Beyond the Obstructive Paradigm: Unveiling the Complex Landscape of Nonobstructive Coronary Artery Disease
Abstract
:1. Introduction
2. Epidemiological Data
3. Mechanistic Insights
3.1. Coronary Plaque Disruption
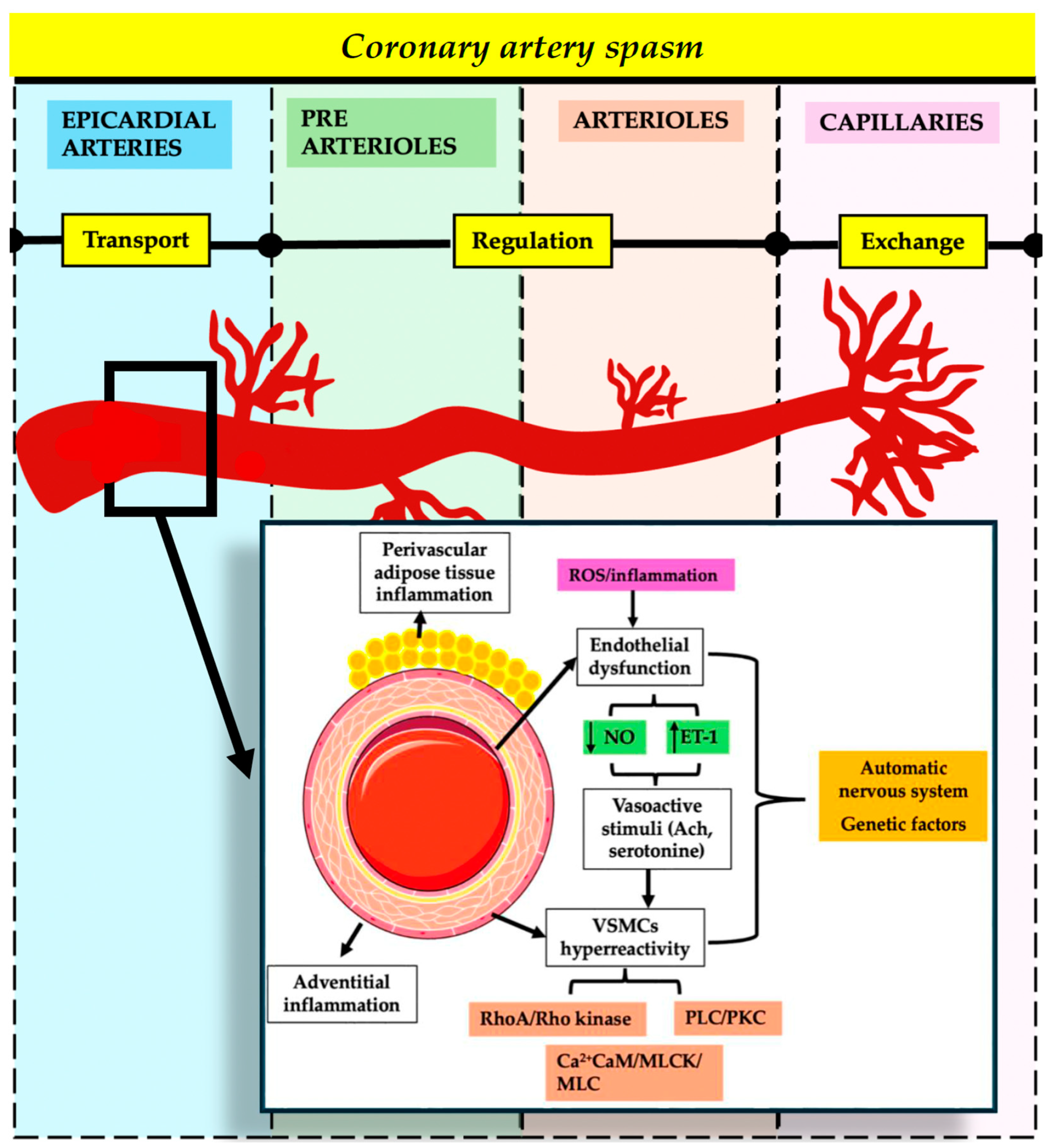
3.2. Coronary Artery Spasm
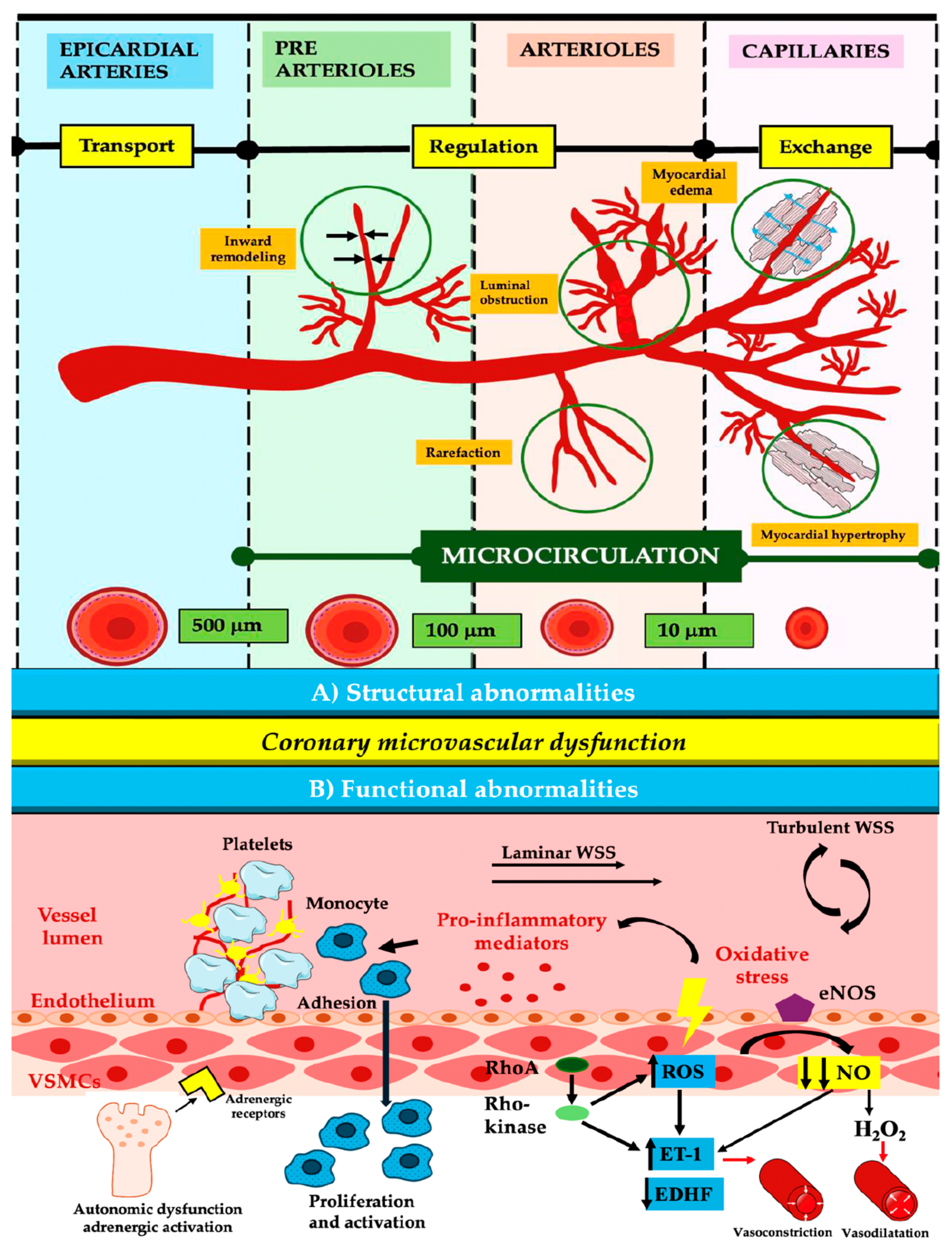
3.3. Coronary Microvascular Dysfunction
4. Clinical Implications
5. Integration of Multimodal Imaging
5.1. Coronary Computed Tomography Angiography
5.2. Cardiac Magnetic Resonance
5.3. Single-Photon Emission Computed Tomography and Positron Emission Tomography
5.4. Invasive Investigations
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef]
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Cardiovasc. Comput. Tomogr. 2022, 16, 54–122. [Google Scholar] [CrossRef] [PubMed]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.A.; Shah, S.M.; Widmer, R.J.; Kobayashi, Y.; Miner, S.E.S.; Taqueti, V.R.; Jeremias, A.; Albadri, A.; Blair, J.A.; Kearney, K.E.; et al. Comprehensive Management of ANOCA, Part 1-Definition, Patient Population, and Diagnosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1245–1263. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA: The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of Ischemic Heart Disease. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986. [Google Scholar] [CrossRef]
- Gulati, M.; Khan, N.; George, M.; Berry, C.; Chieffo, A.; Camici, P.G.; Crea, F.; Kaski, J.C.; Marzilli, M.; Merz, C.N.B. Ischemia with no obstructive coronary artery disease (INOCA): A patient self-report quality of life survey from INOCA international. Int. J. Cardiol. 2023, 371, 28–39. [Google Scholar] [CrossRef]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef]
- Kanaji, Y.; Ahmad, A.; Sara, J.D.S.; Ozcan, I.; Akhiyat, N.; Prasad, A.; Raphael, C.E.; Kakuta, T.; Lerman, L.O.; Lerman, A. Coronary Vasomotor Dysfunction Is Associated with Cardiovascular Events in Patients with Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2024, 17, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Gitto, M.; Gentile, F.; Nowbar, A.N.; Chieffo, A.; Al-Lamee, R. Gender-Related Differences in Clinical Presentation and Angiographic Findings in Patients with Ischemia and No Obstructive Coronary Artery Disease (INOCA): A Single-Center Observational Registry. Int. J. Angiol. 2020, 29, 250–255. [Google Scholar] [CrossRef]
- Wu, M.D.; Moccetti, F.; Brown, E.; Davidson, B.P.; Atkinson, T.; Belcik, J.T.; Giraud, G.; Duell, P.B.; Fazio, S.; Tavori, H.; et al. Lipoprotein Apheresis Acutely Reverses Coronary Microvascular Dysfunction in Patients with Severe Hypercholesterolemia. JACC Cardiovasc. Imaging 2019, 12, 1430–1440. [Google Scholar] [CrossRef]
- Conde, I.M.; Salazar, M.; Pereira, V.H.; Vieira, C.; Braga, C.G.; Oliveira, C. Early versus late cardiac magnetic resonance in the diagnosis of MINOCA. Rev. Port. Cardiol. 2024, 43, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.Y.; Goc, N.; Schwann, A.N.; Cherlin, E.J.; Kunnirickal, S.J.; Odanovic, N.; Curry, L.A.; Shah, S.M.; Spatz, E.S. Illness Perception and the Impact of a Definitive Diagnosis on Women with Ischemia and No Obstructive Coronary Artery Disease: A Qualitative Study. Circ. Cardiovasc. Qual. Outcomes 2023, 16, 521–529. [Google Scholar] [CrossRef]
- La, S.; Tavella, R.; Wu, J.; Pasupathy, S.; Zeitz, C.; Worthley, M.; Sinhal, A.; Arstall, M.; Spertus, J.A.; Beltrame, J.F. Angina and Non-Obstructive Coronary Artery (ANOCA) Patients with Coronary Vasomotor Disorders. Life 2023, 13, 2190. [Google Scholar] [CrossRef] [PubMed]
- Grigorian-Shamagian, L.; Oteo, J.F.; Gutierrez-Barrios, A.; Abdul-Jawad Altisent, O.; Amat-Santos, I.; Cisnal, A.F.; Roa, J.; Arellano Serrano, C.; Fadeuilhe, E.; Cortes, C.; et al. Endothelial dysfunction in patients with angina and non-obstructed coronary arteries is associated with an increased risk of mayor cardiovascular events. Results of the Spanish ENDOCOR registry. Int. J. Cardiol. 2023, 370, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Camma, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction with Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; Elias-Smale, S.E.; van den Oord, S.C.; Ong, P.; de Vos, A.M.J.; van de Hoef, T.P.; Paradies, V.; Smits, P.C.; van Royen, N.; et al. Assessing Microvascular Dysfunction in Angina with Unobstructed Coronary Arteries: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1471–1479. [Google Scholar] [CrossRef]
- Boivin-Proulx, L.A.; Haddad, K.; Lombardi, M.; Chong, A.Y.; Escaned, J.; Mukherjee, S.; Forcillo, J.; Potter, B.J.; Coutinho, T.; Pacheco, C. Pathophysiology of Myocardial Infarction with Nonobstructive Coronary Artery Disease: A Contemporary Systematic Review. CJC Open 2024, 6, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Jang, I.K. Plaque erosion and acute coronary syndromes: Phenotype, molecular characteristics and future directions. Nat. Rev. Cardiol. 2021, 18, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Fan, H.; Zhu, Y.; Chen, Y.; Shen, L. Coronary functional assessment in non-obstructive coronary artery disease: Present situation and future direction. Front. Cardiovasc. Med. 2022, 9, 934279. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Libby, P.; Jang, I.K. New Insights Into Plaque Erosion as a Mechanism of Acute Coronary Syndromes. JAMA 2021, 325, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Rallidis, L.S.; Xenogiannis, I.; Brilakis, E.S.; Bhatt, D.L. Causes, Angiographic Characteristics, and Management of Premature Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2431–2449. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Pompei, G.; Ganzorig, N.; Brown, S.; Beltrame, J.; Kunadian, V. Vasospastic angina: A review on diagnostic approach and management. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241230400. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, J.; Vink, C.E.M.; Schipaanboord, D.J.M.; Eringa, E.C.; den Ruijter, H.M.; Feenstra, R.G.T.; Boerhout, C.K.M.; Beijk, M.A.M.; de Waard, G.A.; Ong, P.; et al. Meta-analysis and systematic review of coronary vasospasm in ANOCA patients: Prevalence, clinical features and prognosis. Front. Cardiovasc. Med. 2023, 10, 1129159. [Google Scholar] [CrossRef]
- Fu, B.; Wei, X.; Lin, Y.; Chen, J.; Yu, D. Pathophysiologic Basis and Diagnostic Approaches for Ischemia with Non-obstructive Coronary Arteries: A Literature Review. Front. Cardiovasc. Med. 2022, 9, 731059. [Google Scholar] [CrossRef]
- Theberge, E.T.; Vikulova, D.N.; Pimstone, S.N.; Brunham, L.R.; Humphries, K.H.; Sedlak, T.L. The Importance of Nontraditional and Sex-Specific Risk Factors in Young Women with Vasomotor Nonobstructive vs Obstructive Coronary Syndromes. CJC Open 2024, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, R.E.; Maas, A.H. The Role of Mental Stress in Ischaemia with No Obstructive Coronary Artery Disease and Coronary Vasomotor Disorders. Eur. Cardiol. 2021, 16, e37. [Google Scholar] [CrossRef] [PubMed]
- Meeder, J.G.; Hartzema-Meijer, M.J.; Jansen, T.P.J.; Konst, R.E.; Damman, P.; Elias-Smale, S.E. Outpatient Management of Patients with Angina with No Obstructive Coronary Arteries: How to Come to a Proper Diagnosis and Therapy. Front. Cardiovasc. Med. 2021, 8, 716319. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities-from bench to bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A. Pathophysiology of coronary microvascular dysfunction. Vascul. Pharmacol. 2023, 153, 107239. [Google Scholar] [CrossRef] [PubMed]
- Francia, P.; delli Gatti, C.; Bachschmid, M.; Martin-Padura, I.; Savoia, C.; Migliaccio, E.; Pelicci, P.G.; Schiavoni, M.; Luscher, T.F.; Volpe, M.; et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 2004, 110, 2889–2895. [Google Scholar] [CrossRef]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Luscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; et al. Assessment and pathophysiology of microvascular disease: Recent progress and clinical implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Deng, J. Research progress on the molecular mechanism of coronary microvascular endothelial cell dysfunction. Int. J. Cardiol. Heart Vasc. 2021, 34, 100777. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef]
- Patel, N.; Greene, N.; Guynn, N.; Sharma, A.; Toleva, O.; Mehta, P.K. Ischemia but no obstructive coronary artery disease: More than meets the eye. Climacteric 2024, 27, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Papafaklis, M.I.; Takahashi, S.; Antoniadis, A.P.; Coskun, A.U.; Tsuda, M.; Mizuno, S.; Andreou, I.; Nakamura, S.; Makita, Y.; Hirohata, A.; et al. Effect of the local hemodynamic environment on the de novo development and progression of eccentric coronary atherosclerosis in humans: Insights from PREDICTION. Atherosclerosis 2015, 240, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Zhang, H.; Abdu, F.A.; Liu, L.; Singh, S.; Lv, X.; Shi, T.; Mareai, R.M.; Mohammed, A.Q.; Yin, G.; et al. Effect of nonobstructive coronary stenosis on coronary microvascular dysfunction and long-term outcomes in patients with INOCA. Clin. Cardiol. 2023, 46, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Luo, X.; Liao, B.; Li, G.; Feng, J. Insights into SGLT2 inhibitor treatment of diabetic cardiomyopathy: Focus on the mechanisms. Cardiovasc. Diabetol. 2023, 22, 86. [Google Scholar] [CrossRef]
- Solberg, O.G.; Stavem, K.; Ragnarsson, A.; Beitnes, J.O.; Skardal, R.; Seljeflot, I.; Ueland, T.; Aukrust, P.; Gullestad, L.; Aaberge, L. Index of microvascular resistance to assess the effect of rosuvastatin on microvascular function in women with chest pain and no obstructive coronary artery disease: A double-blind randomized study. Catheter. Cardiovasc. Interv. 2019, 94, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Juni, R.P.; Kuster, D.W.D.; Goebel, M.; Helmes, M.; Musters, R.J.P.; van der Velden, J.; Koolwijk, P.; Paulus, W.J.; van Hinsbergh, V.W.M. Cardiac Microvascular Endothelial Enhancement of Cardiomyocyte Function Is Impaired by Inflammation and Restored by Empagliflozin. JACC Basic Transl. Sci. 2019, 4, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, G.; Li, Z.; Li, D.; Chen, R.; Huang, C.; Li, Y.; Li, B.; Yu, H.; Chu, X.M. MINOCA biomarkers: Non-atherosclerotic aspects. Clin. Chim. Acta 2023, 551, 117613. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Talib, J.; Stanley, C.P.; Rashid, I.; Michaelsson, E.; Lindstedt, E.L.; Croft, K.D.; Kettle, A.J.; Maghzal, G.J.; Stocker, R. Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1448–1457. [Google Scholar] [CrossRef]
- Hjort, M.; Eggers, K.M.; Lindhagen, L.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenback, C.; Frick, M.; Henareh, L.; et al. Increased Inflammatory Activity in Patients 3 Months after Myocardial Infarction with Nonobstructive Coronary Arteries. Clin. Chem. 2019, 65, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Fairman, G.; Robichaud, S.; Ouimet, M. Metabolic Regulators of Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Fatima, L.; Goyal, A.; Yakkali, S.; Jain, H.; Raza, F.A.; Peer, T.; Kanagala, S.G.; Sohail, A.H.; Malik, J. Precision medicine in Myocardial Infarction with Non-obstructive Coronary Disease (MINOCA): A comprehensive review. Curr. Probl. Cardiol. 2024, 49, 102185. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Baron, T.; Hjort, M.; Nordenskjold, A.M.; Tornvall, P.; Lindahl, B. Clinical and prognostic implications of C-reactive protein levels in myocardial infarction with nonobstructive coronary arteries. Clin. Cardiol. 2021, 44, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Suda, A.; Nishimiya, K.; Godo, S.; Yasuda, S.; Shimokawa, H. Pathophysiology and Diagnosis of Coronary Functional Abnormalities. Eur. Cardiol. 2021, 16, e30. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Prasad, A.; Nesbitt, L.; Loeffler, D.; Herrmann, J.; Lerman, L.O.; Lerman, A. Local Production of Soluble Urokinase Plasminogen Activator Receptor and Plasminogen Activator Inhibitor-1 in the Coronary Circulation Is Associated with Coronary Endothelial Dysfunction in Humans. J. Am. Heart Assoc. 2018, 7, e009881. [Google Scholar] [CrossRef] [PubMed]
- Brolin, E.B.; Agewall, S.; Cederlund, K.; Ekenback, C.; Henareh, L.; Malmqvist, K.; Ruck, A.; Svensson, A.; Tornvall, P. Plasma biomarker levels and non-obstructive coronary artery disease determined by coronary computed tomography angiography. Clin. Physiol. Funct. Imaging 2018, 38, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Oshita, C.; Uchimura, Y. Clinical Characteristics and Prognosis of Patients with Multi-Vessel Coronary Spasm in Comparison with Those in Patients with Single-Vessel Coronary Spasm. J. Cardiovasc. Dev. Dis. 2022, 9, 204. [Google Scholar] [CrossRef]
- Feenstra, R.G.T.; Jansen, T.P.J.; Matthijs Boekholdt, S.; Brouwer, J.E.; Klees, M.I.; Appelman, Y.; Wittekoek, M.E.; van de Hoef, T.P.; de Winter, R.J.; Piek, J.J.; et al. Efficacy and safety of the endothelin-1 receptor antagonist macitentan in epicardial and microvascular vasospasm; a proof-of-concept study. Int. J. Cardiol. Heart Vasc. 2023, 47, 101238. [Google Scholar] [CrossRef]
- Ford, T.J.; Corcoran, D.; Padmanabhan, S.; Aman, A.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Maguire, J.J.; Watkins, S.; Eteiba, H.; et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur. Heart J. 2020, 41, 3239–3252. [Google Scholar] [CrossRef]
- Pasupathy, S.; Tavella, R.; Zeitz, C.; Edwards, S.; Worthley, M.; Arstall, M.; Beltrame, J.F. Anti-Anginal Efficacy of Zibotentan in the Coronary Slow-Flow Phenomenon. J. Clin. Med. 2024, 13, 1337. [Google Scholar] [CrossRef] [PubMed]
- Al-Badri, A.; Tahhan, A.S.; Sabbak, N.; Alkhoder, A.; Liu, C.; Ko, Y.A.; Vaccarino, V.; Martini, A.; Sidoti, A.; Goodwin, C.; et al. Soluble Urokinase-Type Plasminogen Activator Receptor and High-Sensitivity Troponin Levels Predict Outcomes in Nonobstructive Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015515. [Google Scholar] [CrossRef] [PubMed]
- Solomonica, A.; Bagur, R.; Choudhury, T.; Lavi, S. Familial Spontaneous Coronary Artery Dissection and the SMAD-3 Mutation. Am. J. Cardiol. 2019, 124, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Pitliya, A.; Datta, S.; Kalayci, A.; Kahe, F.; Sharfaei, S.; Jafarizade, M.; Goudarzi, S.; Chi, G. Eosinophilic inflammation in spontaneous coronary artery dissection: A potential therapeutic target? Med. Hypotheses 2018, 121, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Karakayali, M.; Altunova, M.; Yakisan, T.; Aslan, S.; Omar, T.; Artac, I.; Ilis, D.; Arslan, A.; Cagin, Z.; Karabag, Y.; et al. The Relationship between the Systemic Immune-Inflammation Index and Ischemia with Non-Obstructive Coronary Arteries in Patients Undergoing Coronary Angiography. Arq. Bras. Cardiol. 2024, 121, e20230540. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Quesada, O.; Al-Badri, A.; Fleg, J.L.; Volgman, A.S.; Pepine, C.J.; Merz, C.N.B.; Shaw, L.J. Ischemia and no obstructive coronary arteries in patients with stable ischemic heart disease. Int. J. Cardiol. 2022, 348, 1–8. [Google Scholar] [CrossRef] [PubMed]
- AlBadri, A.; Lai, K.; Wei, J.; Landes, S.; Mehta, P.K.; Li, Q.; Johnson, D.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: A report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS ONE 2017, 12, e0177684. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Bairey Merz, C.N.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Toya, T.; Albers, D.; Sebaali, F.; Lewis, B.R.; Bois, J.; Gulati, R.; Prasad, A.; Best, P.J.M.; Bell, M.R.; et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients with Angina and Nonobstructive Coronary Arteries. Circ. Res. 2022, 130, 326–338. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Liang, K.; Nakou, E.; Del Buono, M.G.; Montone, R.A.; D’Amario, D.; Bucciarelli-Ducci, C. The Role of Cardiac Magnetic Resonance in Myocardial Infarction and Non-obstructive Coronary Arteries. Front. Cardiovasc. Med. 2021, 8, 821067. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021, 17, e875–e887. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Jang, I.K.; Beltrame, J.F.; Sicari, R.; Meucci, M.C.; Bode, M.; Gaibazzi, N.; Niccoli, G.; Bucciarelli-Ducci, C.; Crea, F. The evolving role of cardiac imaging in patients with myocardial infarction and non-obstructive coronary arteries. Prog. Cardiovasc. Dis. 2021, 68, 78–87. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, T.; Asch, F.M.; Davidson, B.; Delgado, V.; DeMaria, A.; Dilsizian, V.; Gaemperli, O.; Garcia, M.J.; Kamp, O.; Lee, D.C.; et al. Non-Invasive Imaging in Coronary Syndromes: Recommendations of the European Association of Cardiovascular Imaging and the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Comput. Tomogr. 2022, 16, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Moreno, P.; Dominguez, A.C.; Tamis-Holland, J.E. The Imaging Toolbox to Assess Patients with Suspected Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease (MINOCA). Curr. Cardiol. Rep. 2020, 22, 134. [Google Scholar] [CrossRef]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADS(TM) Coronary Artery Disease—Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J. Cardiovasc. Comput. Tomogr. 2016, 10, 269–281. [Google Scholar] [CrossRef]
- Occhipinti, G.; Bucciarelli-Ducci, C.; Capodanno, D. Diagnostic pathways in myocardial infarction with non-obstructive coronary artery disease (MINOCA). Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 813–822. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Wei, J.; Cook-Wiens, G.; Pepine, C.J.; Handberg, E.M.; Shaw, L.J.; Budoff, M.; Merz, C.N.B. Comparison of risk profiles of participants in the Women’s IschemiA TRial to reduce events in non-ObstRuctive CAD (WARRIOR) trial, using coronary computed tomography angiography vs invasive coronary angiography. Prog. Cardiovasc. Dis. 2024, 84, 90–93. [Google Scholar] [CrossRef]
- Fan, H.P.; Rui, J.Q.; Xin, C.X.; Zhou, Y.; Jin, J.; Hu, X.F. Medium-Term Prognostic Implications of Cardiac Magnetic Resonance Imaging in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Luis, S.A.; Luis, C.R.; Habibian, M.; Lwin, M.T.; Gadowski, T.C.; Chan, J.; Hamilton-Craig, C.; Raffel, O.C. Prognostic Value of Cardiac Magnetic Resonance Imaging in Acute Coronary Syndrome Patients with Troponin Elevation and Nonobstructive Coronary Arteries. Mayo Clin. Proc. 2021, 96, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.Y.; Chiang, J.B.; Ng, P.P.; Chow, B.C.K.; Cheng, Y.W.; Wong, C.Y. Utility of cardiac magnetic resonance imaging in troponin-positive chest pain with non-obstructive coronary arteries: Literature review. Hong Kong Med. J. 2021, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.P.J.; Elias-Smale, S.E.; van den Oord, S.; Gehlmann, H.; Dimitiriu-Leen, A.; Maas, A.; Konst, R.E.; van Royen, N.; Damman, P. Sex Differences in Coronary Function Test Results in Patient with Angina and Nonobstructive Disease. Front. Cardiovasc. Med. 2021, 8, 750071. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rahman, H.; Perera, D. Coronary microvascular dysfunction and heart failure with preserved ejection fraction: What are the mechanistic links? Curr. Opin. Cardiol. 2023, 38, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Zampella, E.; Mannarino, T.; D’Antonio, A.; Assante, R.; Gaudieri, V.; Buongiorno, P.; Panico, M.; Cantoni, V.; Green, R.; Nappi, C.; et al. Prediction of outcome by (82)Rb PET/CT in patients with ischemia and nonobstructive coronary arteries. J. Nucl. Cardiol. 2023, 30, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Lampas, E.; Syrmali, K.; Nikitas, G.; Papadakis, E.C.; Patsilinakos, S.P. Five-year morbidity and mortality of patients with ischemia with non-obstructive coronary artery disease and myocardial single-photon emission computed tomography perfusion defects. Rev. Port. Cardiol. 2023, 42, 519–524. [Google Scholar] [CrossRef]
- Armillotta, M.; Amicone, S.; Bergamaschi, L.; Angeli, F.; Rinaldi, A.; Paolisso, P.; Stefanizzi, A.; Sansonetti, A.; Impellizzeri, A.; Bodega, F.; et al. Predictive value of Killip classification in MINOCA patients. Eur. J. Intern. Med. 2023, 117, 57–65. [Google Scholar] [CrossRef]
- Zhou, W.; Lee, J.C.Y.; Leung, S.T.; Lai, A.; Lee, T.F.; Chiang, J.B.; Cheng, Y.W.; Chan, H.L.; Yiu, K.H.; Goh, V.K.; et al. Long-Term Prognosis of Patients with Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc. Imaging 2021, 14, 602–611. [Google Scholar] [CrossRef]
- Kong, H.; Cao, J.; Tian, J.; Yong, J.; An, J.; Song, X.; He, Y. Relationship between coronary microvascular dysfunction (CMD) and left ventricular diastolic function in patients with symptoms of myocardial ischemia with non-obstructive coronary artery disease (INOCA) by cardiovascular magnetic resonance feature-tracking. Clin. Radiol. 2024, 79, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Florian, A. Diastolic dysfunction in women with ischemia and non-obstructive coronary arteries (INOCA)—Could non-invasive imaging reveal the missing piece of the puzzle? Int. J. Cardiol. 2021, 334, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Holtzman, J.N.; Juszczynski, C.; Khan, N.; Kaur, G.; Varma, B.; Gulati, M. Ischemia with No Obstructive Arteries (INOCA): A Review of the Prevalence, Diagnosis and Management. Curr. Probl. Cardiol. 2023, 48, 101420. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, A.G.; Baritussio, A.; De Garate, E.; Drobni, Z.; Biglino, G.; Singhal, P.; Milano, E.G.; Angelini, G.D.; Dorman, S.; Strange, J.; et al. Prognostic Role of CMR and Conventional Risk Factors in Myocardial Infarction with Nonobstructed Coronary Arteries. JACC Cardiovasc. Imaging 2019, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; von Knobelsdorff-Brenkenhoff, F.; Bernhardt, P.; Carbone, I.; Muellerleile, K.; Aldrovandi, A.; Francone, M.; Desch, S.; Gutberlet, M.; Strohm, O.; et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011, 306, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Pathik, B.; Raman, B.; Mohd Amin, N.H.; Mahadavan, D.; Rajendran, S.; McGavigan, A.D.; Grover, S.; Smith, E.; Mazhar, J.; Bridgman, C.; et al. Troponin-positive chest pain with unobstructed coronary arteries: Incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1146–1152. [Google Scholar] [CrossRef]
- Williams, M.G.L.; Liang, K.; De Garate, E.; Spagnoli, L.; Fiori, E.; Dastidar, A.; Benedetto, U.; Biglino, G.; Johnson, T.W.; Luscher, T.; et al. Peak Troponin and CMR to Guide Management in Suspected ACS and Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2022, 15, 1578–1587. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Foa, A.; Paolisso, P.; Renzulli, M.; Angeli, F.; Fabrizio, M.; Bartoli, L.; Armillotta, M.; Sansonetti, A.; Amicone, S.; et al. Prognostic Role of Early Cardiac Magnetic Resonance in Myocardial Infarction with Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2024, 17, 149–161. [Google Scholar] [CrossRef]
- Steffen Johansson, R.; Tornvall, P.; Sorensson, P.; Nickander, J. Reduced stress perfusion in myocardial infarction with nonobstructive coronary arteries. Sci. Rep. 2023, 13, 22094. [Google Scholar] [CrossRef]
- Crea, F.; Bairey Merz, C.N.; Beltrame, J.F.; Berry, C.; Camici, P.G.; Kaski, J.C.; Ong, P.; Pepine, C.J.; Sechtem, U.; Shimokawa, H. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur. Heart J. 2019, 40, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation against Invasive Coronary Physiology. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Fernandes, J.L.; Moon, J.C. Automated Quantitative Stress Perfusion in a Clinical Routine. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Gonzalez, A.G.; Tio, R.A.; de Groot, J.C.; Boersma, H.H.; Prakken, N.H.; De Jongste, M.J.L.; Alexanderson-Rosas, E.; Slart, R. Long-term prognostic value of quantitative myocardial perfusion in patients with chest pain and normal coronary arteries. J. Nucl. Cardiol. 2019, 26, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammar, T.; Carlsson, M.; Jogi, J.; Arheden, H.; Engblom, H. Myocardial perfusion by CMR coronary sinus flow shows sex differences and lowered perfusion at stress in patients with suspected microvascular angina. Clin. Physiol. Funct. Imaging 2022, 42, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Park, J.; Hwang, D.; Rhee, T.M.; Kim, J.; Park, J.; Kim, H.Y.; Jung, H.W.; Cho, Y.K.; et al. Physiological and Clinical Assessment of Resting Physiological Indexes. Circulation 2019, 139, 889–900. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Ai, H.; Feng, Y.; Gong, Y.; Zheng, B.; Jin, Q.; Zhang, H.P.; Sun, F.; Li, J.; Chen, Y.; Huo, Y.; et al. Coronary Angiography-Derived Index of Microvascular Resistance. Front. Physiol. 2020, 11, 605356. [Google Scholar] [CrossRef] [PubMed]
- Abdu, F.A.; Liu, L.; Mohammed, A.Q.; Yin, G.; Xu, B.; Zhang, W.; Xu, S.; Lv, X.; Fan, R.; Feng, C.; et al. Prognostic impact of coronary microvascular dysfunction in patients with myocardial infarction with non-obstructive coronary arteries. Eur. J. Intern. Med. 2021, 92, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Feng, W.; Zhou, H.; Peng, W.; Wang, X. Diagnostic and prognostic value of angiography-derived index of microvascular resistance: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1360648. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Renteria, H.; Wang, L.; Chipayo-Gonzales, D.; van de Hoef, T.P.; Travieso, A.; Espejo, C.; Nunez-Gil, I.J.; Macaya, F.; Gonzalo, N.; Escaned, J. Angiography-derived assessment of coronary microcirculatory resistance in patients with suspected myocardial ischaemia and non-obstructive coronary arteries. EuroIntervention 2023, 18, e1348–e1356. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Takahashi, T.; Rios, S.A.; Latib, A.; Lee, J.M.; Fearon, W.F.; Kobayashi, Y. Diagnostic performance and prognostic impact of coronary angiography-based Index of Microcirculatory Resistance assessment: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2022, 99, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Peregrina, E.; Garcia-Garcia, H.M.; Sans-Rosello, J.; Sanz-Sanchez, J.; Kotronias, R.; Scarsini, R.; Echavarria-Pinto, M.; Tebaldi, M.; De Maria, G.L. Angiography-derived versus invasively-determined index of microcirculatory resistance in the assessment of coronary microcirculation: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2022, 99, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjold, A.M.; Agewall, S.; Atar, D.; Baron, T.; Beltrame, J.; Bergstrom, O.; Erlinge, D.; Gale, C.P.; Lopez-Pais, J.; Jernberg, T.; et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am. Heart J. 2021, 231, 96–104. [Google Scholar] [CrossRef]
- Rodriguez, C., II; Perez-Aybar, A.E.; Roman-Ramos, J.A. MINOCA: A Working Diagnosis. Cureus 2023, 15, e49695. [Google Scholar] [CrossRef]
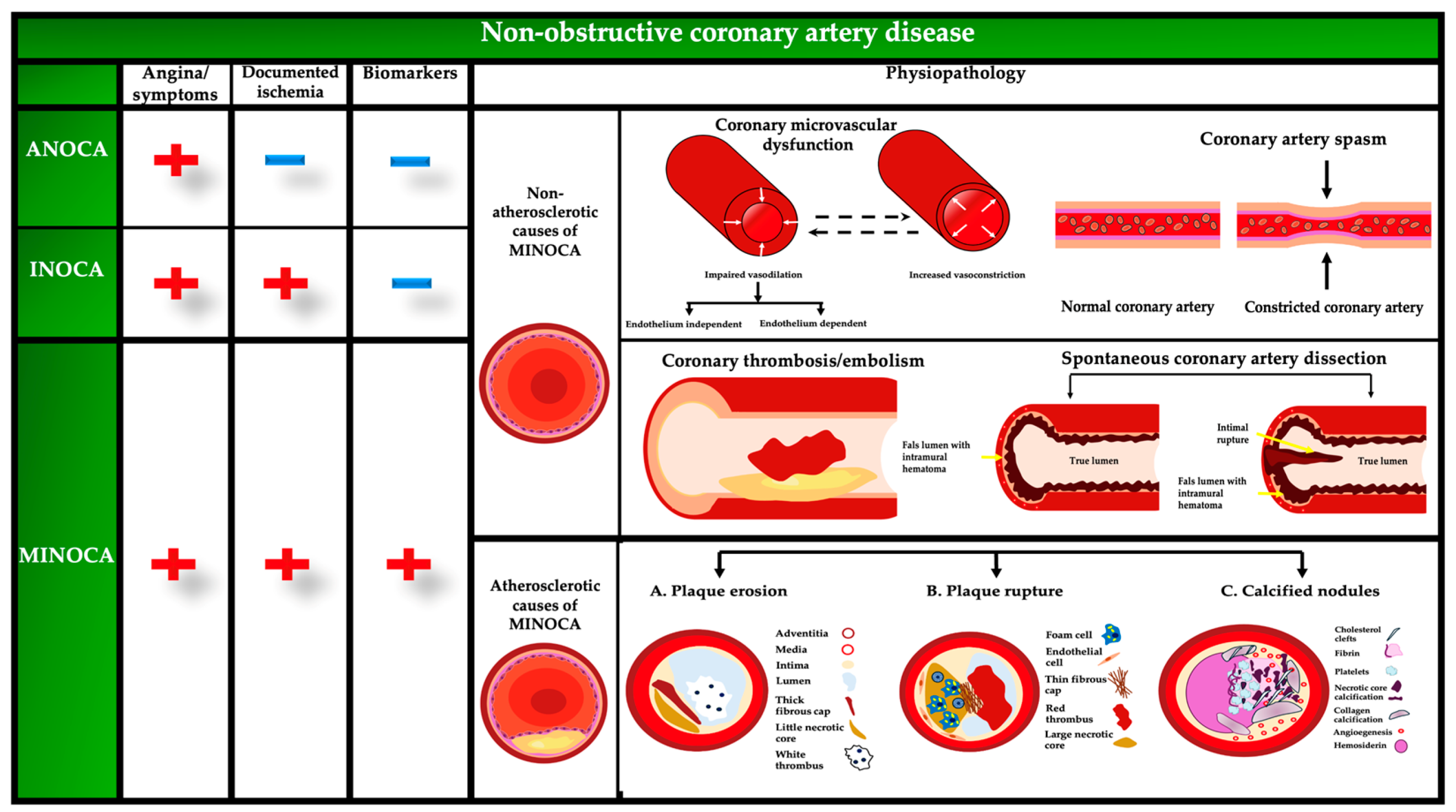

| Type | Mechanisms | Distribution of Mechanisms |
|---|---|---|
| ANOCA |
| |
| INOCA |
| |
| MINOCA |
|
| Type | Imaging Aspects |
|---|---|
| INOCA | |
| ANOCA | - |
| MINOCA |
|
| VSA | - |
| CMD |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudurachi, A.; Anghel, L.; Tudurachi, B.-S.; Zăvoi, A.; Ceasovschih, A.; Sascău, R.A.; Stătescu, C. Beyond the Obstructive Paradigm: Unveiling the Complex Landscape of Nonobstructive Coronary Artery Disease. J. Clin. Med. 2024, 13, 4613. https://doi.org/10.3390/jcm13164613
Tudurachi A, Anghel L, Tudurachi B-S, Zăvoi A, Ceasovschih A, Sascău RA, Stătescu C. Beyond the Obstructive Paradigm: Unveiling the Complex Landscape of Nonobstructive Coronary Artery Disease. Journal of Clinical Medicine. 2024; 13(16):4613. https://doi.org/10.3390/jcm13164613
Chicago/Turabian StyleTudurachi, Andreea, Larisa Anghel, Bogdan-Sorin Tudurachi, Alexandra Zăvoi, Alexandr Ceasovschih, Radu Andy Sascău, and Cristian Stătescu. 2024. "Beyond the Obstructive Paradigm: Unveiling the Complex Landscape of Nonobstructive Coronary Artery Disease" Journal of Clinical Medicine 13, no. 16: 4613. https://doi.org/10.3390/jcm13164613









