The New Frontiers of Fetal Imaging: MRI Insights into Cardiovascular and Thoracic Structures
Abstract
1. Introduction
2. Chest MRI
2.1. Imaging Acquisition
- T1-weighted 3D GRE: T1 contrast optimization at 3 T involves adjusting repetition time (TR), echo time (TE), and flip angle, with fat-saturated T1 3D GRE Dixon sequences used. Parallel imaging combined with T1-weighted 3D GRE sequences allows acquisitions during breath-holding to minimize fetal motion artifacts.
- Single-shot fast spin-echo (SS-FSE): A preferred technique for fetal imaging is the free-breathing T2-weighted SS-FSE sequence, despite susceptibility to fetal motion artifacts. Dielectric artifact, influenced by body region and patient physiology, is a limitation at 3 T. The artifact worsens with larger maternal diameters and amniotic fluid volumes. Mitigation techniques include saturation bands, increased flip angle, and prescan-B1 filter application. However, these may increase radiofrequency power deposition and require longer TR. Adjusting fetal positioning relative to the body coil can optimize artifact reduction.
- Balanced steady-state-free precession (bSSFP): bSSFP sequences offer a high signal-to-noise ratio and T2/T1 image contrast, beneficial for heart and vessel evaluation due to bright-blood signal. Adjusting offset frequency mitigates banding artifacts, altering image contrasts. Real-time bSSFP imaging captures cardiac motion, ideal for uncooperative patients, achieving temporal resolution of 1.5 phases [5].
2.2. Clinical Application
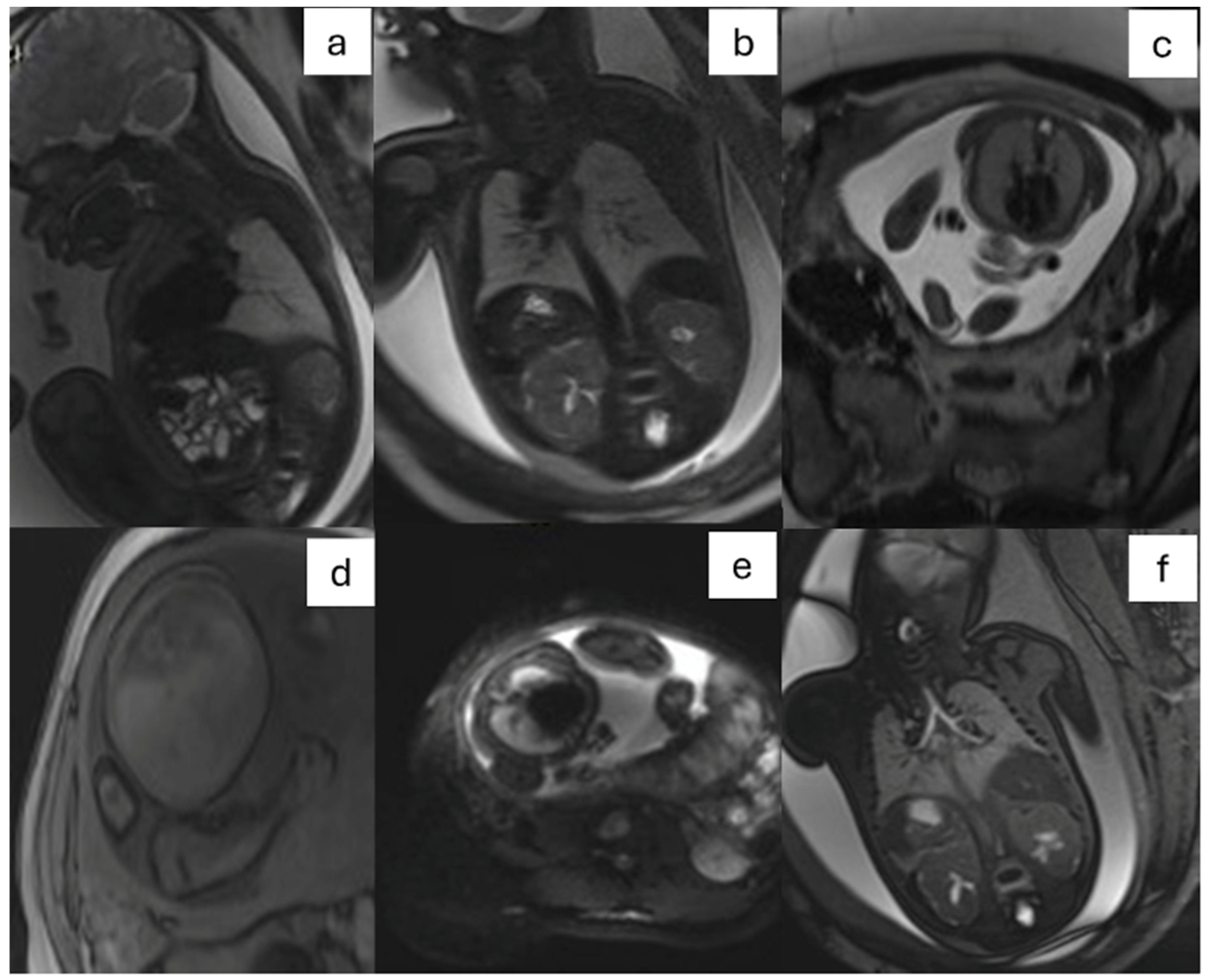
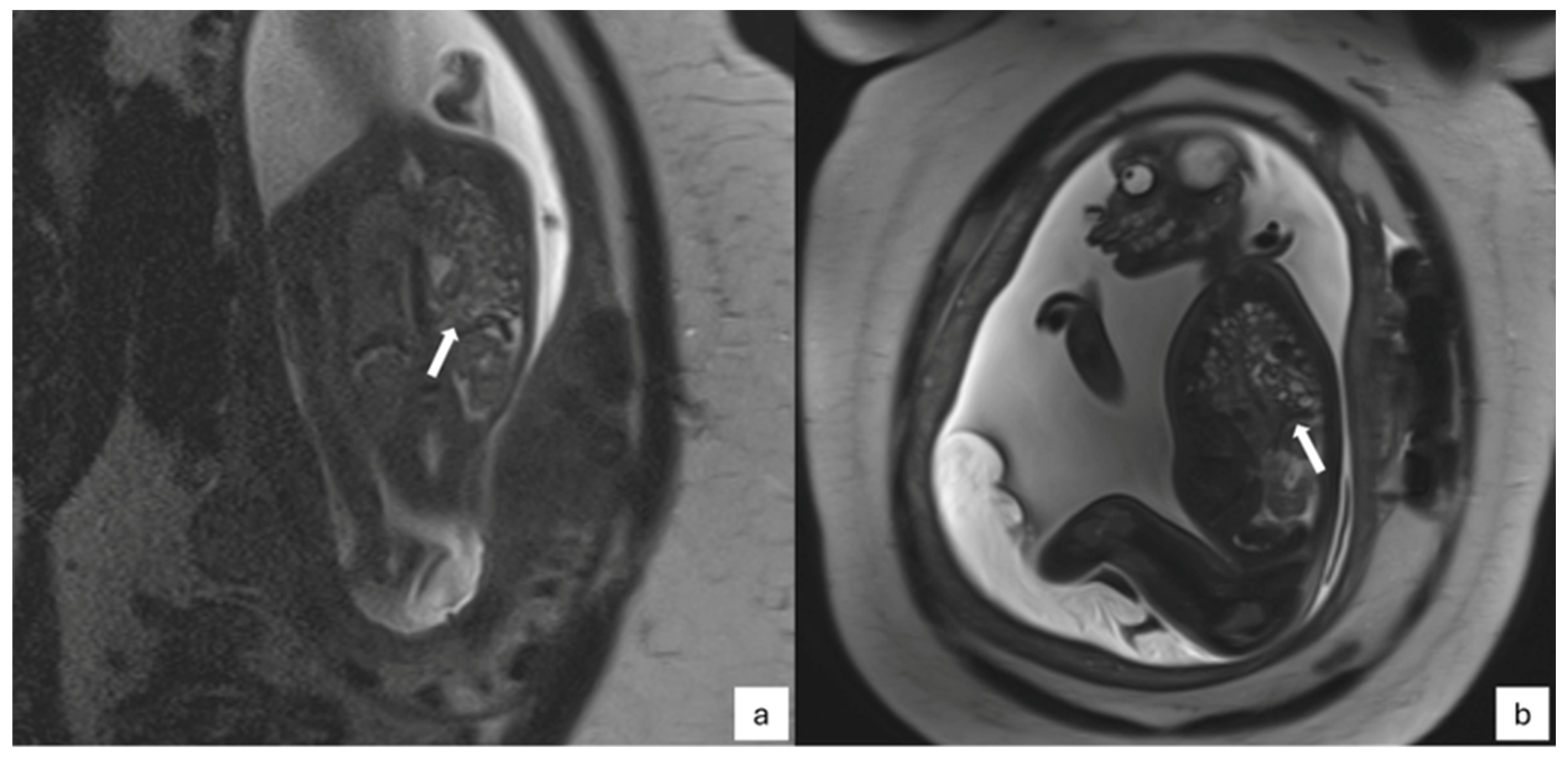
- CPAM: This is the most frequent malformation, consisting of communication with the normal tracheobronchial tree. They can present in solid or cystic form and are classified into 5 subcategories (Figure 2).
- Bronchopulmonary sequestration (BPS): It is the second most frequent cause of lung disease, characterized by the presence of lung parenchyma not communicating with the tracheobronchial tree. BPS is classified into two categories, extra-lobar or intra-lobar, depending on the presence of an independent pleura.
- Congenital lobar overinflation (CLO): It represents hyperinflation of a lung segment or lobe caused by bronchial obstruction.
- Congenital bronchogenic cyst: A fluid-containing lesion with thin walls often located near the carina.
- Congenital upper airway obstruction syndrome (CHAOS): It is caused by obstruction of the larynx or trachea from an extrinsic or intrinsic cause, resulting in fluid entrapment in the lungs and dilatation of the trachea.
- Bronchial atresia: This malformation results in secondary changes to the distal lung parenchyma that appears homogeneously hyperintense in T2-weighted sequences. It is subcategorized into two groups, proximal type and peripheral type.
- CDH: It is defined as a herniation of abdominal organs in the thorax through an orifice of the diaphragm caused by delayed or abnormal separation of the thoracic and abdominal compartments (Figure 3).
3. Cardiac Magnetic Resonance Imaging
3.1. Fetal Cardiac Gating
3.2. Image Acquisition
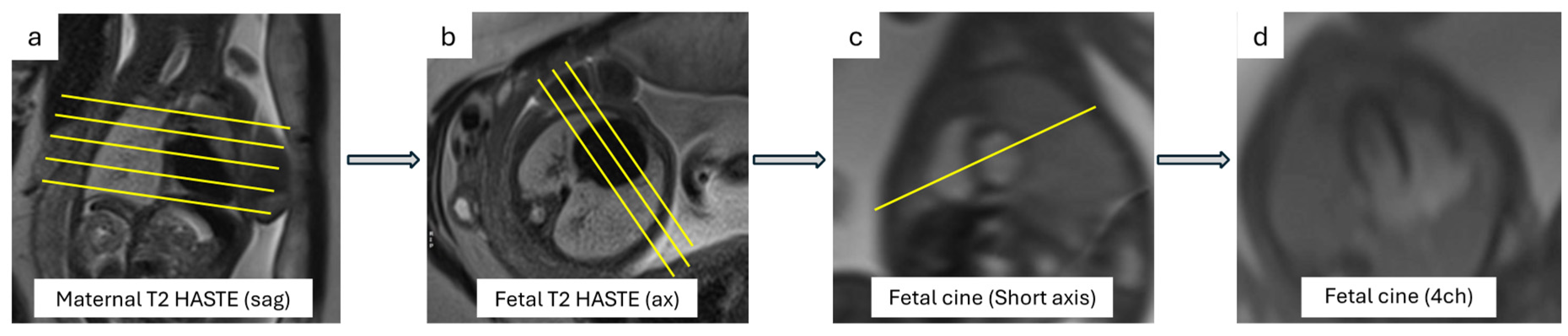
3.2.1. Cardiac Planes
3.2.2. Cine Images
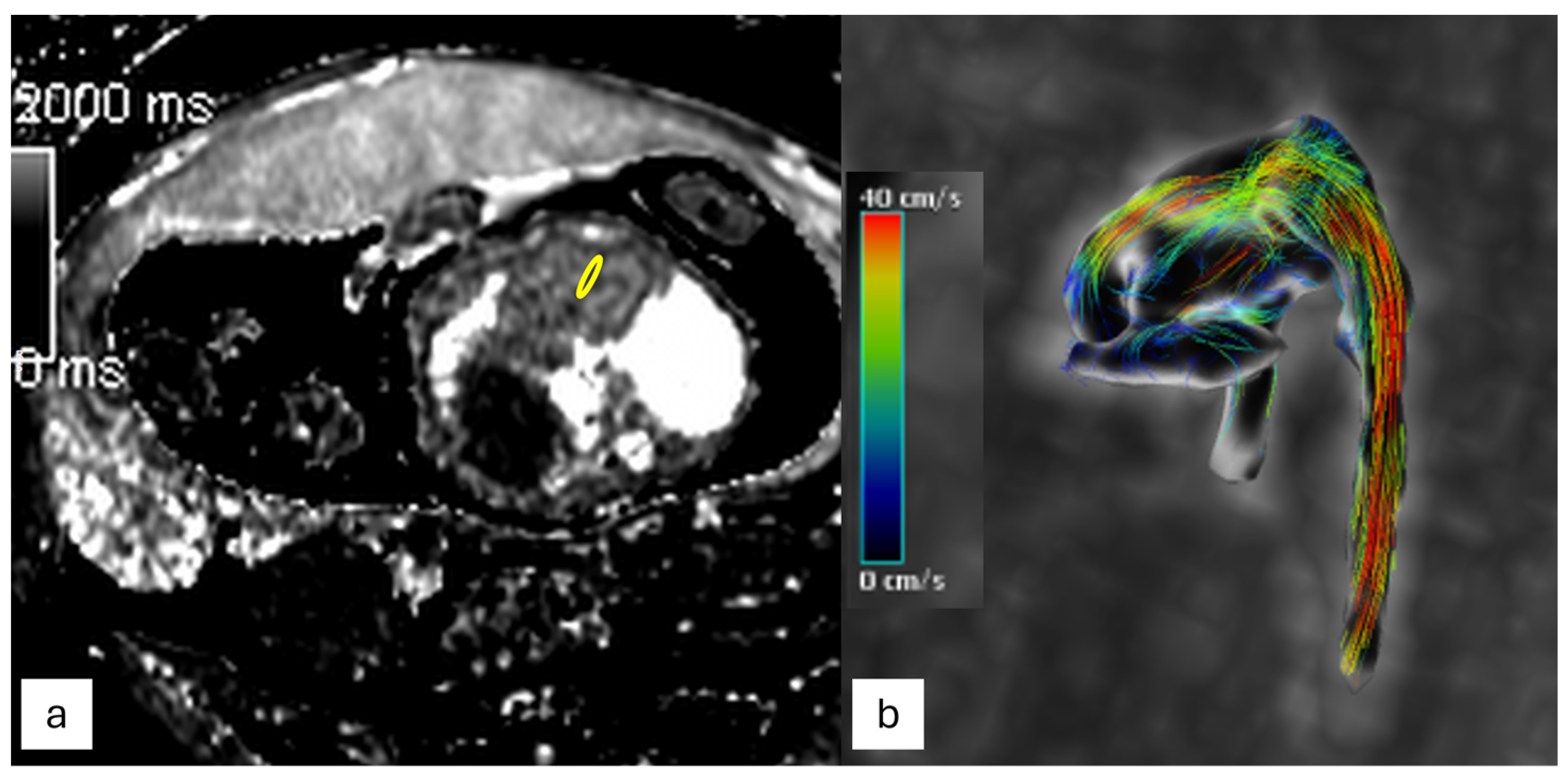
3.2.3. Flow Imaging
3.2.4. T1 and T2 Mapping
3.3. Clinical Applications
4. Conclusions
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Wilde, J.P.; Rivers, A.W.; Price, D.L. A Review of the Current Use of Magnetic Resonance Imaging in Pregnancy and Safety Implications for the Fetus. Prog. Biophys. Mol. Biol. 2005, 87, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, G.; Capuani, S.; Antonelli, A.; Camilli, A.; Ciulla, S.; Petrillo, R.; Satta, S.; Grimm, R.; Giancotti, A.; Ricci, P.; et al. IntraVoxel Incoherent Motion (IVIM) MRI of Fetal Lung and Kidney: Can the Perfusion Fraction Be a Marker of Normal Pulmonary and Renal Maturation? Eur. J. Radiol. 2021, 139, 109726. [Google Scholar] [CrossRef] [PubMed]
- Capuani, S.; Guerreri, M.; Antonelli, A.; Bernardo, S.; Porpora, M.G.; Giancotti, A.; Catalano, C.; Manganaro, L. Diffusion and Perfusion Quantified by Magnetic Resonance Imaging Are Markers of Human Placenta Development in Normal Pregnancy. Placenta 2017, 58, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Capuani, S.; Ercolani, G.; Dolciami, M.; Ciulla, S.; Celli, V.; Kuehn, B.; Piccioni, M.G.; Giancotti, A.; Porpora, M.G.; et al. Human Placental Microperfusion and Microstructural Assessment by Intra-Voxel Incoherent Motion MRI for Discriminating Intrauterine Growth Restriction: A Pilot Study. J. Matern. Fetal Neonatal Med. 2024, 35, 9667–9674. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Capuani, S.; Gennarini, M.; Miceli, V.; Ninkova, R.; Balba, I.; Galea, N.; Cupertino, A.; Maiuro, A.; Ercolani, G.; et al. Fetal MRI: What’s New? A Short Review. Eur. Radiol. Exp. 2023, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Vena, F.; Manganaro, L.; D’Ambrosio, V.; Masciullo, L.; Ventriglia, F.; Ercolani, G.; Bertolini, C.; Catalano, C.; Di Mascio, D.; D’Alberti, E.; et al. Neuroimaging and Cerebrovascular Changes in Fetuses with Complex Congenital Heart Disease. J. Clin. Med. 2022, 11, 6740. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, T.M.; Bissell, M.M.; Kording, F.; Geipel, A.; Isaak, A.; Strizek, B.S.; Hart, C.; Barker, A.J.; Luetkens, J.A. Fetal Cardiac MRI Using Doppler US Gating: Emerging Technology and Clinical Implications. Radiol. Cardiothorac. Imaging 2024, 6, e230182. [Google Scholar] [CrossRef] [PubMed]
- Marini, D.; Xu, J.; Sun, L.; Jaeggi, E.; Seed, M. Current and Future Role of Fetal Cardiovascular MRI in the Setting of Fetal Cardiac Interventions. Prenat. Diagn. 2020, 40, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Prayer, D.; Malinger, G.; De Catte, L.; De Keersmaecker, B.; Gonçalves, L.F.; Kasprian, G.; Laifer-Narin, S.; Lee, W.; Millischer, A.-E.; Platt, L.; et al. ISUOG Practice Guidelines (Updated): Performance of Fetal Magnetic Resonance Imaging. Ultrasound Obstet. Gynecol. 2023, 61, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Colleran, G.C.; Kyncl, M.; Garel, C.; Cassart, M. Fetal Magnetic Resonance Imaging at 3 Tesla—The European Experience. Pediatr. Radiol. 2022, 52, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Antonelli, A.; Bernardo, S.; Capozza, F.; Petrillo, R.; Satta, S.; Vinci, V.; Saldari, M.; Maccioni, F.; Ballesio, L.; et al. Highlights on MRI of the Fetal Body. Radiol. Medica 2018, 123, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zamora, I.J.; Sheikh, F.; Cassady, C.I.; Olutoye, O.O.; Mehollin-Ray, A.R.; Ruano, R.; Lee, T.C.; Welty, S.E.; Belfort, M.A.; Ethun, C.G.; et al. Fetal MRI Lung Volumes Are Predictive of Perinatal Outcomes in Fetuses with Congenital Lung Masses. J. Pediatr. Surg. 2014, 49, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.; Nicolaides, K.H.; Keller, R.L.; Benachi, A.; Peralta, C.F.A.; Favre, R.; Moreno, O.; Tibboel, D.; Lipitz, S.; Eggink, A.; et al. Observed to Expected Lung Area to Head Circumference Ratio in the Prediction of Survival in Fetuses with Isolated Diaphragmatic Hernia. Ultrasound Obstet. Gynecol. 2007, 30, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kilian, A.K.; Schaible, T.; Hofmann, V.; Brade, J.; Neff, K.W.; Büsing, K.A. Congenital Diaphragmatic Hernia: Predictive Value of MRI Relative Lung-to-Head Ratio Compared with MRI Fetal Lung Volume and Sonographic Lung-to-Head Ratio. Am. J. Roentgenol. 2009, 192, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ward, V.L. MR Imaging in the Prenatal Diagnosis of Fetal Chest Masses. Acad. Radiol. 2002, 9, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Rubesova, E.; Barth, R. MR Assessment of Normal Fetal Lung Volumes: A Literature Review. Am. J. Roentgenol. 2010, 194, W212–W217. [Google Scholar] [CrossRef] [PubMed]
- Kastenholz, K.E.; Weis, M.; Hagelstein, C.; Weiss, C.; Kehl, S.; Schaible, T.; Neff, K.W. Correlation of Observed-to-Expected MRI Fetal Lung Volume and Ultrasound Lung-to-Head Ratio at Different Gestational Times in Fetuses With Congenital Diaphragmatic Hernia. Am. J. Roentgenol. 2016, 206, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Rubesova, E. Why Do We Need More Data on MR Volumetric Measurements of the Fetal Lung? Pediatr. Radiol. 2016, 46, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Sandaite, I.; Claus, F.; De Keyzer, F.; Donè, E.; Van Mieghem, T.; Gucciardo, L.; DeKoninck, P.; Jani, J.; Cannie, M.; Deprest, J.A. Examining the Relationship between the Lung-to-Head Ratio Measured on Ultrasound and Lung Volumetry by Magnetic Resonance in Fetuses with Isolated Congenital Diaphragmatic Hernia. Fetal Diagn. Ther. 2011, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.; Cannie, M.; Sonigo, P.; Robert, Y.; Moreno, O.; Benachi, A.; Vaast, P.; Gratacos, E.; Nicolaides, K.H.; Deprest, J. Value of Prenatal Magnetic Resonance Imaging in the Prediction of Postnatal Outcome in Fetuses with Diaphragmatic Hernia. Ultrasound Obstet. Gynecol. 2008, 32, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Dighe, M.K.; Peterson, S.E.; Dubinsky, T.J.; Perkins, J.; Cheng, E. EXIT Procedure: Technique and Indications with Prenatal Imaging Parameters for Assessment of Airway Patency. RadioGraphics 2011, 31, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S. Management of Fetal Lung Lesions. Clin. Perinatol. 2009, 36, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Euser, A.G.; Meyers, M.L.; Zaretsky, M.V.; Crombleholme, T.M. Comparison of Congenital Pulmonary Airway Malformation Volume Ratios Calculated by Ultrasound and Magnetic Resonance Imaging. J. Matern. -Fetal Neonatal Med. 2016, 29, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.W.; Seed, M.; Macgowan, C.K. Accelerated MRI of the Fetal Heart Using Compressed Sensing and Metric Optimized Gating. Magn. Reson. Med. 2017, 77, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; Fogel, M.A. Fetal Cardiac MRI: Doppler US-Gated Cine Imaging in Complex Congenital Heart Disease. Radiol. Cardiothorac. Imaging 2023, 5, e220314. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.W.; van Amerom, J.F.P.; Marini, D.; Seed, M.; Macgowan, C.K. Fetal Cardiac MRI. Top. Magn. Reson. Imaging 2019, 28, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Tavares de Sousa, M.; Lenz, A.; Herrmann, J.; Zhang, S.; Kording, F.; Hergert, B.; Adam, G.; Bannas, P.; Schoennagel, B.P. Fetal 4D Flow MRI of the Great Thoracic Vessels at 3 Tesla Using Doppler-Ultrasound Gating: A Feasibility Study. Eur. Radiol. 2022, 33, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Tavares de Sousa, M.; Hecher, K.; Yamamura, J.; Kording, F.; Ruprecht, C.; Fehrs, K.; Behzadi, C.; Adam, G.; Schoennagel, B.P. Dynamic Fetal Cardiac Magnetic Resonance Imaging in Four-chamber View Using Doppler Ultrasound Gating in Normal Fetal Heart and in Congenital Heart Disease: Comparison with Fetal Echocardiography. Ultrasound Obstet. Gynecol. 2019, 53, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Schoennagel, B.P.; Yamamura, J.; Kording, F.; Fischer, R.; Bannas, P.; Adam, G.; Kooijman, H.; Ruprecht, C.; Fehrs, K.; Tavares de Sousa, M. Fetal Dynamic Phase-Contrast MR Angiography Using Ultrasound Gating and Comparison with Doppler Ultrasound Measurements. Eur. Radiol. 2019, 29, 4169–4176. [Google Scholar] [CrossRef]
- Peterson, D.M.; Beck, B.L.; Duensing, G.R.; Fitzsimmons, J.R. Common Mode Signal Rejection Methods for MRI: Reduction of Cable Shield Currents for High Static Magnetic Field Systems. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2003, 19, 1–8. [Google Scholar] [CrossRef]
- Kording, F.; Schoennagel, B.P.; de Sousa, M.T.; Fehrs, K.; Adam, G.; Yamamura, J.; Ruprecht, C. Evaluation of a Portable Doppler Ultrasound Gating Device for Fetal Cardiac MR Imaging: Initial Results at 1.5T and 3T. Magn. Reson. Med. Sci. 2018, 17, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Haris, K.; Hedström, E.; Kording, F.; Bidhult, S.; Steding-Ehrenborg, K.; Ruprecht, C.; Heiberg, E.; Arheden, H.; Aletras, A.H. Free-breathing Fetal Cardiac MRI with Doppler Ultrasound Gating, Compressed Sensing, and Motion Compensation. J. Magn. Reson. Imaging 2020, 51, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, T.M.; Hart, C.; Zhang, S.; Katemann, C.; Isaak, A.; Pieper, C.C.; Kuetting, D.; Faridi, B.; Strizek, B.; Attenberger, U.; et al. Fetal Cardiac Cine MRI with Doppler US Gating in Complex Congenital Heart Disease. Radiol. Cardiothorac. Imaging 2023, 5, e220129. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Reffo, E.; Castaldi, B.; Cattapan, I.; Avesani, M.; Biffanti, R.; Cavaliere, A.; Cerutti, A.; Di Salvo, G. Utility of Fetal Cardiac Resonance Imaging in Prenatal Clinical Practice: Current State of the Art. Diagnostics 2023, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Desmond, A.; Nguyen, K.; Watterson, C.T.; Sklansky, M.; Satou, G.M.; Prosper, A.E.; Garg, M.; Van Arsdell, G.S.; Finn, J.P.; Afshar, Y. Integration of Prenatal Cardiovascular Magnetic Resonance Imaging in Congenital Heart Disease. J. Am. Heart Assoc. 2023, 12, e030640. [Google Scholar] [CrossRef] [PubMed]
- Kording, F.; Yamamura, J.; de Sousa, M.T.; Ruprecht, C.; Hedström, E.; Aletras, A.H.; Ellen Grant, P.; Powell, A.J.; Fehrs, K.; Adam, G.; et al. Dynamic Fetal Cardiovascular Magnetic Resonance Imaging Using Doppler Ultrasound Gating. J. Cardiovasc. Magn. Reson. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Piek, M.; Ryd, D.; Töger, J.; Testud, F.; Hedström, E.; Aletras, A.H. Fetal 3D Cardiovascular Cine Image Acquisition Using Radial Sampling and Compressed Sensing. Magn. Reson. Med. 2023, 89, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Ryd, D.; Fricke, K.; Bhat, M.; Arheden, H.; Liuba, P.; Hedström, E. Utility of Fetal Cardiovascular Magnetic Resonance for Prenatal Diagnosis of Complex Congenital Heart Defects. JAMA Netw. Open 2021, 4, e213538. [Google Scholar] [CrossRef] [PubMed]
- Minocha, P.K.; Englund, E.K.; Friesen, R.M.; Fujiwara, T.; Smith, S.A.; Meyers, M.L.; Browne, L.P.; Barker, A.J. Reference Values for Fetal Cardiac Dimensions, Volumes, Ventricular Function and Left Ventricular Longitudinal Strain Using Doppler Ultrasound Gated Cardiac Magnetic Resonance Imaging in Healthy Third Trimester Fetuses. J. Magn. Reson. Imaging 2023, 60, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, T.M.; Hart, C.; Zhang, S.; Katemann, C.; Sprinkart, A.M.; Isaak, A.; Attenberger, U.; Pieper, C.C.; Kuetting, D.; Geipel, A.; et al. Deep Learning Denoising Reconstruction for Improved Image Quality in Fetal Cardiac Cine MRI. Front. Cardiovasc. Med. 2024, 11, 1323443. [Google Scholar] [CrossRef]
- Udine, M.; Loke, Y.-H.; Goudar, S.; Donofrio, M.T.; Truong, U.; Krishnan, A. The Current State and Potential Innovation of Fetal Cardiac MRI. Front. Pediatr. 2023, 11, 1219091. [Google Scholar] [CrossRef] [PubMed]
- Barber, N.; Freud, L. Advances in Fetal Cardiac Imaging and Intervention. CJC Pediatr. Congenit. Heart Dis. 2024, 3, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.A.; van Amerom, J.F.P.; Uus, A.; Lloyd, D.F.A.; van Poppel, M.P.M.; Price, A.N.; Tournier, J.-D.; Mohanadass, C.A.; Jackson, L.H.; Malik, S.J.; et al. Fetal Whole Heart Blood Flow Imaging Using 4D Cine MRI. Nat. Commun. 2020, 11, 4992. [Google Scholar] [CrossRef] [PubMed]
- Ebel, S.; Kühn, A.; Köhler, B.; Behrendt, B.; Riekena, B.; Preim, B.; Denecke, T.; Grothoff, M.; Gutberlet, M. Quantitative 4D Flow MRI-Derived Thoracic Aortic Normal Values of 2D Flow MRI Parameters in Healthy Volunteers. RöFo-Fortschritte Auf Dem Geb. Röntgenstrahlen Bildgeb. Verfahr. 2024, 196, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and Treatment of Fetal Cardiac Disease. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.F.A.; van Amerom, J.F.P.; Pushparajah, K.; Simpson, J.M.; Zidere, V.; Miller, O.; Sharland, G.; Allsop, J.; Fox, M.; Lohezic, M.; et al. An Exploration of the Potential Utility of Fetal Cardiovascular MRI as an Adjunct to Fetal Echocardiography. Prenat. Diagn. 2016, 36, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Vachon-Marceau, C.; Guerra, V.; Jaeggi, E.; Chau, V.; Ryan, G.; Van Mieghem, T. In-utero Treatment of Large Symptomatic Rhabdomyoma with Sirolimus. Ultrasound Obstet. Gynecol. 2019, 53, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, T.M.; Hart, C.; Luetkens, J.A. Fetal Cardiac MRI of Complex Interrupted Aortic Arch. Radiology 2023, 307, e223224. [Google Scholar] [CrossRef] [PubMed]
- Dargahpour Barough, M.; Tavares de Sousa, M.; Hergert, B.; Fischer, R.; Huber, L.; Seliger, J.M.; Kaul, M.G.; Adam, G.; Herrmann, J.; Bannas, P.; et al. Myocardial Strain Assessment in the Human Fetus by Cardiac MRI Using Doppler Ultrasound Gating and Feature Tracking. Eur. Radiol. 2024, 34, 4920–4927. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Leo, I.; Lisignoli, V.; Boyle, S.; Bucciarelli-Ducci, C.; Secinaro, A.; Montanaro, C. Cardiovascular Magnetic Resonance from Fetal to Adult Life—Indications and Challenges: A State-of-the-Art Review. Children 2023, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Mervak, B.M.; Altun, E.; McGinty, K.A.; Hyslop, W.B.; Semelka, R.C.; Burke, L.M. MRI in Pregnancy: Indications and Practical Considerations. J. Magn. Reson. Imaging 2019, 49, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Di Grezia, G.; Cuccurullo, V.; Sardu, C.; Iovino, F.; Comune, R.; Ruggiero, A.; Chirico, M.; La Forgia, D.; Fanizzi, A.; et al. MRI in Pregnancy and Precision Medicine: A Review from Literature. J. Pers. Med. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]




| Sequence | TA (ms) | FOV (cm) | TR (ms) | TE (ms) | Flip Angle (Degrees) | Voxel Size (mm3) | Slices | Acceleration | Other |
|---|---|---|---|---|---|---|---|---|---|
| T1 3D GRE saturated | 0:19 | 24 × 30 | 6 | 1.3 | 9 | 1.2 × 1.2 × 3 | 30 | Grappa = 3 | Saturation method DIXON, breath-holding |
| T2 SS-FSE | 0:55 | 27 × 30 | 1500 | 152 | 135 | 0.8 × 0.8 × 2.5 | 25 | Grappa = 2 | Parallel saturation bands, free breathing |
| bSSFP | 0:22 | 30 × 38 | 500 | 1.9 | 46 | 1.1 × 1.1 × 3 | 50 | Grappa = 2 | Offset frequency after scout evaluation, free breathing |
| IVIM DWI | 2:50 | 38 × 30 | 4500 | 67 | - | 1.7 × 1.7 × 3.5 | 20 | Grappa = 2 | B-values: 0, 10, 30, 50, 70, 100, 200, 400, 700, 1000 Avgs: 2, 2, 2, 2, 2, 3, 3, 4, 6, 9 Diffusion mode: 3D diagonal |
| Sequence | T2-W Haste | T1-W Flash 2D | DWI | True-FISP |
|---|---|---|---|---|
| TR (ms) | 1000 | 6 | 5300 | 3 |
| TE (ms) | 119 | 3 | 79 | 1 |
| Slice thickness (mm) | 3 | 3.5 | 4 | 4 |
| FOV (mm) | 203 × 270 | 500 × 313 | 380 × 380 | 400 × 300 |
| Matrix | 256 × 134 | 256 × 112 | 192 × 192 | 256 × 144 |
| Flip angle | 150° | 10° | 90° | 60° |
| Concatenations | 1 | 2 | 1 | 1 |
| b values (s/mm2) | - | - | 50,200,700 | - |
| TI (ms) | - | - | 185 | - |
| Acquisition time (s) | 16–20 | 30 | 45 | 15 |
| Potential Clinical Indication | The Role of fCMR |
|---|---|
| Cardiac malformations | |
| Anomalous pulmonary venous drainage | -Systemic venous anatomy and connections to the cardiac chambers -Number and normal/abnormal connections of pulmonary veins |
| Atrial/ventricular septal defect | -Relationship to neighboring structures and outflow tracts -Ventricular size and balance, volumes, function |
| Tetralogy of Fallot, Transposition of Great Artery, Double outlet right ventricle (TOF, TGA, DORV) | -Ventricular size, and function, better 3D evaluation of cardiac structure relationships -Outflow tracts, great vessel size, and relationships |
| Hypoplastic left heart syndrome (HLHS) | -Ventricular size and volumes. -Pulmonary parenchymal changes in the presence of a restrictive septum (secondary lymphangiectasia) |
| Malformation of the great vessels (aortic coarctation, aortic arch anomalies, vascular rings) | -Arch anatomy, branching, dimensions, and flow patterns -Vascular relationships of the great vessels to the bronchopulmonary tree and esophagus (deviation, compression, etc.) |
| Myocardial anomalies | |
| Cardiomyopathies | -Myocardial and valvar function -Extracardiac anomalies |
| Cardiac masses | -Assessment of function, size of mass, and relation to neighboring structures -Potential of tissue characterization -Renal and brain assessment in tuberous sclerosis |
| Cardiovascular assessment in systemic conditions | |
| Hydrops fetalis | -Cardiovascular assessment and function |
| Viral infections | -Cardiovascular status (anatomy, function) -Teratogenic effects of CMV, HIV, toxoplasma, etc. |
| Heterotaxy and situs | -Thoracic and abdominal visceral situs -Venous anomalies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cundari, G.; Galea, N.; Di Mascio, D.; Gennarini, M.; Ventriglia, F.; Curti, F.; Dodaro, M.; Rizzo, G.; Catalano, C.; Giancotti, A.; et al. The New Frontiers of Fetal Imaging: MRI Insights into Cardiovascular and Thoracic Structures. J. Clin. Med. 2024, 13, 4598. https://doi.org/10.3390/jcm13164598
Cundari G, Galea N, Di Mascio D, Gennarini M, Ventriglia F, Curti F, Dodaro M, Rizzo G, Catalano C, Giancotti A, et al. The New Frontiers of Fetal Imaging: MRI Insights into Cardiovascular and Thoracic Structures. Journal of Clinical Medicine. 2024; 13(16):4598. https://doi.org/10.3390/jcm13164598
Chicago/Turabian StyleCundari, Giulia, Nicola Galea, Daniele Di Mascio, Marco Gennarini, Flavia Ventriglia, Federica Curti, Martina Dodaro, Giuseppe Rizzo, Carlo Catalano, Antonella Giancotti, and et al. 2024. "The New Frontiers of Fetal Imaging: MRI Insights into Cardiovascular and Thoracic Structures" Journal of Clinical Medicine 13, no. 16: 4598. https://doi.org/10.3390/jcm13164598
APA StyleCundari, G., Galea, N., Di Mascio, D., Gennarini, M., Ventriglia, F., Curti, F., Dodaro, M., Rizzo, G., Catalano, C., Giancotti, A., & Manganaro, L. (2024). The New Frontiers of Fetal Imaging: MRI Insights into Cardiovascular and Thoracic Structures. Journal of Clinical Medicine, 13(16), 4598. https://doi.org/10.3390/jcm13164598










