Prenatal Manifestation of Transient Abnormal Myelopoiesis: Case Report and Review of the Literature
Abstract
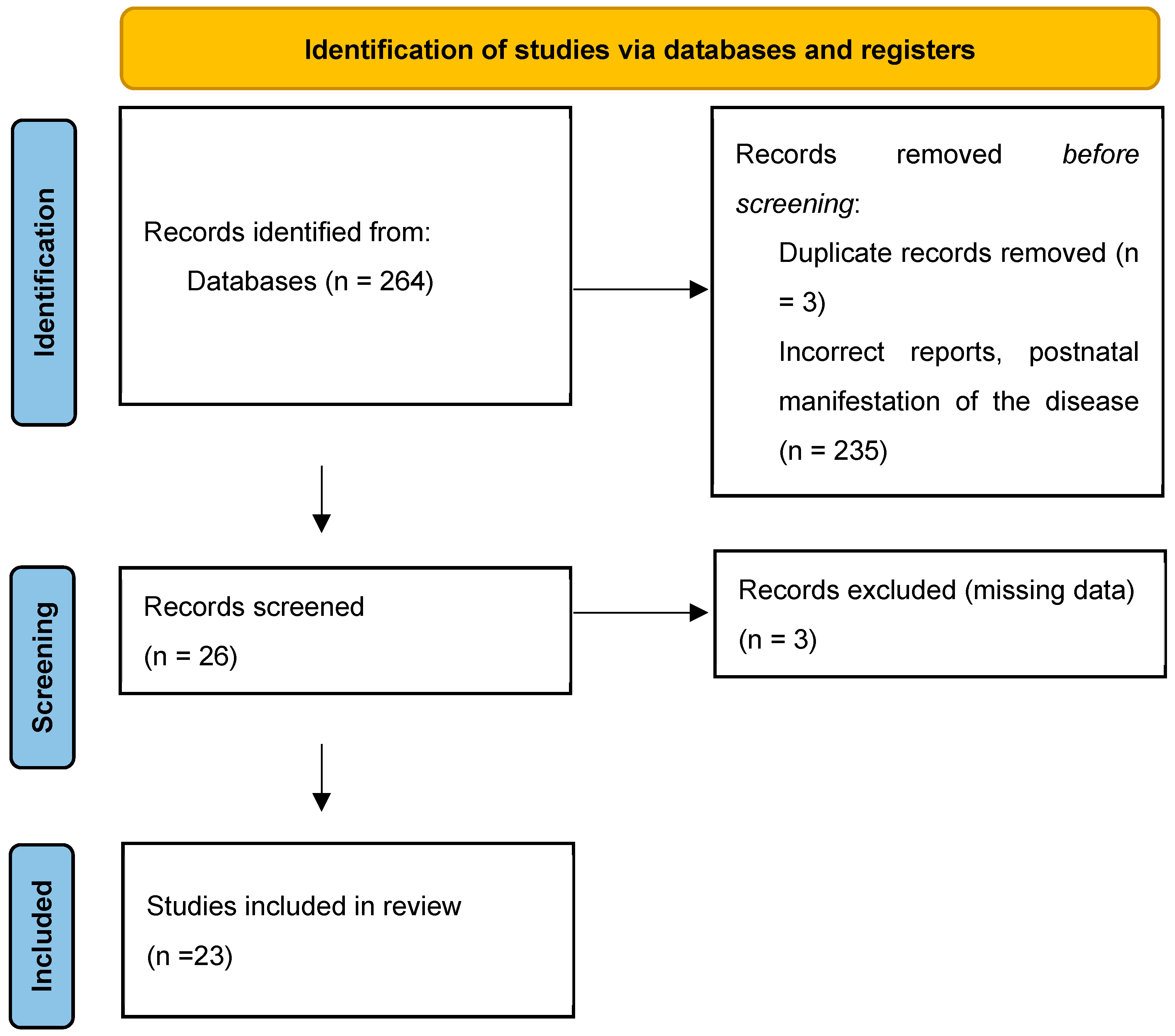
:1. Introduction
2. Materials and Methods
3. Case Presentation
4. Literature Review
5. Discussion
6. Conclusions
Implication for Clinical Practice
- High MCA PSV without infection or immunization can be a symptom of TAM in fetuses presenting hepatosplenomegaly.
- TAM is a possible cause of non-immune hydrops fetalis, fetal anemia, and hepatosplenomegaly.
- This case illustrates the importance of performing an ultrasound examination that includes doppler studies even if the fetus is growing normally in patients presenting with reduced fetal movements.
- Do not assume that TAM occurs only in DS neonates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebire, N.J.; Jauniaux, E. Fetal and placental malignancies: Prenatal diagnosis and management. Ultrasound Obstet. Gynecol. 2009, 33, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Taee, N.; Faraji-Goodarzi, M.; Safdari, M.; Bajelan, A. Transient abnormal myelopoiesis in pediatrics with trisomy 21. Clin. Case Rep. 2020, 9, 605–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zipursky, A. Transient leukaemia—A benign form of leukaemia in newborn infants with trisomy 21. Br. J. Haematol. 2003, 120, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Choudhry, V.P. Childhood myelodysplastic syndrome. Indian J. Pediatr. 2013, 80, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Hasegawa, D.; Hirabayashi, S.; Kamiya, T.; Yoshida, K.; Yonekawa, S.; Ogawa, C.; Hosoya, R.; Toki, T.; Terui, K.; et al. Acute megakaryoblastic leukemia with acquired trisomy 21 and GATA1 mutations in phenotypically normal children. Eur. J. Pediatr. 2015, 174, 525–531. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, H.; Hopman, A.H.; Kraakman, K.C.; de Jong, D. Spontaneous remission in congenital leukemia is not related to (mosaic) trisomy 21: Case presentation and literature review. Pediatr. Hematol. Oncol. 2004, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.; De Jong, G.; Mansvelt, E. Prenatal diagnosis of congenital leukemia in a fetus at 25 weeks’ gestation with Down syndrome: Case report and review of the literature. Ultrasound Obstet. Gynecol. 2003, 21, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Chelghoum, Y.; Vey, N.; Raffoux, E.; Huguet, F.; Pigneux, A.; Witz, B.; Pautas, C.; de Botton, S.; Guyotat, D.; Lioure, B.; et al. Acute leukemia during pregnancy: A report on 37 patients and a review of the literature. Cancer 2005, 104, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Salloum, D.; Stanirowski, P.J.; Symonides, A.; Krajewski, P.; Bomba-Opoń, D.; Wielgoś, M. Enlarged Abdominal Lymph Node as a Cause of Polyhydramnios in the Course of Congenital Neonatal Leukaemia: A Case Report and Review of the Literature on Foetal Abdominal Tumours with Coexisting Polyhydramnios. J. Clin. Med. 2022, 11, 6598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foucar, K.; Friedman, K.; Llewellyn, A.; McConnell, T.; Aisenbrey, G.; Argubright, K.; Ballinger, L. Prenatal diagnosis of transient myeloproliferative disorder via percutaneous umbilical blood sampling. Report of two cases in fetuses affected by Down’s syndrome. Am. J. Clin. Pathol. 1992, 97, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.; Deter, R.L.; Carpenter, R.L.; Rahman, F.; Zimmerman, R.; Moise, K.J., Jr.; Dorman, K.F.; Ludomirsky, A.; Gonzalez, R.; Gomez, R.; et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N. Engl. J. Med. 2000, 342, 9–14. [Google Scholar] [CrossRef]
- Prefumo, F.; Fichera, A.; Fratelli, N.; Sartori, E. Fetal anemia: Diagnosis and management. Best. Pract. Res. Clin. Obstet. Gynaecol. 2019, 58, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Wagner, T.; Malisius, R.; Gembruch, U. Prenatal diagnosis of a transient myeloproliferative disorder in trisomy 21. Prenat Diagn. 1998, 18, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.; Chaoui, R.; Wauer, R.; Bollmann, R. Fetal hepatosplenomegaly: An isolated sonographic sign of trisomy 21 in a case of myeloproliferative disorder. Ultrasound Obstet. Gynecol. 1998, 11, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, S.K.; Sorensen, T.K.; Baker, E.R. Trisomy 21, fetal hydrops, and anemia: Prenatal diagnosis of transient myeloproliferative disorder? Obstet. Gynecol. 1993, 82, 703–705. [Google Scholar] [PubMed]
- Macones, G.A.; Johnson, A.; Tilley, D.; Wade, R.; Wapner, R. Fetal hepatosplenomegaly associated with transient myeloproliferative disorder in trisomy 21. Fetal Diagn. Ther. 1995, 10, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Strobelt, N.; Ghidini, A.; Locatelli, A.; Vergani, P.; Mariani, S.; Biondi, A. Intrauterine diagnosis and management of transient myeloproliferative disorder. Am. J. Perinatol. 1995, 12, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Smoleniec, J. Antenatal diagnosis of transient abnormal myelopoiesis associated with Down syndrome. Aust. N. Z. J. Obstet. Gynaecol. 1999, 39, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, C.; Eguchi, Y.; Kohmura, Y.; Minakami, H.; Sato, I. Isolated pericardial effusion and transient abnormal myelopoiesis in a fetus with Down’s syndrome. J. Obstet. Gynaecol. Res. 2000, 26, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Smrcek, J.M.; Baschat, A.A.; Germer, U.; Gloeckner-Hofmann, K.; Gembruch, U. Fetal hydrops and hepatosplenomegaly in the second half of pregnancy: A sign of myeloproliferative disorder in fetuses with trisomy 21. Ultrasound Obstet. Gynecol. 2001, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Yamada, N.; Watanabe, H.; Okuno, S.; Fujiki, Y.; Kubo, T. Hypoechoic hepatomegaly associated with transient abnormal myelopoiesis provides clues to trisomy 21 in the third-trimester fetus. Ultrasound Obstet. Gynecol. 2001, 17, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; Greco, P.; Gentile, A.; Ingravallo, G.; Loverro, G.; Selvaggi, L. Fetal liver hyperechogenicity on sonography may be a serendipitous sign of a transient myeloproliferating disorder. Prenat. Diagn. 2003, 23, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Azancot, A.; Diehl, R.; Dorgeret, S.; Sebag, G.; Baumann, C.; Vuillard, E.; Machado, L.; Luton, D.; Oury, J.F. Isolated pericardial effusion in the human fetus: A report of three cases. Prenat. Diagn. 2003, 23, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Hosoya, N.; Sato, A.; Tanaka, T. Is the degree of fetal hepatosplenomegaly with transient abnormal myelopoiesis closely related to the postnatal severity of hematological abnormalities in Down syndrome? Ultrasound Obstet. Gynecol. 2004, 24, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Tamura, N.; Ishii, K.; Takakuwa, K.; Matsunaga, M.; Sudo, N.; Tanaka, K. Four cases of fetal hypoechoic hepatomegaly associated with Trisomy 21 and transient abnormal myelopoiesis. Prenat. Diagn. 2007, 27, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Hojo, S.; Tsukimori, K.; Kitade, S.; Nakanami, N.; Hikino, S.; Hara, T.; Wake, N. Prenatal sonographic findings and hematological abnormalities in fetuses with transient abnormal myelopoiesis with Down syndrome. Prenat. Diagn. 2007, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Malin, G.L.; Kilby, M.D.; Velangi, M. Transient abnormal myelopoiesis associated with Down syndrome presenting as severe hydrops fetalis: A case report. Fetal Diagn. Ther. 2010, 27, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Tsai, F.J.; Chern, S.R.; Chang, T.Y.; Hsu, C.Y.; Lin, H.H.; Wang, W. Prenatal diagnosis of 46,XX,DER(13;21)(Q10;Q10),+21 and transient abnormal myelopoiesis in a fetus with hepatosplenomegaly and spontaneous resolution of fetal ascites. Taiwan. J. Obstet. Gynecol. 2009, 48, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Rijhsinghani, A. Elevated delta OD 450 due to transient abnormal myelopoiesis in a Down syndrome fetus with hepatosplenomegaly on ultrasound. Prenat. Diagn. 2014, 34, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Traisrisilp, K.; Charoenkwan, P.; Tongprasert, F.; Srisupundit, K.; Tongsong, T. Hemodynamic assessment of hydrops foetalis secondary to transient myeloproliferative disorder associated with foetal Down syndrome: A case report and literature review. J. Obstet. Gynaecol. 2016, 36, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Dosedla, E.; Turcsanyiova, Z.; Calda, P.; Kolenova, A. Transient Myeloproliferative Syndrome in Newborn without Down Syndrome Phenotype: A Unique Case Report: Actual Gynecology and Obstetrics, Transient Myeloproliferative Syndrome in Newborn without Down Syndrome Phenotype: A Unique Case Report|Actual Gynecology and Obstetrics. Available online: https://www.actualgyn.com/en/article/2019/222 (accessed on 4 February 2024).
- Rizzo, A.; Perotti, G.; Fiandrino, G.; Spinillo, A.; Stronati, M.; Iasci, A. Prenatal diagnosis of transient abnormal myelopoiesis in three fetuses with Down syndrome: Heterogeneous ultrasonographic findings and outcomes. Ultrasound Obstet. Gynecol. 2018, 51, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, J.; Yoshimoto, N.; Ohsawa, A.; Matsuzawa, S.; Katsuragi, S. Fetal Distress and Neonatal Death After Thoracoamniotic Shunting Therapy Due to Hydrops Associated with Transient Abnormal Myelopoiesis. Cureus 2022, 14, e28991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, H.; Hu, J.; Liu, L.; Lv, L.; Lu, J.; Yang, J.; Lu, J.; Chen, Z.; Yang, C.; Chen, D.; et al. Prenatal diagnosis of Down syndrome combined with transient abnormal myelopoiesis in foetuses with a GATA1 gene variant: Two case reports. Mol. Cytogenet. 2023, 16, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heazell, A.E.; Frøen, J.F. Methods of fetal movement counting and the detection of fetal compromise. J. Obstet. Gynaecol. 2008, 28, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.D.; Rayburn, W.F. Antenatal evaluation of the fetus using fetal movement monitoring. Clin. Obstet. Gynecol. 2002, 45, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Frøen, J.F.; Tveit, J.V.; Saastad, E.; Børdahl, P.E.; Stray-Pedersen, B.; Heazell, A.E.; Flenady, V.; Fretts, R.C. Management of decreased fetal movements. Semin. Perinatol. 2008, 32, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kovo, M.; Barda, G.; Gluck, O.; Koren, L.; Bar, J.; Weiner, E. Reduced fetal movements at term, low-risk pregnancies: Is it associated with adverse pregnancy outcomes? Ten years of experience from a single tertiary center. Arch. Gynecol. Obstet. 2020, 301, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sharma, A.; Nallaswamy, V.; Jayagopal, N.; Bhatti, N. Obstetric outcome in women complaining of reduced fetal movements. J. Obstet. Gynaecol. 2007, 27, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Skornick-Rapaport, A.; Maslovitz, S.; Kupferminc, M.; Lessing, J.B.; Many, A. Proposed management for reduced fetal movements: Five years’ experience in one medical center. J. Matern. Fetal Neonatal Med. 2011, 24, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, G.J.; Novikova, N. Management of reported decreased fetal movements for improving pregnancy outcomes. Cochrane Database Syst. Rev. 2012, 4, CD009148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, G.J.; Lee, E.S. Prenatal diagnosis of transient abnormal myelopoiesis in a Down syndrome fetus. Korean J. Radiol. 2009, 10, 190–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamblyn, J.A.; Norton, A.; Spurgeon, L.; Donovan, V.; Bedford Russell, A.; Bonnici, J.; Perkins, K.; Vyas, P.; Roberts, I.; Kilby, M.D. Prenatal therapy in transient abnormal myelopoiesis: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F67–F71. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Nizery, L.; Tunstall, O.; Vyas, P.; Roberts, I. Transient Abnormal Myelopoiesis and AML in Down Syndrome: An Update. Curr. Hematol. Malig. Rep. 2016, 11, 333–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohkawa, T.; Miyamoto, S.; Sugie, M.; Tomizawa, D.; Imai, K.; Nagasawa, M.; Morio, T.; Mizutani, S.; Takagi, M. Transient abnormal myelopoiesis in non-Down syndrome neonate. Pediatr. Int. 2015, 57, e14–e17. [Google Scholar] [CrossRef] [PubMed]
| Author | GA (w) | Hydrops Fetalis | Hepatosplenomegaly | Dopplers | Intrauterine Intervention | Birth (w) | Outcome | Fetal Karyotype | Blast Karyotype | Complete Blood Count |
|---|---|---|---|---|---|---|---|---|---|---|
| Baschat et al., 1998 [13] | 26 | yes | yes | N/A | N/A | 31 | IUD | Trisomy 21 | Trisomy 21 | Anemia, Leukocytosis |
| Hartung et al., 1988 [14] | 31 | no | yes | Increased PI UA Decreased PI MCA | N/A | 32 | Alive | Trisomy 21 | N/A | Leukocythosis |
| Hendricks et al., 1993 [15] | 26 | yes | no | N/A | C blood transfusion | 29 | IUD | Trisomy 21 | N/A | Anemia |
| Hendricks et al., 1993 [15] | 29 | yes | no | N/A | FBS | 31 | IUD | Trisomy 21 | N/A | Leukocytosis Anemia Thrombocytopenia |
| Macones et al., 1995 [16] | 28 | yes | yes | N/A | FBS | 29 | IUD | Trisomy 21 | N/A | Leukocytosis Thrombocytopenia |
| Macones et al., 1995 [16] | 30 | yes | yes | N/A | FBS | 33 | Alive | Trisomy 21 | N/A | Leukocytosis Anemia |
| Strobelt et al., 1995 [17] | 31 | no | yes | N/A | FBS | 35 | Alive | Trisomy 21 | Trisomy 21 | Leukocytosis |
| Siva et al., 1999 [18] | N/A | no | no | N/A | N/A | 38 | Alive | Trisomy 21 | N/A | N/A |
| Hirashima et al., 2000 [19] | 34 | no | no | N/A | N/A | 35 | Alive | Trisomy 21 | N/A | N/A |
| Smercek et al., 2001 [20] | 30 | yes | yes | UA AEDV | FBS | N/A | IUD | Trisomy 21 | Trisomy 21/CD 34+ | Leukocytosis Anemia Thrombocytopenia |
| Smercek et al., 2001 [20] | 26 | yes | yes | High UA PI | FBS | 31 | IUD | Trisomy 21 | Trisomy 21/CD 34 | Leukocytosis Anemia Thrombocytopenia |
| Smercek et al., 2001 [20] | 28 | yes | yes | NA | FBS | N/A | IUD | Mosaic Trisomy 21 | Trisomy 21 | Leukocytosis Anemia Thrombocytopenia |
| Smercek et al., 2001 [20] | 29 | yes | yes | N/A | N/A | 29 | IUD | - | N/A | N/A |
| Hamada et al., 2001 [21] | 35 | no | yes | N/A | N/A | 36 | NND | Trisomy 21 | N/A | N/A |
| Hamada et al., 2001 [21] | 35 | no | yes | N/A | N/A | 38 | NND | Trisomy 21 | Trisomy 21 | N/A |
| Vimercati et al., 2003 [22] | 23 | no | yes | N/A | FBS | N/A | TOP | Trisomy 21 | N/A | normal |
| Azancot et al., 2003 [23] | 31 | no | no | N/A | FBS | 32 | TOP | Trisomy 21 | CD 13, CD 33, CD 34, CD 7 | Leukocytosis Thrombocytopenia |
| Ogawa et al., 2004 [24] | 28 | no | yes | N/A | N/A | 30 | Alive | Trisomy 21 | N/A | N/A |
| Ogawa et al., 2004 [24] | 32 | no | yes | N/A | N/A | 39 | Alive | Trisomy 21 | N/A | N/A |
| Ogawa et al., 2004 [24] | 28 | no | no | N/A | N/A | 37 | Alive | Trisomy 21 | N/A | N/A |
| Kikuchi et al., 2007 [25] | 34 | no | yes | UA AEDV | N/A | 35 | NND | Trisomy 21 | N/A | N/A |
| Kikuchi et al., 2007 [25] | 37 | no | yes | N/A | N/A | 38 | NND | Mosaic Trisomy 21 | N/A | N/A |
| Kikuchi et al., 2007 [25] | 28 | no | yes | N/A | N/A | 40 | Alive | Trisomy 21 | N/A | N/A |
| Kikuchi et al., 2007 [25] | 31 | no | yes | N/A | N/A | 40 | Alive | Trisomy 21 | N/A | N/A |
| Hojo et al., 2007 [26] | 33 | no | no | N/A | FBS | 35 | Alive | Trisomy 21 | N/A | Leukocytosis Thrombocytopenia |
| Hojo et al., 2007 [26] | 28 | yes | no | N/A | FBS | 32 | Alive | Trisomy 21 | N/A | Leukocytosis Thrombocytopenia |
| Hojo et al., 2007 [26] | 33 | yes | no | N/A | FBS | 37 | Alive | Trisomy 21 | N/A | Leukocytosis Thrombocytopenia |
| Hojo et al., 2007 [26] | 28 | yes | yes | N/A | FBS | 30 | IUD | Trisomy 21 | N/A | Leukocytosis Anemia Thrombocytopenia |
| Hojo et al., 2007 [26] | 28 | yes | yes | N/A | FBS | 31 | NND | Trisomy 21 | N/A | Leukocytosis Anemia |
| Hojo et al., 2007 [26] | 37 | yes | yes | N/A | FBS | 37 | NND | Trisomy 21 | N/A | Leukocytosis Anemia Thrombocytopenia |
| Hojo et al., 2007 [26] | 32 | yes | yes | UA AEDV | N/A | 32 | NND | Trisomy 21 | N/A | Leukocytosis Anemia |
| Gwang et al., 2009 [27] | 28 | yes | yes | High MCA PSV | FBS | 29 | IUD | Trisomy 21 | N/A | Leukocytosis Anemia Thrombocytopenia |
| Chen et al., 2009 [28] | 32 | no | yes | N/A | FBS | 36 | IUD | 46,XX,der(13; 21)(q10;q10),+21 (mosaic trisomy) | N/A | Leukocytosis |
| Malin et al., 2010 [27] | 29 | yes | no | MCA PSV high | C blood transfusion | 32 | NND | Trisomy 21 | CD 34 CD 33 CD117 CD 41 CD 42 CD 61 GATA 1 + | Leukocytosis Anemia Thrombocytopenia |
| Mancuso et al., 2014 [29] | 32 | yes | yes | N/A | N/A | 36 | NND | Trisomy 21 | N/A | - |
| Traisrisilp et al., 2016 [30] | 31 | yes | yes | Pulsation UV | FBS | 33 | NND | Trisomy 21 | N/A | Leukocytosis Anemia Thrombocytopenia |
| Dosedla et al., 2019 [31] | 35 | no | yes | N/A | N/A | 35 | Alive | Normal | GATA 1 + | N/A |
| Rizzo et al., 2017 [32] | 32 | no | yes | High MCA PSV | N/A | 34 | NND | Trisomy 21 | GATA 1 + | N/A |
| Rizzo et al., 2017 [32] | 33 | no | yes | incorrect | N/A | 35 | Alive | Trisomy 21 | GATA 1 + | N/A |
| Rizzo et al., 2017 [32] | N/A | no | no | N/A | N/A | 39 | Alive | Trisomy 21 | GATA 1 + | N/A |
| Muraoka et al., 2022 [33] | 30 | yes | yes | High MCA PSV | Thoracocentesis | N/A | NND | Trisomy 21 | N/A | N/A |
| Tang et al., 2023 [34] | 36 | no | yes | N/A | FBS | N/A | IUD | Trisomy 21 | GATA 1 + | Leukocytosis Thrombocytopenia |
| Tang et al., 2023 [34] | 36 | yes | no | N/A | FBS | N/A | TOP | Trisomy 21 | GATA 1 + | Leukocytosis Thrombocytopenia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walasik, I.; Litwińska-Korcz, E.; Szpotańska, M.; Stanirowski, P.; Księżopolska, A.; Ludwin, A.; Litwińska, M. Prenatal Manifestation of Transient Abnormal Myelopoiesis: Case Report and Review of the Literature. J. Clin. Med. 2024, 13, 4584. https://doi.org/10.3390/jcm13164584
Walasik I, Litwińska-Korcz E, Szpotańska M, Stanirowski P, Księżopolska A, Ludwin A, Litwińska M. Prenatal Manifestation of Transient Abnormal Myelopoiesis: Case Report and Review of the Literature. Journal of Clinical Medicine. 2024; 13(16):4584. https://doi.org/10.3390/jcm13164584
Chicago/Turabian StyleWalasik, Izabela, Ewelina Litwińska-Korcz, Monika Szpotańska, Paweł Stanirowski, Aleksandra Księżopolska, Artur Ludwin, and Magdalena Litwińska. 2024. "Prenatal Manifestation of Transient Abnormal Myelopoiesis: Case Report and Review of the Literature" Journal of Clinical Medicine 13, no. 16: 4584. https://doi.org/10.3390/jcm13164584






