Circulating Cell-Free Mitochondrial DNA as a Novel Biomarker for Intra-Amniotic Infection in Obstetrics: A Pilot Trial
Abstract
1. Background
2. Materials and Methods
2.1. Sample Size Estimation
2.2. Sample Analysis
2.3. Statistical Analysis
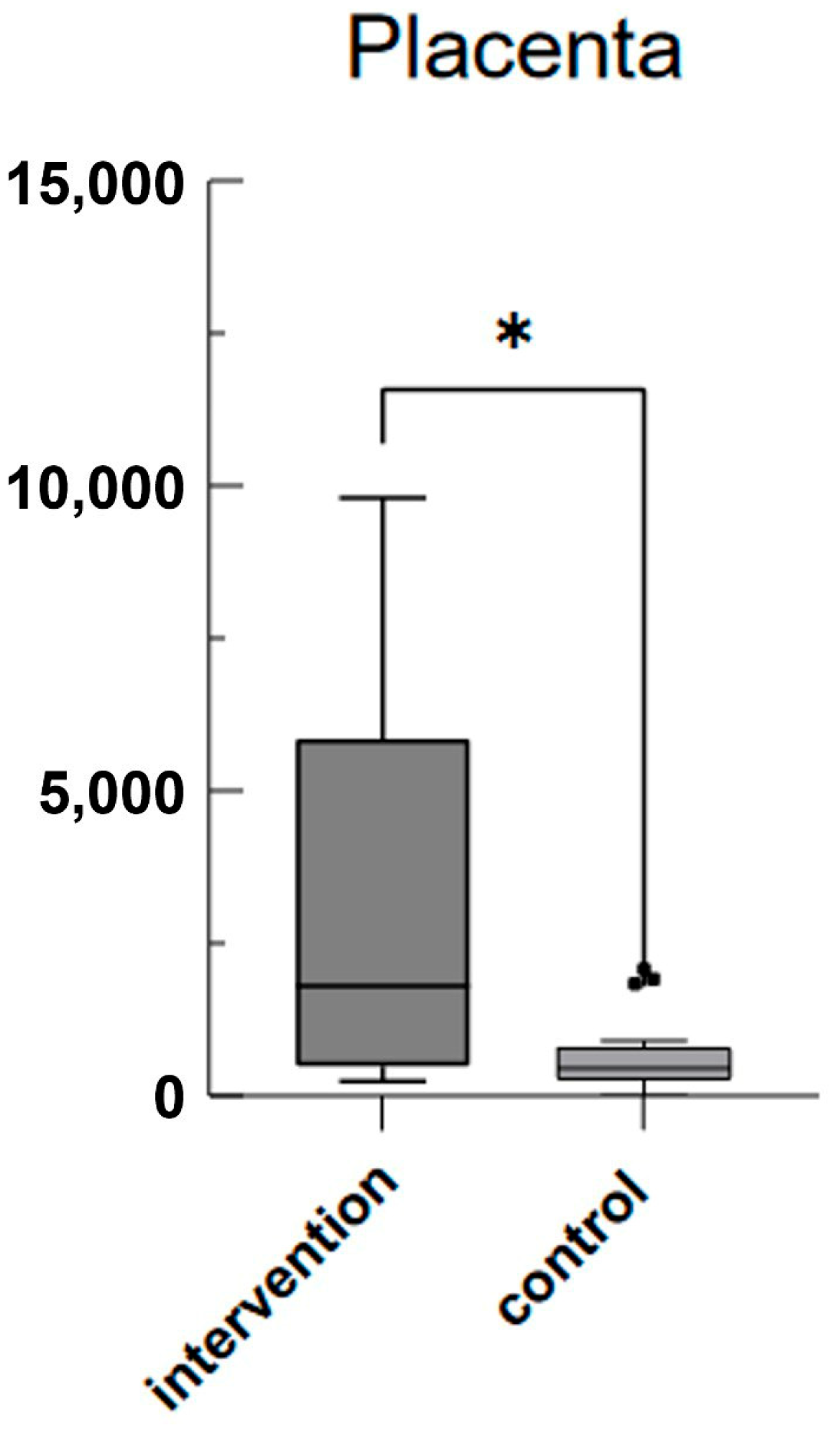
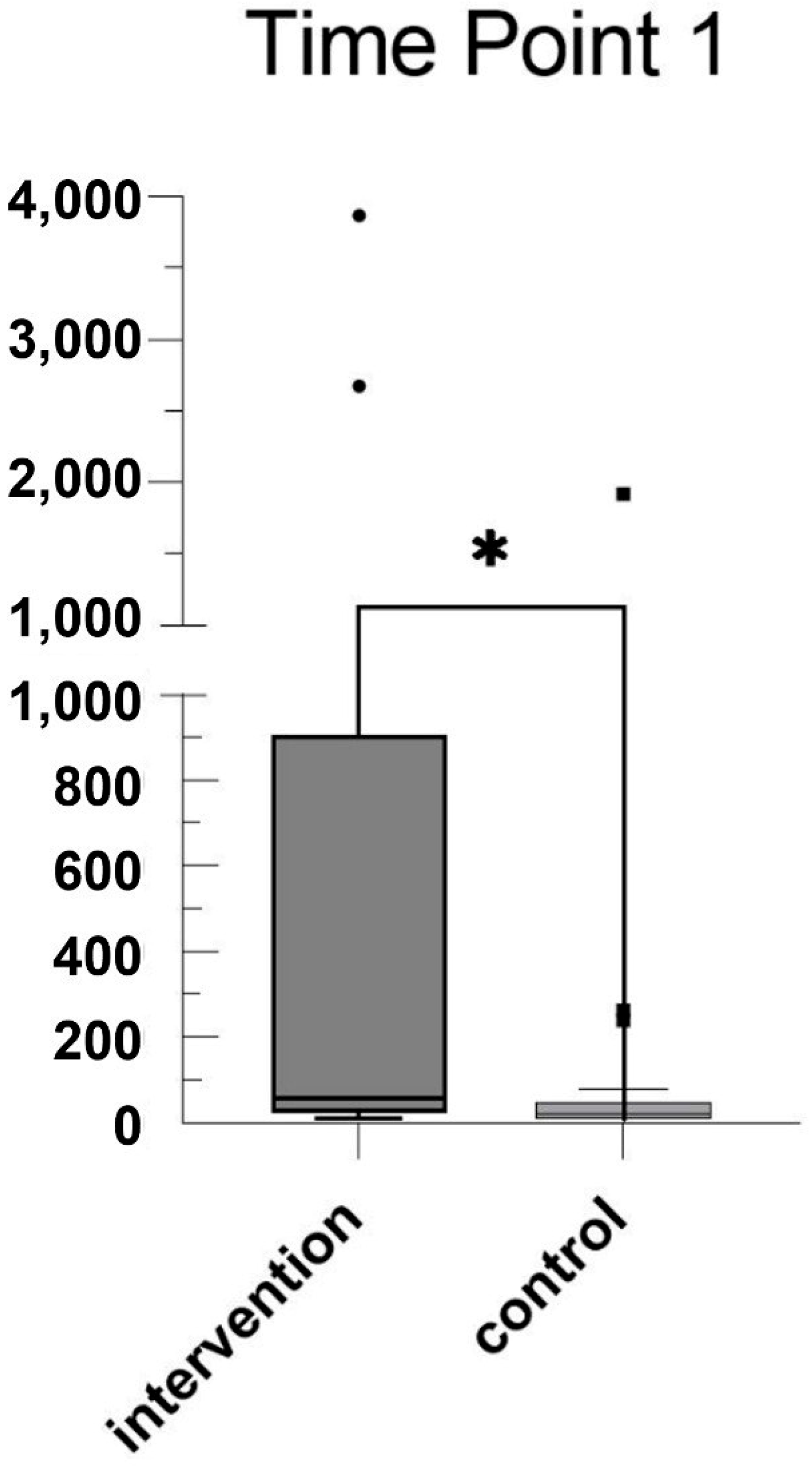
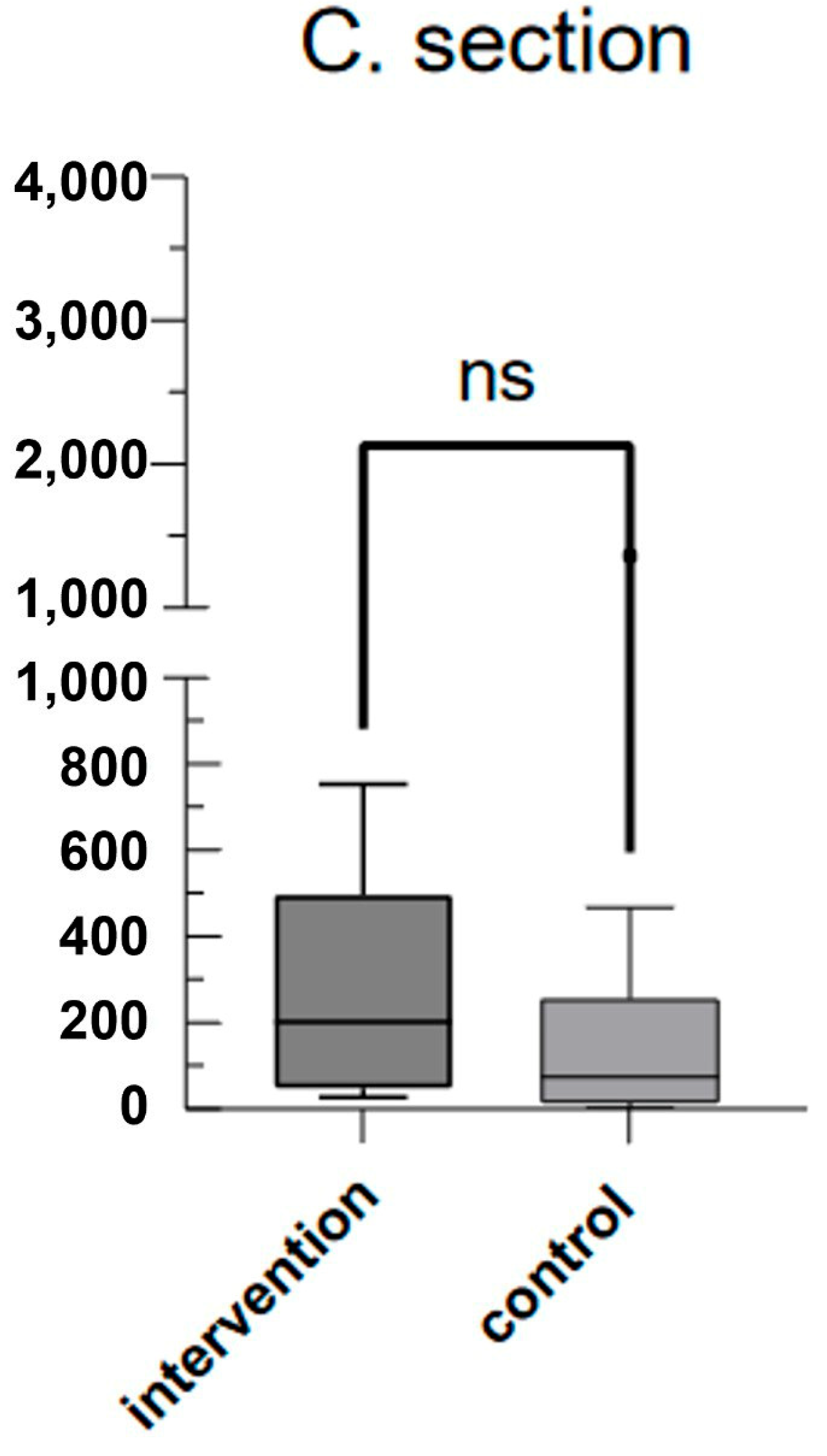
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, A.N.; Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 2013, 13, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Shahni, R.; Rodriguez-de-Ledesma, A.; Laftah, A.; Cunningham, P. Mitochondrial DNA as a non-invasive biomarker: Accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem. Biophys. Res. Commun. 2011, 412, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012, 4, a011403. [Google Scholar] [CrossRef] [PubMed]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Ruhittel, S.; Hohensinner, P.J.; Koller, L.; Kaun, C.; Lenz, M.; Bauer, B.; Wutzlhofer, L.; Draxler, D.F.; Maurer, G.; et al. Mitochondrial DNA and Toll-Like Receptor-9 Are Associated with Mortality in Critically Ill Patients. Crit. Care Med. 2015, 43, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, F.; Lattuada, D.; Garretto, A.; Massari, M.; Mandò, C.; Somigliana, E.; Cetin, I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am. J. Obstet. Gynecol. 2010, 203, e1–e365. [Google Scholar] [CrossRef] [PubMed]
- Cushen, S.C.; Sprouse, M.L.; Blessing, A.; Sun, J.; Jarvis, S.S.; Okada, Y.; Fu, Q.; Romero, S.A.; Phillips, N.R.; Goulopoulou, S. Cell-free mitochondrial DNA increases in maternal circulation during healthy pregnancy: A prospective, longitudinal study. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2020, 318, R445–R452. [Google Scholar] [CrossRef] [PubMed]
- Marschalek, J.; Wohlrab, P.; Ott, J.; Wojta, J.; Speidl, W.; Klein, K.U.; Kiss, H.; Pateisky, P.; Zeisler, H.; Kuessel, L. Maternal serum mitochondrial DNA (mtDNA) levels are elevated in preeclampsia—A matched case-control study. Pregnancy Hypertens 2018, 14, 195–199. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Cushen, S.C.; Phillips, N.R.; Goulopoulou, S. Circulating Cell-Free Mitochondrial DNA in Pregnancy. Physiology 2022, 37, 187–196. [Google Scholar] [CrossRef]
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns with a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice. Committee Opinion No. 712: Intrapartum Management of Intraamniotic Infection. Obstet. Gynecol. 2017, 130, e95–e101. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Bin Moon, J.; Shim, S.-S.; Kim, M.; Kim, G.; Jun, J.K. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Magee, F.; Qiu, Z.; Houbé, J.; Von Dadelszen, P.; Lee, S.K. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am. J. Obstet. Gynecol. 2005, 193, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, P.S.; Lieman, J.M.; Brumfield, C.G.; Carlo, W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2005, 192, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Rouse, D.J.; Landon, M.; Leveno, K.J.; Leindecker, S.; Varner, M.W.; Caritis, S.N.; O’Sullivan, M.J.; Wapner, R.J.; Meis, P.J.; Miodovnik, M.; et al. The maternal-fetal medicine units cesarean registry: Chorioamnionitis at term and its duration—Relationship to outcomes. Am. J. Obstet. Gynecol. 2004, 191, 211–216. [Google Scholar] [CrossRef]
- Hauth, J.C.; Gilstrap, L.C.; Hankins, G.D.; Connor, K.D. Term maternal and neonatal complications of acute chorioamnionitis. Obstet. Gynecol. 1985, 66, 59–62. [Google Scholar]
- Impey, L.; Greenwood, C.; MacQuillan, K.; Reynolds, M.; Sheil, O. Fever in labour and neonatal encephalopathy: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 594–597. [Google Scholar] [CrossRef]
- Helmo, F.R.; Alves, E.A.R.; Moreira, R.A.d.A.; Severino, V.O.; Rocha, L.P.; Monteiro, M.L.G.d.R.; dos Reis, M.A.; Etchebehere, R.M.; Machado, J.R.; Corrêa, R.R.M. Intrauterine infection, immune system and premature birth. J. Matern. -Fetal Neonatal Med. 2018, 31, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; García, J.A.; García-Corzo, L.; Ortiz, F.; Acuña-Castroviejo, D. Mitochondrial DNA and inflammatory diseases. Hum. Genet. 2012, 131, 161–173. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Clinical Practice Update: Update on Criteria for Suspected Diagnosis of Intraamniotic Infection. Obstet. Gynecol. 2024, 144, e17–e19. [Google Scholar] [CrossRef]
- Del Arroyo, A.; Sanchez, J.; Patel, S.; Phillips, S.; Reyes, A.; Cubillos, C.; Fernando, R.; David, A.; Reeve, A.; Sodha, S.; et al. Role of leucocyte caspase-1 activity in epidural-related maternal fever: A single-centre, observational, mechanistic cohort study. Br. J. Anaesth. 2019, 122, 92–102. [Google Scholar] [CrossRef] [PubMed]
| IAI n (%) or Mean ± SD | Control n (%) or Mean ± SD | p-Value | |
|---|---|---|---|
| Patients | 12 (100%) | 21 (100%) | ns |
| Maternal age (years) | 32.08 (5.47) | 32.14 (5.30) | ns |
| Maternal weight (kg) | 74.92 (14.85) | 79.72 (11.81) | ns |
| Maternal BMI | 27.44 (5.81) | 29.77 (4.55) | ns |
| Nicotine | - | - | |
| yes | 4 (19%) | ||
| no | 12 (100%) | 17(81%) | |
| Neonatal birth weight (grams) | 911.3 (231) | 3243 (491) | <0.0001 |
| GA at timepoint 1 (compl. weeks) | 24.75 (0.97) | 36.14 (0.48) | <0.0001 |
| GA at delivery (compl. weeks) | 26.25 (1.55) | 38.05 (0.50) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiner, S.; Wohlrab, P.; Rosicky, I.; Schukro, R.P.; Klein, K.U.; Wojta, J.; Speidl, W.; Kiss, H.; Muin, D.A. Circulating Cell-Free Mitochondrial DNA as a Novel Biomarker for Intra-Amniotic Infection in Obstetrics: A Pilot Trial. J. Clin. Med. 2024, 13, 4616. https://doi.org/10.3390/jcm13164616
Zeiner S, Wohlrab P, Rosicky I, Schukro RP, Klein KU, Wojta J, Speidl W, Kiss H, Muin DA. Circulating Cell-Free Mitochondrial DNA as a Novel Biomarker for Intra-Amniotic Infection in Obstetrics: A Pilot Trial. Journal of Clinical Medicine. 2024; 13(16):4616. https://doi.org/10.3390/jcm13164616
Chicago/Turabian StyleZeiner, Sebastian, Peter Wohlrab, Ingo Rosicky, Regina Patricia Schukro, Klaus Ulrich Klein, Johann Wojta, Walter Speidl, Herbert Kiss, and Dana Anaïs Muin. 2024. "Circulating Cell-Free Mitochondrial DNA as a Novel Biomarker for Intra-Amniotic Infection in Obstetrics: A Pilot Trial" Journal of Clinical Medicine 13, no. 16: 4616. https://doi.org/10.3390/jcm13164616
APA StyleZeiner, S., Wohlrab, P., Rosicky, I., Schukro, R. P., Klein, K. U., Wojta, J., Speidl, W., Kiss, H., & Muin, D. A. (2024). Circulating Cell-Free Mitochondrial DNA as a Novel Biomarker for Intra-Amniotic Infection in Obstetrics: A Pilot Trial. Journal of Clinical Medicine, 13(16), 4616. https://doi.org/10.3390/jcm13164616







