurement 1 (HM1): from point A to the most anterior point of the postoperative ridge (dark green);
- ▪
- Horizontal measurement 2 (HM2): from point B to the most anterior point of the postoperative ridge (light green/yellow);
- ▪
- Vertical measurement (VM): from the midpoint of MH1, named C, to the most coronal point of the postoperative ridge (dark blue).
- ▪
- The presence of pain not relieved by the use of Tier I and II analgesics during the one-week postoperative follow-up appointment;
- ▪
- Membrane exposure;
- ▪
- The presence of infectious phenomena related to the operative site (site suppuration);
- ▪
- The presence of nerve complications—hypoesthesia of the inferior alveolar nerve.
- ▪
- The implant success rate was evaluated according to—clinical absence of inflammation of the peri-implant mucosa, absence of periodontal pocketing greater than 2 mm upon peri-implant probing, and implants in function;
- ▪
- Bone resorption was assessed by 2D intraoral radiographs on the bone level in relation to the implant neck at 3 months and 1 year after implant placement.
3. Results
- Two out of five patients (40%) experienced postoperative pain at the one-week postoperative follow-up appointment;
- No patient experienced membrane exposure, infectious phenomena, or nerve complications;
- The implant success rate was 100%;
- Radiographic analysis around the loaded dental implants at 3-month and 1-year follow-ups reported, respectively, 0.2 mm and 0.44 mm of peri-implant bone loss (Table 4);
4. Discussion
- ▪
- In the horizontal direction, for all membranes combined, Sanz-Sanchez et al. [18] (2015) reported a mean horizontal gain of 3.90 mm (95% CI, 3.52–4.28 mm), and Elnayef et al. (2018) a gain of 3.61 ± 0.27 mm [19]. With resorbable collagen membranes only, Wessing et al. (2018) found lower results (2.27 ± 1.68 mm) among 460 patients [20].
- ▪
- ▪
- Focusing on the posterior mandibular sector, for all membranes combined, our results approached those of Elnayef et al.‘s systematic review, which reported a mean vertical gain of 3.83 ± 0.49 mm from a sample of 62 patients [19]. With non-resorbable membranes only, Robert et al. (2023) showed, in their systematic review, a mean vertical gain of 4.7 mm (minimum: 1.5 mm–maximum: 5.24 mm) [22].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A. Vertical and Horizontal Ridge Augmentation: New Perspectives; Quintessence Publishing: Berlin, Germany, 2017; 390p. [Google Scholar]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.F.; Rocchietta, I.; Buti, J.; D’Aiuto, F. Comparative evidence of different surgical techniques for the management of vertical alveolar ridge defects in terms of complications and efficacy: A systematic review and network meta-analysis. J. Clin. Periodontol. 2023, 50, 1487–1519. [Google Scholar] [CrossRef]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, J.; Smith, L.E.H.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Boyapati, L. «PASS» principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández-Alfaro, F. Physicochemical characterization of barrier membranes for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rinna, C.; Ungari, C.; Saltarel, A.; Cassoni, A.; Reale, G. Orbital floor restoration. J. Craniofac. Surg. 2005, 16, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Rinna, C.; Reale, G.; Foresta, E.; Mustazza, M.C. Medial orbital wall reconstruction with swine bone cortex. J. Craniofac. Surg. 2009, 20, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Senese, O.; Boutremans, E.; Gossiaux, C.; Loeb, I.; Dequanter, D. Retrospective analysis of 79 patients with orbital floor fracture: Outcomes and patient-reported satisfaction. Arch. Craniofac. Surg. 2018, 19, 108–113. [Google Scholar] [CrossRef]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.R.H.; Lu, X.J.; Lai, W.M.C.; Fu, J.H. Clinical and histological sequelae of surgical complications in horizontal guided bone regeneration: A systematic review and proposal for management. Int. J. Implant Dent. 2020, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265–273. [Google Scholar]
- Gallo, P.; Díaz-Báez, D. Management of 80 Complications In Vertical And Horizontal Ridge Augmentation with Nonresorbable Membrane (d-PTFE): A Cross-Sectional Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.L.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.A.; Sohrabi, C.; Mathew, G.; Franchi, T.; Kerwan, A.; O’Neill, N.; Thoma, A.; Beamish, A.J.; Noureldin, A.; Rao, A.; et al. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting of CasESeries in Surgery (PROCESS) Guidelines. Int. J. Surg. 2020, 84, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-analysis. J. Dent. Res. 2015, 94 (Suppl. S9), 128S–142S. [Google Scholar] [CrossRef] [PubMed]
- Elnayef, B.; Monje, A.; Gargallo-Albiol, J.; Galindo-Moreno, P.; Wang, H.L.; Hernández-Alfaro, F. Vertical Ridge Augmentation in the Atrophic Mandible: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 319–339. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Aloy-Prósper, A.; Arias-Herrera, S. Vertical augmentation of the atrofic posterior mandibular ridges with onlay grafts: Intraoral blocks vs. guided bone regeneration. Systematic review. J. Clin. Exp. Dent. 2023, 15, e357–e365. [Google Scholar] [CrossRef] [PubMed]
- Foti, V.; Savio, D.; Rossi, R. One-Time Cortical Lamina: A New Technique for Horizontal Ridge Augmentation. A Case Series. J. Biomed. Eng. Med. Imaging 2021, 8, 22–30. [Google Scholar] [CrossRef]
- Rossi, R.; Conti, A.; Bertazzo, D.; Pilloni, A. Horizontal Ridge Augmentation with the Cortical Lamina Technique: A Case Report. Mod. Res. Dent. 2019, 4, 408–411. [Google Scholar]
- Rossi, R.; Ghezzi, C.; Tomecek, M. Cortical lamina: A new device for the treatment of moderate and severe tridimensional bone and soft tissue defects. Int. J. Esthet. Dent. 2020, 15, 454–473. [Google Scholar] [PubMed]
- Stucker, S.; Chen, J.; Watt, F.E.; Kusumbe, A.P. Bone Angiogenesis and Vascular Niche Remodeling in Stress, Aging, and Diseases. Front. Cell Dev. Biol. 2020, 8, 602269. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Sato, H.; Holmes, P.; Turri, A. Intra-surgical vs. radiographic bone level assessments in measuring peri-implant bone loss. Clin. Oral Implant. Res. 2017, 28, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Güven, S.Ş.; Cabbar, F.; Güler, N. Local and systemic factors associated with marginal bone loss around dental implants: A retrospective clinical study. Quintessence Int. 2020, 51, 128–141. [Google Scholar]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Andriola, F.D.O.; Haas Junior, O.L.; Guijarro-Martinez, R.; Hernandez-Alfaro, F.; Oliveira, R.B.D.; Pagnoncelli, R.M.; Swennen, G.R. Computed tomography imaging superimposition protocols to assess outcomes in orthognathic surgery: A systematic review with comprehensive recommendations. Dentomaxillofac. Radiol. 2022, 51, 20210340. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, A.; Leonardi, R. Automatic cephalometric landmark identification with artificial intelligence: An umbrella review of systematic reviews. J. Dent. 2024, 146, 105056. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, J.; Gracea, R.S.; Vanheers, M.; Winderickx, N.; Preda, F.; Shujaat, S.; Jacobs, R. Can artificial intelligence-driven cephalometric analysis replace manual tracing? A systematic review and meta-analysis. Eur. J. Orthod. 2024, 46, cjae029. [Google Scholar] [CrossRef] [PubMed]
- Fokas, G.; Vaughn, V.M.; Scarfe, W.C.; Bornstein, M.M. Accuracy of linear measurements on CBCT images related to presurgical implant treatment planning: A systematic review. Clin. Oral Implants Res. 2018, 29 (Suppl. S16), 393–415. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, C.D.O.; Masterson, D.; Motta, A.F.J.; Motta, A.T. Reliability and reproducibility of three-dimensional cephalometric landmarks using CBCT: A systematic review. J. Appl. Oral Sci. 2015, 23, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sam, A.; Currie, K.; Oh, H.; Flores-Mir, C.; Lagravére-Vich, M. Reliability of different three-dimensional cephalometric landmarks in cone-beam computed tomography: A systematic review. Angle Orthod. 2019, 89, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Schlicher, W.; Nielsen, I.; Huang, J.C.; Maki, K.; Hatcher, D.C.; Miller, A.J. Consistency and precision of landmark identification in three-dimensional cone beam computed tomography scans. Eur. J. Orthod. 2012, 34, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Nijkamp, P.; Verheij, H.; Tairie, J.; Vink, C.; van der Stelt, P.; van Beek, H. Precision of identifying cephalometric landmarks with cone beam computed tomography in vivo. Eur. J. Orthod. 2013, 35, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zamora, N.; Llamas, J.M.; Cibrián, R.; Gandia, J.L.; Paredes, V. A study on the reproducibility of cephalometric landmarks when undertaking a three-dimensional (3D) cephalometric analysis. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e678–e688. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, E.; Lee, W.F.; Lin, C.Y.; Huang, H.M.; Lin, C.T.; Feng, S.W.; Chang, W.J. A Novel Porcine Graft for Regeneration of Bone Defects. Materials 2015, 8, 2523–2536. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; Chang, W.J. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Hwang, S.H.; Kim, Y.N.; Kim, H.J.; Bae, E.B.; Huh, J.B. Bone Reconstruction Using Two-Layer Porcine-Derived Bone Scaffold Composed of Cortical and Cancellous Bones in a Rabbit Calvarial Defect Model. Int. J. Mol. Sci. 2022, 23, 2647. [Google Scholar] [CrossRef] [PubMed]


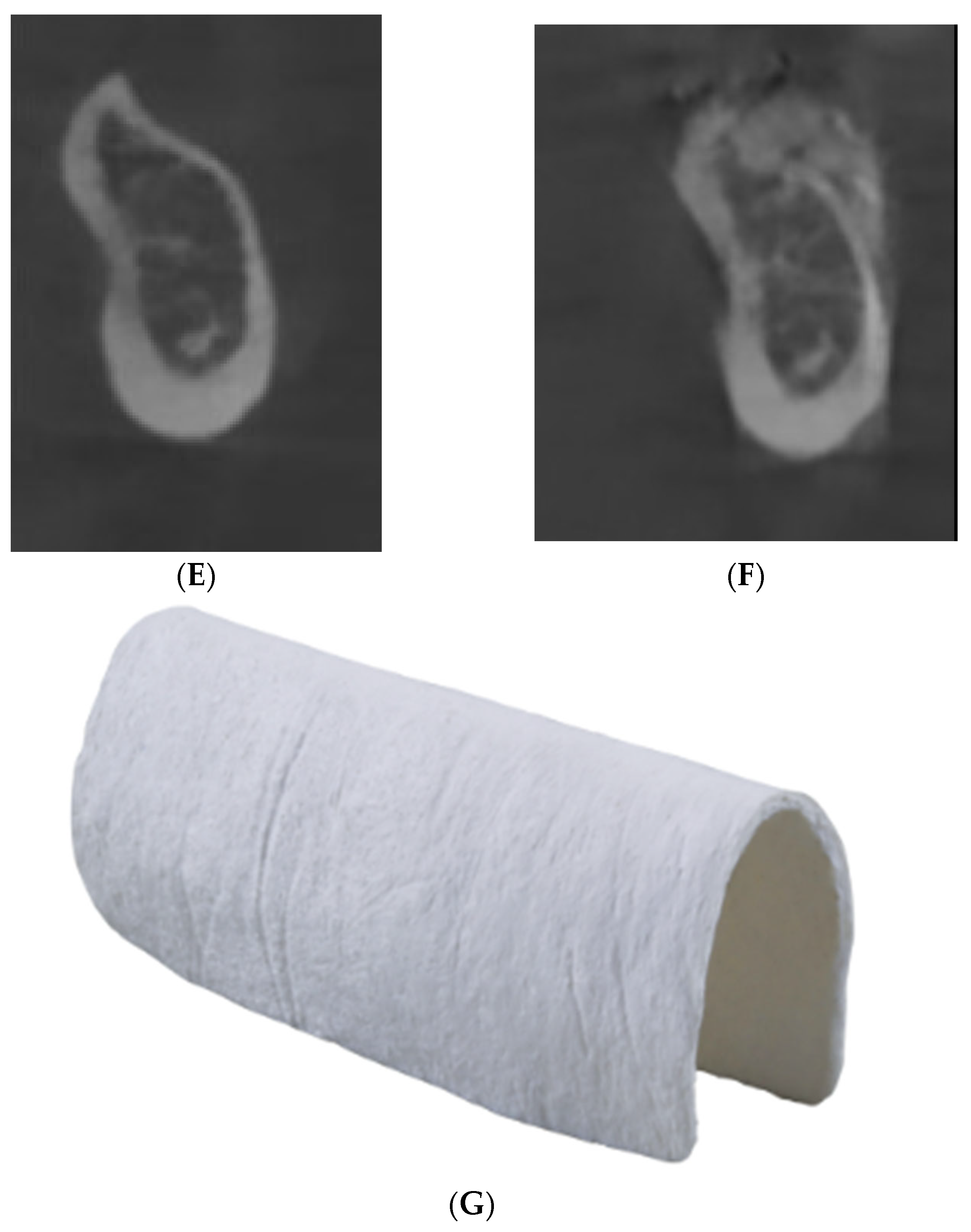
| Landmarks | Description |
|---|---|
| BONE | |
| Point B | Most inferior point on the midline of the image of the anterior concavity of the mandible |
| Point Menton | Most medial point and lowest part of the mandible |
| DENTAL | |
| Apices of tooth 41, 31 | Most apical point on part of the tooth |
| Free edges 42, 41, 31,32 | Most coronal point on part of the incisors on a sagittal section |
| Central fossae 36, 46 | Point most apical part of the cuspid fossa |
| Measure Time | General Mean (±SD) | |||
|---|---|---|---|---|
| R0 | R1 | |||
| Horizontal gain (mm) | Mean HM1 | 3.84 | 3.85 | 3.84 ± 1.05 |
| Mean HM2 | 3.88 | 3.73 | 3.81 ± 0.90 | |
| Mean HM1 + HM2 | 3.86 | 3.79 | 3.83 ± 1.41 | |
| Vertical gain (mm) | Mean VM | 4.14 | 4.20 | 4.17 ± 1.86 |
| Types of Measure | Lin Concordance Coefficient between R0 and R1 |
|---|---|
| HM1 | 0.998 |
| HM2 | 0.996 |
| VM | 0.998 |
| Mean | 0.997 |
| Peri-Implant Bone Loss (mm) | ||
|---|---|---|
| Implants Sites | T1 | T2 |
| 1 | 0.1 | 0.3 |
| 2 | 0 | 0.4 |
| 3 | 0.3 | 0.4 |
| 4 | 0.2 | 0.2 |
| 5 | 0.4 | 0.8 |
| 6 | 0.3 | 0.6 |
| 7 | 0.1 | 0.4 |
| Mean peri-implant bone loss (mm) | 0.2 | 0.44 |
| Patient | Cawood and Howell | Horizontal Mean Gain | Vertical Mean Gain | Pain | Membrane Exposure | Infection | Nerve Trouble | Failure |
|---|---|---|---|---|---|---|---|---|
| 1 | V | 5.11 | 3.93 | Y | N | N | N | N |
| 2 | V | 5.25 | 1.42 | Y | N | N | N | N |
| V | 4.3 | 2.57 | ||||||
| 3 | VI | 1.26 | 9.35 | N | N | N | N | N |
| 4 | IV | 3.56 | 3.32 | N | N | N | N | N |
| 5 | V | 3.65 | 4.05 | N | N | N | N | N |
| V | 3.66 | 4.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debortoli, C.; Falguiere, A.; Campana, F.; Catherine, J.-H.; Tardivo, D.; Lan, R. Utilization of a Cortical Xenogeneic Membrane for Guided Bone Regeneration: A Retrospective Case Series. J. Clin. Med. 2024, 13, 4575. https://doi.org/10.3390/jcm13154575
Debortoli C, Falguiere A, Campana F, Catherine J-H, Tardivo D, Lan R. Utilization of a Cortical Xenogeneic Membrane for Guided Bone Regeneration: A Retrospective Case Series. Journal of Clinical Medicine. 2024; 13(15):4575. https://doi.org/10.3390/jcm13154575
Chicago/Turabian StyleDebortoli, Cyril, Arthur Falguiere, Fabrice Campana, Jean-Hugues Catherine, Delphine Tardivo, and Romain Lan. 2024. "Utilization of a Cortical Xenogeneic Membrane for Guided Bone Regeneration: A Retrospective Case Series" Journal of Clinical Medicine 13, no. 15: 4575. https://doi.org/10.3390/jcm13154575





