Mechanical Circulatory Support with Impella: Principles, Evidence, and Daily Practice
Abstract
:1. Introduction
2. Use in High-Risk PCI
| Name | Year | Setting | N | Intervention vs. Control | Endpoints | Results |
|---|---|---|---|---|---|---|
| Randomized controlled trial | ||||||
| O’Neill et al. [8] | 2012 | Non-emergent HR-PCI | 452 | Impella 2.5 vs. IABP | 30- and 90-day MAE | Non-inferiority |
| Observational registry | ||||||
| Cohen et al. [11] | 2015 | Non-emergent HR-PCI | 555 | Impella 2.5 | In-hospital MAE | Comparable with P-II pts |
| Burzotta et al. [12] | 2019 | Non-emergent HR-PCI | 79 | Impella 2.5/CP | LVEF at 180 days | +26% |
| Baumann et al. [22] | 2019 | Non-emergent HR-PCI | 157 | Impella 2.5/CP | 6-month MACE | 22.8% |
| Chieffo et al. [15] | 2020 | Non-emergent HR-PCI | 177 | Impella 2.5/CP | 1-year all-cause death | 15.6% |
| Wollmuth et al. [13] | 2022 | Non-emergent HR-PCI | 251 | Impella 2.5/CP | LVEF at 90 days | +29% |
| Matched/adjusted analysis | ||||||
| Kovacic et al. [9] | 2015 | Non-emergent HR-PCI | 325 | Impella 2.5 vs. IABP | 90-day MAE | Impella was better |
| Azzalini et al. [18] | 2020 | Non-emergent HR-PCI | 474 | Impella 2.5/CP vs. no MCS | 1 year MACE | No differences |
| Lansky et al. [19] | 2022 | Non-emergent HR-PCI | 2156 | Impella 2.5/CP vs. IABP | In-hospital mortality | Impella was better |
| Van den Buijs et al. [20] | 2022 | Non-emergent HR-PCI | 41 | Impella CP vs. VA-ECMO | 30-day mortality | No differences |
| Panoulas et al. [14] | 2024 | Non-emergent HR-PCI | 344 | Impella 2.5/CP vs. IABP | LVEF at 90 days | Impella was better |
3. Use in CS
| Name | Year | Setting | N | Intervention vs. Control | Endpoints | Results |
|---|---|---|---|---|---|---|
| Randomized controlled trial | ||||||
| ISAR-Shock [25] | 2008 | CS | 26 | Impella LP2.5 vs. IABP | Change in CI after 30 min | Impella was better |
| IMPRESS [26] | 2017 | CS-AMI (STEMI) | 48 | Impella vs. IABP | 30-day all-cause mortality | No differences |
| IMPELLA-STIC [27] | 2020 | CS-AMI (STEMI) | 12 | Impella LP 5.0 vs. IABP | Change in CPI after 12 h | No differences |
| DANGER SHOCK [28] | 2024 | CS-AMI (STEMI) | 360 | Impella CP vs. standard of care | 180-day all-cause mortality | Impella was better |
| Retrospective matched/adjusted analysis | ||||||
| IABP-SHOCK II [35] | 2019 | CS-AMI (N/STEMI) | 474 | Impella vs. entire cohort of IABP-SHOCK II | 30-day all-cause mortality | No differences |
| IABP-SHOCK II [35] | 2019 | CS-AMI (N/STEMI) | 230 | Impella vs. IABP cohort of IABP-SHOCK II | 30-day all-cause mortality | No differences |
| Karami et al. [40] | 2020 | CS | 128 | Impella CP/5.0 vs. ECMO | 30-day all-cause mortality | No differences |
| Schrage et al. [33] | 2020 | ECLS-treated CS | 510 | VA ECMO and Impella vs. VA ECMO | 30-day all-cause mortality | Impella was better |
| Dhruva et al. [36] | 2020 | CS-AMI (N/STEMI) | 3360 | Impella vs. IABP | In-hospital all-cause death | pLVAD was worse |
| Scherer et al. [39] | 2020 | CS | 140 | Impella CP vs. no ELCS | 1-year and 5-year all-cause mortality | No differences |
| Wernly et al. [37] | 2021 | CS | 149 | Impella 2.5 vs. ECLS | 30-day all-cause mortality | No differences |
| Sieweke et al. [38] | 2021 | rCS after OHCA | 30 | Impella vs. standard of care | 30-day all-cause mortality | Impella was better |
4. Adverse Events
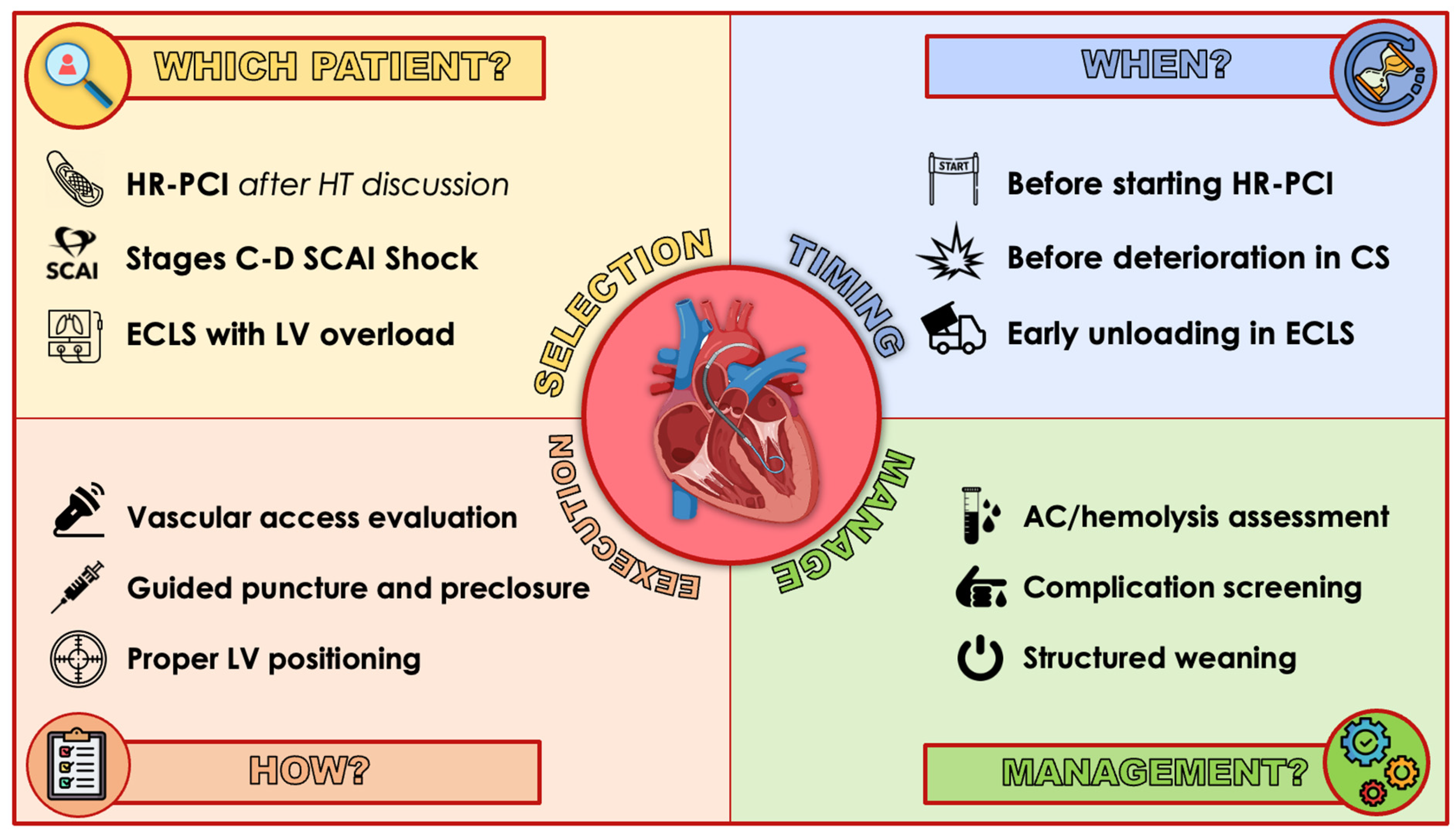
5. Best Practice
5.1. Patient and Device Selection
5.2. Timing
5.3. Access
5.4. Device Positioning
5.5. Anticoagulation
5.6. Bleeding Management
5.7. Daily Monitoring
5.8. Weaning
6. Gaps in Knowledge
7. Conclusions
Funding
Conflicts of Interest
Abbreviations and Acronyms (Alphabetical Order)
| AKI | acute kidney injury |
| BCIS-JS | British Cardiovascular Intervention Society Myocardial Jeopardy Score |
| CAD | coronary artery disease |
| CHIP | complex high-risk indicated procedure |
| CS | cardiogenic shock |
| ECMO | extracorporeal veno-arterial membrane oxygenation |
| EF | ejection fraction |
| HR-PCI | high-risk PCI |
| IABP | intra-aortic balloon pump |
| LV | left ventricle |
| LVEDP | left ventricle end-diastolic pressure |
| MAE | major adverse event |
| MACE | major cardiovascular event |
| MACCEs | major adverse cardiac and cerebrovascular events |
| MCS | mechanical circulatory support |
| PCI | percutaneous coronary intervention |
| PCWP | pulmonary capillary wedge pressure |
| pLVAD | percutaneous left ventricular assist device |
| RCT | randomized controlled trial |
| SYNTAX | Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery |
References
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Kellnar, A.; Krieg, K.; Binzenhöfer, L.; Scherer, C.; Zimmer, S.; Schrage, B.; Fichtner, S.; Petzold, T.; Braun, D.; et al. Percutaneous Transvalvular Microaxial Flow Pump Support in Cardiology. Circulation 2022, 145, 1254–1284. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, M.; Johnson, A.; Kabir, R.; Bhogal, S.; Wermers, J.P.; Bernardo, N.L.; Ben-Dor, I.; Hashim, H.; Satler, L.F.; Sheikh, F.H.; et al. Randomized Trials of Percutaneous Microaxial Flow Pump Devices. J. Am. Coll. Cardiol. 2022, 80, 2028–2049. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Dudek, D.; Hassager, C.; Combes, A.; Gramegna, M.; Halvorsen, S.; Huber, K.; Kunadian, V.; Maly, J.; Møller, J.E.; et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. EuroIntervention 2021, 17, e274–e286. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Burzotta, F.; Pappalardo, F.; Briguori, C.; Garbo, R.; Masiero, G.; Nicolini, E.; Ribichini, F.; Trani, C.; Álvarez, B.C.; et al. Clinical expert consensus document on the use of percutaneous left ventricular assist support devices during complex high-risk indicated PCI. Int. J. Cardiol. 2019, 293, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.W.; Anderson, M.; Schreiber, T.; Popma, J.; Lombardi, W.; Maini, B.; Moller, J.E.; Schäfer, A.; Dixon, S.R.; Hall, S.; et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am. Heart J. 2018, 199, 115–121. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Džavík, V.; et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Kini, A.; Banerjee, S.; Dangas, G.; Massaro, J.; Mehran, R.; Popma, J.; O’Neill, W.W.; Sharma, S.K. Patients with 3-Vessel Coronary Artery Disease and Impaired Ventricular Function Undergoing PCI with Impella 2.5 Hemodynamic Support Have Improved 90-Day Outcomes Compared to Intra-Aortic Balloon Pump: A Sub-Study of The PROTECT II Trial. J. Interv. Cardiol. 2015, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Kini, A.S.; Sharma, S.K.; Henriques, J.P.; Claessen, B.E.; Dixon, S.R.; Massaro, J.M.; Palacios, I.; Popma, J.J.; Ohman, E.M.; et al. Impact of Hemodynamic Support With Impella 2.5 Versus Intra-Aortic Balloon Pump on Prognostically Important Clinical Outcomes in Patients Undergoing High-Risk Percutaneous Coronary Intervention (from the PROTECT II Randomized Trial). Am. J. Cardiol. 2013, 113, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.G.; Matthews, R.; Maini, B.; Dixon, S.; Vetrovec, G.; Wohns, D.; Palacios, I.; Popma, J.; Ohman, E.M.; Schreiber, T.; et al. Percutaneous left ventricular assist device for high-risk percutaneous coronary interventions: Real-world versus clinical trial experience. Am. Heart J. 2015, 170, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Russo, G.; Ribichini, F.; Piccoli, A.; D’amario, D.; Paraggio, L.; Previ, L.; Pesarini, G.; Porto, I.; Leone, A.M.; et al. Long-Term Outcomes of Extent of Revascularization in Complex High Risk and Indicated Patients Undergoing Impella-Protected Percutaneous Coronary Intervention: Report from the Roma-Verona Registry. J. Interv. Cardiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wollmuth, J.; Patel, M.P.; Dahle, T.; Bharadwaj, A.; Waggoner, T.E.; Chambers, J.W.; Ruiz-Rodriguez, E.; Mahmud, E.; Thompson, C.; Morris, D.L. Ejection Fraction Improvement Following Contemporary High-Risk Percutaneous Coronary Intervention: RESTORE EF Study Results. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100350. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Escaned, J.; Hill, J.M.; Barker, E.; Butler, K.; Almedhychy, A.; Tsintzos, S.I.; O’neill, W.W. Predictors of left ventricular ejection fraction in high-risk percutaneous coronary interventions. Front. Cardiovasc. Med. 2024, 11, 1342409. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Ancona, M.B.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly: The IMP-IT registry. EuroIntervention 2020, 15, E1343. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Masiero, G.; Burzotta, F.; Pazzanese, V.; Briguori, C.; Trani, C.; Piva, T.; De Marco, F.; Di Biasi, M.; Pagnotta, P.; et al. Timing of Impella implantation and outcomes in cardiogenic shock or high-risk percutaneous coronary revascularization. Catheter. Cardiovasc. Interv. 2021, 98, E222–E234. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.P.; Moses, J.W.; Westenfeld, R.; Palacios, I.; O’Neill, W.W.; Schreiber, T.L.; Lim, M.J.; Kaki, A.; Ghiu, I.; Mehran, R. Impella support and acute kidney injury during high-risk percutaneous coronary intervention: The Global cVAD Renal Protection Study. Catheter. Cardiovasc. Interv. 2019, 95, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Azzalini, L.; Johal, G.S.; Baber, U.; Bander, J.; Moreno, P.R.; Bazi, L.; Kapur, V.; Barman, N.; Kini, A.S.; Sharma, S.K. Outcomes of Impella-supported high-risk nonemergent percutaneous coronary intervention in a large single-center registry. Catheter. Cardiovasc. Interv. 2021, 97, E26–E33. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Tirziu, D.; Moses, J.W.; Pietras, C.; Ohman, E.M.; O’Neill, W.W.; Ekono, M.M.; Grines, C.L.; Parise, H. Impella Versus Intra-Aortic Balloon Pump for High-Risk PCI: A Propensity-Adjusted Large-Scale Claims Dataset Analysis. Am. J. Cardiol. 2022, 185, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Buijs, D.M.v.D.; Wilgenhof, A.; Knaapen, P.; Zivelonghi, C.; Meijers, T.; Vermeersch, P.; Arslan, F.; Verouden, N.; Nap, A.; Sjauw, K.; et al. Prophylactic Impella CP versus VA-ECMO in Patients Undergoing Complex High-Risk Indicated PCI. J. Interv. Cardiol. 2022, 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.; Horn, P.; Voss, F.; Kivel, M.; Wolff, G.; Jung, C.; Zeus, T.; Kelm, M.; Westenfeld, R. Incidence of Acute Kidney Injury Is Lower in High-Risk Patients Undergoing Percutaneous Coronary Intervention Supported with Impella Compared to ECMO. J. Cardiovasc. Transl. Res. 2021, 15, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Werner, N.; Al-Rashid, F.; Schäfer, A.; Bauer, T.; Sotoudeh, R.; Bojara, W.; Shamekhi, J.; Sinning, J.-M.; Becher, T.; et al. Six months follow-up of protected high-risk percutaneous coronary intervention with the microaxial Impella pump: Results from the German Impella registry. Coron. Artery Dis. 2020, 31, 237–242. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Wayangankar, S.A.; Bangalore, S.; McCoy, L.A.; Jneid, H.; Latif, F.; Karrowni, W.; Charitakis, K.; Feldman, D.N.; Dakik, H.A.; Mauri, L.; et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2016, 9, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; Eriksen, E.; Ouweneel, D.M.; E Claessen, B.; Vis, M.M.; Baan, J.; Beijk, M.; Packer, E.J.S.; Sjauw, K.D.; Engstrom, A.; et al. Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: Percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bochaton, T.; Huot, L.; Elbaz, M.; Delmas, C.; Aissaoui, N.; Farhat, F.; Mewton, N.; Bonnefoy, E. Mechanical circulatory support with the Impella® LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: The IMPELLA-STIC randomized study. Arch. Cardiovasc. Dis. 2019, 113, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Masiero, G.; Cardaioli, F.; Tarantini, G. Mechanical circulatory support in cardiogenic shock: A critical appraisal. Expert Rev. Cardiovasc. Ther. 2022, 20, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Westenfeld, R.; Sieweke, J.-T.; Zietzer, A.; Wiora, J.; Masiero, G.; Martinez, C.S.; Tarantini, G.; Werner, N. Complete Revascularisation in Impella-Supported Infarct-Related Cardiogenic Shock Patients Is Associated With Improved Mortality. Front. Cardiovasc. Med. 2021, 8, 678748. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Deseda, G.C.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Lemor, A.; Dehkordi, S.H.H.; Basir, M.B.; Villablanca, P.A.; Jain, T.; Koenig, G.C.; Alaswad, K.; Moses, J.W.; Kapur, N.K.; O’Neill, W. Impella Versus Extracorporeal Membrane Oxygenation for Acute Myocardial Infarction Cardiogenic Shock. Cardiovasc. Revascularization Med. 2020, 21, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.-M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Wernly, B.; Karami, M.; Engström, A.E.; Windecker, S.; Hunziker, L.; Lüscher, T.F.; Henriques, J.P.; Ferrari, M.W.; Binnebößel, S.; Masyuk, M.; et al. Impella versus extracorporal life support in cardiogenic shock: A propensity score adjusted analysis. ESC Heart Fail. 2021, 8, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Sieweke, J.-T.; Akin, M.; Beheshty, J.-A.; Flierl, U.; Bauersachs, J.; Schäfer, A. Unloading in Refractory Cardiogenic Shock After Out-Of-Hospital Cardiac Arrest Due to Acute Myocardial Infarction—A Propensity Score-Matched Analysis. Front. Cardiovasc. Med. 2021, 8, 704312. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Lüsebrink, E.; Kupka, D.; Stocker, T.J.; Stark, K.; Stremmel, C.; Orban, M.; Petzold, T.; Germayer, A.; Mauthe, K.; et al. Long-term clinical outcome of cardiogenic shock patients undergoing impella CP treatment vs. standard of care. J. Clin. Med. 2020, 9, 3803. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; den Uil, C.A.; Ouweneel, D.M.; Scholte, N.T.; Engström, A.E.; Akin, S.; Lagrand, W.K.; Vlaar, A.P.; Jewbali, L.S.; Henriques, J.P. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Redfors, B.; Watson, B.M.; McAndrew, T.; Palisaitis, E.; Francese, D.P.; Razavi, M.; Safirstein, J.; Mehran, R.; Kirtane, A.J.; Généreux, P. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiol. 2017, 2, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Ancona, M.B.; Montorfano, M.; Masiero, G.; Burzotta, F.; Briguori, C.; Pagnesi, M.; Pazzanese, V.; Trani, C.; Piva, T.; De Marco, F.; et al. Device-related complications after Impella mechanical circulatory support implantation: An IMP-IT observational multicentre registry substudy. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, W.W.; Anderson, M.; Burkhoff, D.; Grines, C.L.; Kapur, N.K.; Lansky, A.J.; Mannino, S.; McCabe, J.M.; Alaswad, K.; Daggubati, R.; et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am. Heart J. 2022, 248, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Leick, J.; Werner, N.; Mangner, N.; Panoulas, V.; Aurigemma, C. Optimized patient selection in high-risk protected percutaneous coronary intervention. Eur. Heart J. Suppl. 2022, 24, J4–J10. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, A.; Gąsecka, A.; Jasińska-Gniadzik, K.; Szwed, P.; Grygier, M.; Pawłowski, T.; Sacha, J.; Kochman, J. Roadmap towards an institutional Impella programme for high-risk coronary interventions. ESC Heart Fail. 2023, 10, 2200–2213. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Basir, M.B.; Schreiber, T.L.; Grines, C.L.; Dixon, S.R.; Moses, J.W.; Maini, B.S.; Khandelwal, A.K.; Ohman, E.M.; O’Neill, W.W. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am. J. Cardiol. 2017, 119, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Leon, S.A.; Rosen, J.L.; Ahmad, D.; Austin, M.A.; Vishnevsky, A.; Rajapreyar, I.N.; Ruggiero, N.J.; Rame, J.E.; Entwistle, J.W.; Massey, H.T.; et al. Microaxial circulatory support for percutaneous coronary intervention: A systematic review and meta-analysis. Artif. Organs 2023, 47, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Sardone, A.; Franchin, L.; Moniaci, D.; Colangelo, S.; Colombo, F.; Boccuzzi, G.; Iannaccone, M. Management of Vascular Access in the Setting of Percutaneous Mechanical Circulatory Support (pMCS): Sheaths, Vascular Access and Closure Systems. J. Pers. Med. 2023, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Karatolios, K.; Hunziker, P.; Schibilsky, D. Managing vascular access and closure for percutaneous mechanical circulatory support. Eur. Heart J. Suppl. 2021, 23, A10–A14. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Lefèvre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral Aortic Valve Implantation. JACC Cardiovasc. Interv. 2011, 4, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Burke, M.N.; Lobo, A.S.; Lips, D.L.; Seto, A.H.; Chavez, I.; Sorajja, P.; Abu-Fadel, M.S.; Wang, Y.; Poulouse, A.; et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nakamaru, R.; Kohsaka, S.; Fukutomi, M.; Onishi, T.; Tobaru, T. Access Site–Stratified Analysis of the Incidence, Predictors, and Outcomes of Impella-Supported Patients With Cardiogenic Shock. Am. J. Cardiol. 2023, 205, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Hartung, P.; Dumpies, O.; Obradovic, D.; Wilde, J.; Majunke, N.; Boekstegers, P.; Müller, R.; Seyfarth, M.; Vorpahl, M.; et al. Comparison of a Pure Plug-Based Versus a Primary Suture-Based Vascular Closure Device Strategy for Transfemoral Transcatheter Aortic Valve Replacement: The CHOICE-CLOSURE Randomized Clinical Trial. Circulation 2022, 145, 170–183. [Google Scholar] [CrossRef] [PubMed]
- van Wiechen, M.P.; Tchétché, D.; Ooms, J.F.; Hokken, T.W.; Kroon, H.; Ziviello, F.; Ghattas, A.; Siddiqui, S.; Laperche, C.; Spitzer, E.; et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure. JACC Cardiovasc. Interv. 2020, 14, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Verreault-Julien, L.; Shekiladze, N.; Wollmuth, J.; Rinfret, S. Single-access for Impella-supported percutaneous coronary intervention using a sheathless technique with an 8 Fr guide. Catheter. Cardiovasc. Interv. 2022, 100, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.; Chandrasekaran, U.; Das, S.; Qi, Z.; Corbett, S. Hemolysis associated with Impella heart pump positioning: In vitro hemolysis testing and computational fluid dynamics modeling. Int. J. Artif. Organs 2020, 43, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Baldetti, L.; Beneduce, A.; Romagnolo, D.; Frias, A.; Gramegna, M.; Sacchi, S.; Calvo, F.; Pazzanese, V.; Cappelletti, A.M.; Ajello, S.; et al. Impella Malrotation Within the Left Ventricle Is Associated With Adverse In-Hospital Outcomes in Cardiogenic Shock. JACC Cardiovasc. Interv. 2023, 16, 739–741. [Google Scholar] [CrossRef]
- Balthazar, T.; Vandenbriele, C.; Verbrugge, F.H.; Uil, C.D.; Engström, A.; Janssens, S.; Rex, S.; Meyns, B.; Van Mieghem, N.; Price, S.; et al. Managing Patients With Short-Term Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2021, 77, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Van Edom, C.J.; Gramegna, M.; Baldetti, L.; Beneduce, A.; Castelein, T.; Dauwe, D.; Frederiks, P.; Giustino, G.; Jacquemin, M.; Janssens, S.P.; et al. Management of Bleeding and Hemolysis During Percutaneous Microaxial Flow Pump Support. JACC Cardiovasc. Interv. 2023, 16, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Morine, K.J.; Annamalai, S.K.; O’kelly, R.; Aghili, N.; Pedicini, R.; Breton, C.; Mullin, A.; Hamadeh, A.; Kiernan, M.S.; et al. Increased Plasma-Free Hemoglobin Levels Identify Hemolysis in Patients With Cardiogenic Shock and a Trans valvular Micro-Axial Flow Pump. Artif. Organs 2018, 43, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Mojoli, M.; Barioli, A.; Battistel, M.; Généreux, P. Blood oozing: A cause of life-threatening bleeding without overt source after transcatheter aortic valve replacement. Int. J. Cardiol. 2016, 224, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Antonides, C.F.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: A consensus statement of the mechanical circulatory support academic research consortium. J. Heart Lung Transplant. 2020, 39, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, M.; Galluzzo, A.; Rossello, X.; Sbarra, P.; Petitti, E.; Prever, S.B.; Boccuzzi, G.; D’Ascenzo, F.; Frea, S.; Pidello, S.; et al. Prognostic implications of pulmonary artery catheter monitoring in patients with cardiogenic shock: A systematic review and meta-analysis of observational studies. J. Crit. Care 2022, 69, 154024. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.M.; Ohman, E.M.; O’neill, W.W.; Rab, T.; Cigarroa, J.E. A Practical Approach to Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2016, 9, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Matassini, M.V.; Marini, M.; Angelozzi, A.; Angelini, L.; Shkoza, M.; Compagnucci, P.; Falanga, U.; Battistoni, I.; Pongetti, G.; Francioni, M.; et al. Clinical outcomes and predictors of success with Impella weaning in cardiogenic shock: A single-center experience. Front. Cardiovasc. Med. 2023, 10, 1171956. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kim, R.J.; Moses, J.W.; Stone, G.W.; Udelson, J.E.; Ben-Yehuda, O.; Redfors, B.; Issever, M.O.; Josephy, N.; Polak, S.J.; et al. Primary left ventricular unloading with delayed reperfusion in patients with anterior ST-elevation myocardial infarction: Rationale and design of the STEMI-DTU randomized pivotal trial. Am. Heart J. 2022, 254, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.; Laine, M.; Schurtz, G.; Roubille, F.; Coste, P.; Leurent, G.; Hraiech, S.; Pankert, M.; Gonzalo, Q.; Dabry, T.; et al. Rationale and design of the ULYSS trial: A randomized multicenter evaluation of the efficacy of early Impella CP implantation in acute coronary syndrome complicated by cardiogenic shock. Am. Heart J. 2023, 265, 203–212. [Google Scholar] [CrossRef] [PubMed]
- The RECOVER IV Trial-Full Text View-ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05506449 (accessed on 11 May 2024).
- The PROTECT-EU Study-Full Text View-ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05466552 (accessed on 11 May 2024).
- Study Details|Impella®-Supported PCI in High-Risk Patients with Complex Coronary Artery Disease and Reduced Left Ventricular Function|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04763200 (accessed on 11 May 2024).
- Tarantini, G.; Masiero, G.; Thiele, H.; Iannaccone, M.; Schrage, B.; Hassager, C.; Woitek, F.; Chieffo, A.; Møller, J.E. Timing and treatment strategies according to SCAI classification in cardiogenic shock. Eur. Heart J. Suppl. 2023, 25, I19–I23. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Protect Kidney Trial|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04321148 (accessed on 11 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiero, G.; Arturi, F.; Panza, A.; Tarantini, G. Mechanical Circulatory Support with Impella: Principles, Evidence, and Daily Practice. J. Clin. Med. 2024, 13, 4586. https://doi.org/10.3390/jcm13164586
Masiero G, Arturi F, Panza A, Tarantini G. Mechanical Circulatory Support with Impella: Principles, Evidence, and Daily Practice. Journal of Clinical Medicine. 2024; 13(16):4586. https://doi.org/10.3390/jcm13164586
Chicago/Turabian StyleMasiero, Giulia, Federico Arturi, Andrea Panza, and Giuseppe Tarantini. 2024. "Mechanical Circulatory Support with Impella: Principles, Evidence, and Daily Practice" Journal of Clinical Medicine 13, no. 16: 4586. https://doi.org/10.3390/jcm13164586






