Multidisciplinary Approach in Atrial Fibrillation: As Good as Gold
Abstract
1. Introduction
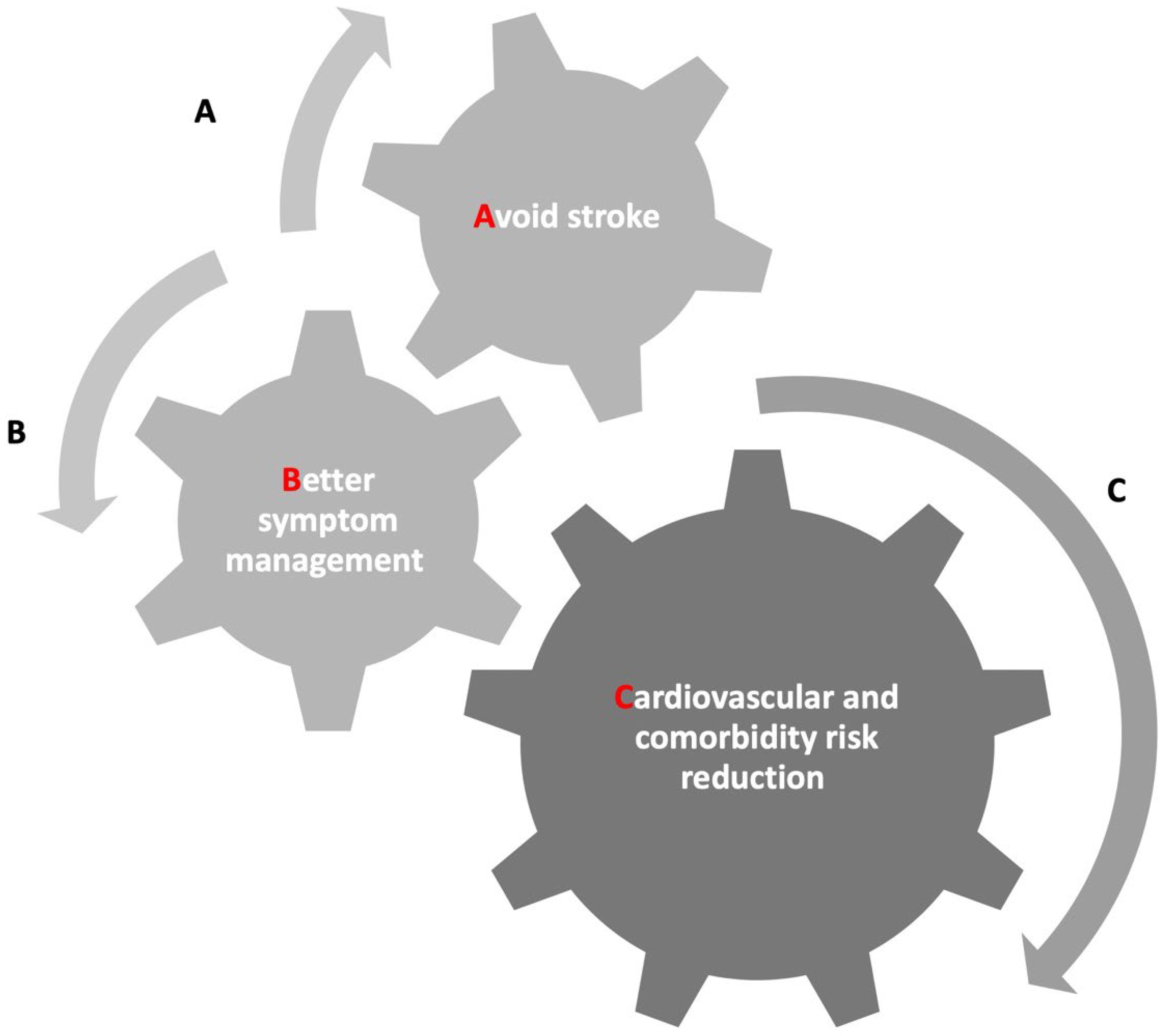
2. Multidisciplinary Assessment of AF
3. Stroke and Cognitive Impairments
4. Frailty and the Elderly
5. Heart Failure
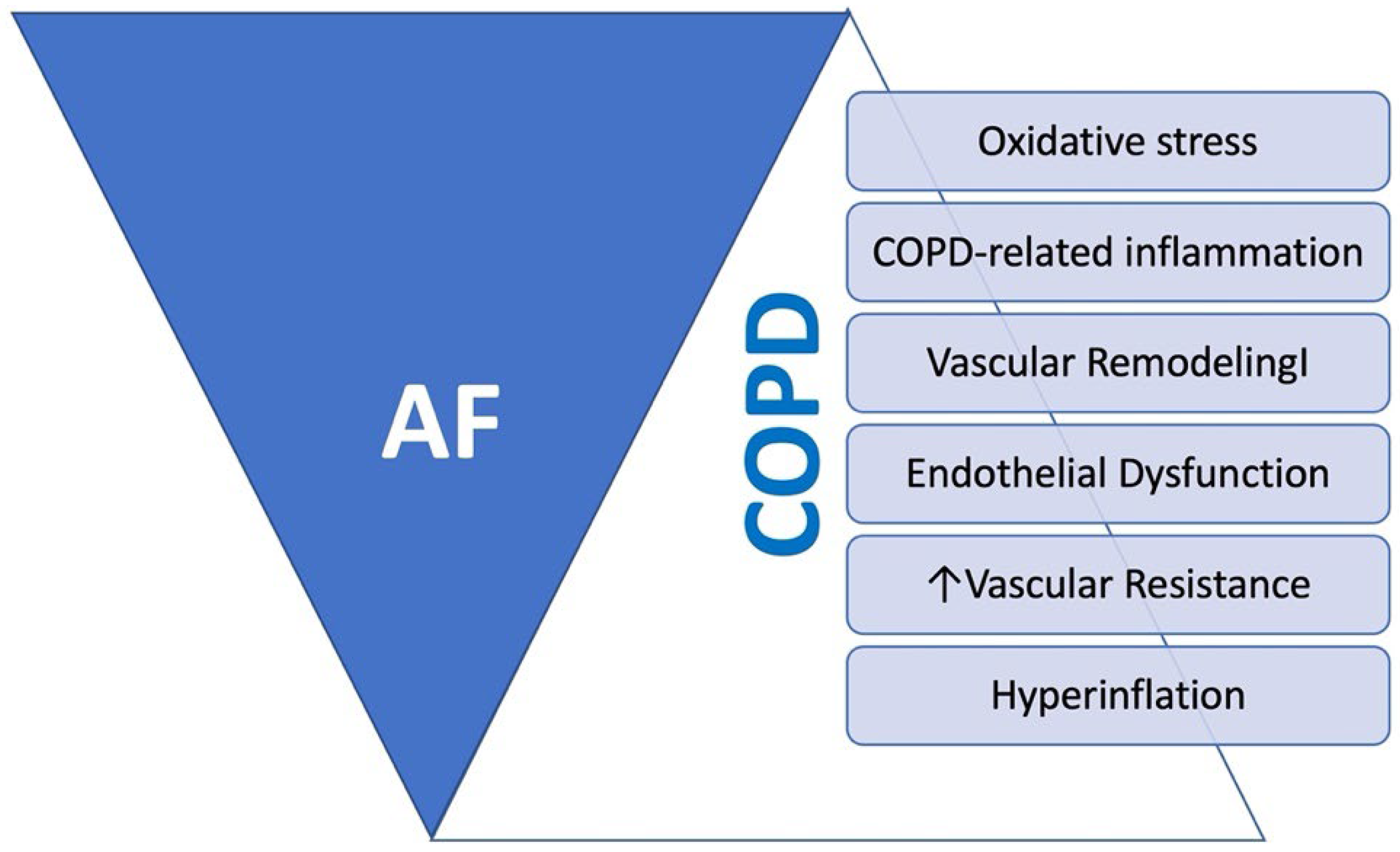
6. Chronic Obstructive Pulmonary Disease (COPD)
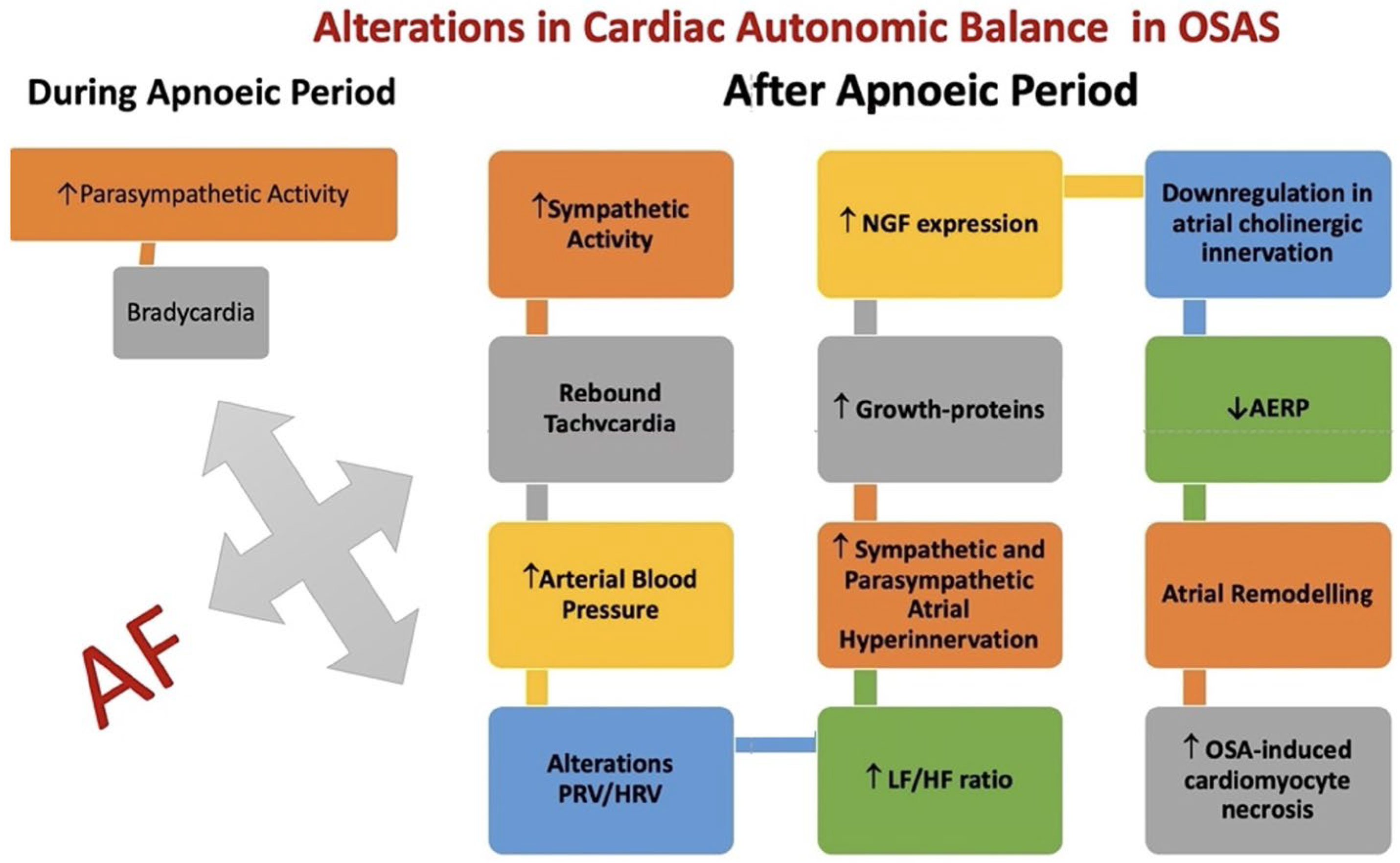
7. Obstructive Sleep Apnea (OSAS)
8. Chronic Liver Diseases
9. Chronic Kidney Disease (CKD)
10. Cancer and Hematological Disorders
11. Atrial Fibrillation in Pregnancy
12. Technological Tools and E-Health (Remote Monitoring and Wearable Tools)
Wearables
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steinberg, B.A.; Kim, S.; Fonarow, G.C.; Thomas, L.; Ansell, J.; Kowey, P.R.; Mahaffey, K.W.; Gersh, B.J.; Hylek, E.; Naccarelli, G.; et al. Drivers of hospitalization for patients with atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2014, 167, 735–742.e2. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Ammentorp, B.; Darius, H.; De Caterina, R.; Le Heuzey, J.Y.; Schilling, R.J.; Schmitt, J.; Zamorano, J.L. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: Primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014, 16, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Goodwin, N. Understanding Integrated Care. Int. J. Integr. Care 2016, 16, 6. [Google Scholar] [CrossRef]
- Bhat, A.; Khanna, S.; Chen, H.H.L.; Gupta, A.; Gan, G.C.H.; Denniss, A.R.; MacIntyre, C.R.; Tan, T.C. Integrated Care in Atrial Fibrillation: A Road Map to the Future. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e007411. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Ponsford, J.; Shelley, A.; Sirpal, A.; Lip, G.Y. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: Effects of an educational intervention programme. The West Birmingham Atrial Fibrillation Project. Int. J. Cardiol. 2006, 110, 354–358. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024, 26, euae043. [Google Scholar] [CrossRef]
- Guerra, J.M.; Moreno Weidmann, Z.; Perrotta, L.; Sultan, A.; Anic, A.; Metzner, A.; Providencia, R.; Boveda, S.; Chun, J. Current management of atrial fibrillation in routine practice according to the last ESC guidelines: An EHRA physician survey—How are we dealing with controversial approaches? EP Eur. 2024, 26, euae012. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.M.; Heidbüchel, H. The management of atrial fibrillation: An integrated team approach—Insights of the 2016 European Society of Cardiology guidelines for the management of atrial fibrillation for nurses and allied health professionals. Eur. J. Cardiovasc. Nurs. 2019, 18, 88–95. [Google Scholar] [CrossRef]
- Chyou, J.Y.; Barkoudah, E.; Dukes, J.W.; Goldstein, L.B.; Joglar, J.A.; Lee, A.M.; Lubitz, S.A.; Marill, K.A.; Sneed, K.B.; Streur, M.M.; et al. Atrial Fibrillation Occurring During Acute Hospitalization: A Scientific Statement from the American Heart Association. Circulation 2023, 147, e676–e698. [Google Scholar] [CrossRef]
- Romiti, G.F.; Proietti, M.; Bonini, N.; Ding, W.Y.; Boriani, G.; Huisman, M.V.; Lip, G.Y. Adherence to the Atrial Fibrillation Better Care (ABC) pathway and the risk of major outcomes in patients with atrial fibrillation: A post-hoc analysis from the prospective GLORIA-AF Registry. EClinicalMedicine 2023, 55, 101757. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Romiti, G.F.; Olshansky, B.; Lane, D.A.; Lip, G.Y.H. Improved Outcomes by Integrated Care of Anticoagulated Patients with Atrial Fibrillation Using the Simple ABC (Atrial Fibrillation Better Care) Pathway. Am. J. Med. 2018, 131, 1359–1366.e6. [Google Scholar] [CrossRef] [PubMed]
- Domek, M.; Gumprecht, J.; Li, Y.G.; Proietti, M.; Rashed, W.; Al Qudaimi, A.; Gumprecht, J.; Zubaid, M.; Lip, G.Y.H. Compliance of atrial fibrillation treatment with the ABC pathway in patients with concomitant diabetes mellitus in the Middle East based on the Gulf SAFE registry. Eur. J. Clin. Investig. 2021, 51, e13385. [Google Scholar] [CrossRef] [PubMed]
- Gumprecht, J.; Domek, M.; Proietti, M.; Li, Y.G.; Asaad, N.; Rashed, W.; Alsheikh-Ali, A.; Zubaid, M.; Lip, G.Y.H. Compliance of Atrial Fibrillation Treatment with the Atrial Fibrillation Better Care (ABC) Pathway Improves the Clinical Outcomes in the Middle East Population: A Report from the Gulf Survey of Atrial Fibrillation Events (SAFE) Registry. J. Clin. Med. 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.; Simovic, S.; Pavlovic, N.; Kocijancic, A.; Paparisto, V.; Music, L.; Trendafilova, E.; Dan, A.R.; Kusljugic, Z.; Dan, G.-A.; et al. Adherence to the ABC (Atrial fibrillation Better Care) pathway in the Balkan region: The BALKAN-AF survey. Pol. Arch. Int. Med. 2020, 130, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.S.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.H.; Lip, G.Y.H.; Joung, B. Application of the simple atrial fibrillation better care pathway for integrated care management in frail patients with atrial fibrillation: A nationwide cohort study. J. Arrhythm. 2020, 36, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lane, D.A.; Wang, L.; Zhang, H.; Wang, H.; Zhang, W.; Wen, J.; Xing, Y.; Wu, F.; Xia, Y.; et al. Mobile Health Technology to Improve Care for Patients with Atrial Fibrillation. J. Am. Coll Cardiol. 2020, 75, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Lip, G.Y.H.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.-A.; Kalarus, Z.; Tavazzi, L.; et al. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2021, 23, 174–183. [Google Scholar] [CrossRef]
- Lip, G.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef]
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Parrini, I.; Canale, M.L.; Giubilato, S.; Cornara, S.; Nesti, M.; Rao, C.M.; et al. Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. J. Clin. Med. 2023, 12, 5955. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.-S.; Guo, Y.; et al. Adherence to the ‘Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb. Haemost. 2022, 122, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Potpara, T.S.; Lip, G.Y.H. Atrial fibrillation: Stroke prevention. Lancet Reg. Health Eur. 2024, 37, 100797. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, S.; La Meir, M.; Lucà, F.; Lorusso, R.; Crudeli, E.; Vasquez, L.; Gensini, G.F.; Maessen, J. Treatment of lone atrial fibrillation: A look at the past, a view of the present and a glance at the future. Eur. J. Cardiothorac. Surg. 2012, 41, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Oliva, F.; Giubilato, S.; Abrignani, M.G.; Rao, C.M.; Cornara, S.; Caretta, G.; Di Fusco, S.A.; Ceravolo, R.; Parrini, I.; et al. Exploring the Perioperative Use of DOACs, off the Beaten Track. J. Clin. Med. 2024, 13, 3076. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Sullivan, R.M.; Olshansky, B.; Lip, G.Y.H. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: The SAMe-TT2R2 score. Chest 2013, 144, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar]
- Saksena, S.; Slee, A.; Waldo, A.L.; Freemantle, N.; Reynolds, M.; Rosenberg, Y.; Rathod, S.; Grant, S.; Thomas, E.; Wyse, D.G. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J. Am. Coll. Cardiol. 2011, 58, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Hagens, V.E.; Ranchor, A.V.; Van Sonderen, E.; Bosker, H.A.; Kamp, O.; Tijssen, J.G.; Kingma, J.; Crijns, H.J.; Van Gelder, I.C. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J. Am. Coll. Cardiol. 2004, 43, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Nesti, M.; Lucà, F.; Duncker, D.; De Sensi, F.; Malaczynska-Rajpold, K.; Behar, J.M.; Waldmann, V.; Ammar, A.; Mirizzi, G.; Garcia, R.; et al. Antiplatelet and Anti-Coagulation Therapy for Left-Sided Catheter Ablations: What Is beyond Atrial Fibrillation? J. Clin. Med. 2023, 12, 6183. [Google Scholar] [CrossRef] [PubMed]
- Rolf, S.; Kornej, J.; Dagres, N.; Hindricks, G. What can rhythm control therapy contribute to prognosis in atrial fibrillation? Heart 2015, 101, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.S.; You, S.C.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: Nationwide cohort study. BMJ 2021, 373, n991. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Mantovan, R.; Macle, L.; De Martino, G.; Chen, J.; Morillo, C.A.; Novak, P.; Calzolari, V.; Guerra, P.G.; Nair, G.; et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): A randomized, multicentre, international trial. Eur. Heart J. 2010, 31, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation. J. Am. Coll. Cardiol. 2024, 83, 109–279. [Google Scholar]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.R.; Neuzil, P.; Kautzner, J.; Petru, J.; Wichterle, D.; Skoda, J.; Cihak, R.; Peichl, P.; Russo, A.D.; Pelargonio, G.; et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: Multicenter results of pulmonary vein remapping studies. Heart Rhythm 2012, 9, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Isakadze, N.; Spragg, D. First-Line Ablation for Persistent AF. JACC: Clin. Electrophysiol. 2024, 10, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Nesti, M.; Luca, F.; Panchetti, L.; Garibaldi, S.; Startari, U.; Mirizzi, G.; Landra, F.; Giannoni, A.; Piacenti, M.; Rossi, A. Impact of Vein of Marshall Ethanol Infusion Combined with Anatomical Ablation for the Treatment of Persistent Atrial Fibrillation: A Long-Term Follow-Up Based on Implantable Loop Recorders. J. Clin. Med. 2023, 12, 6916. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Moretz, K.; Poole, J.E.; Mascette, A.; Rosenberg, Y.; Jeffries, N.; et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am. Heart J. 2018, 199, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Thrall, G.; Lip, G.Y.; Carroll, D.; Lane, D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest 2007, 132, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; Elliott, A.D.; Wong, C.X.; Rangnekar, G.; Middeldorp, M.E.; Mahajan, R.; Lau, D.H.; Sanders, P.; Hendriks, J.M.L. Integrated care in atrial fibrillation: A systematic review and meta-analysis. Heart 2017, 103, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
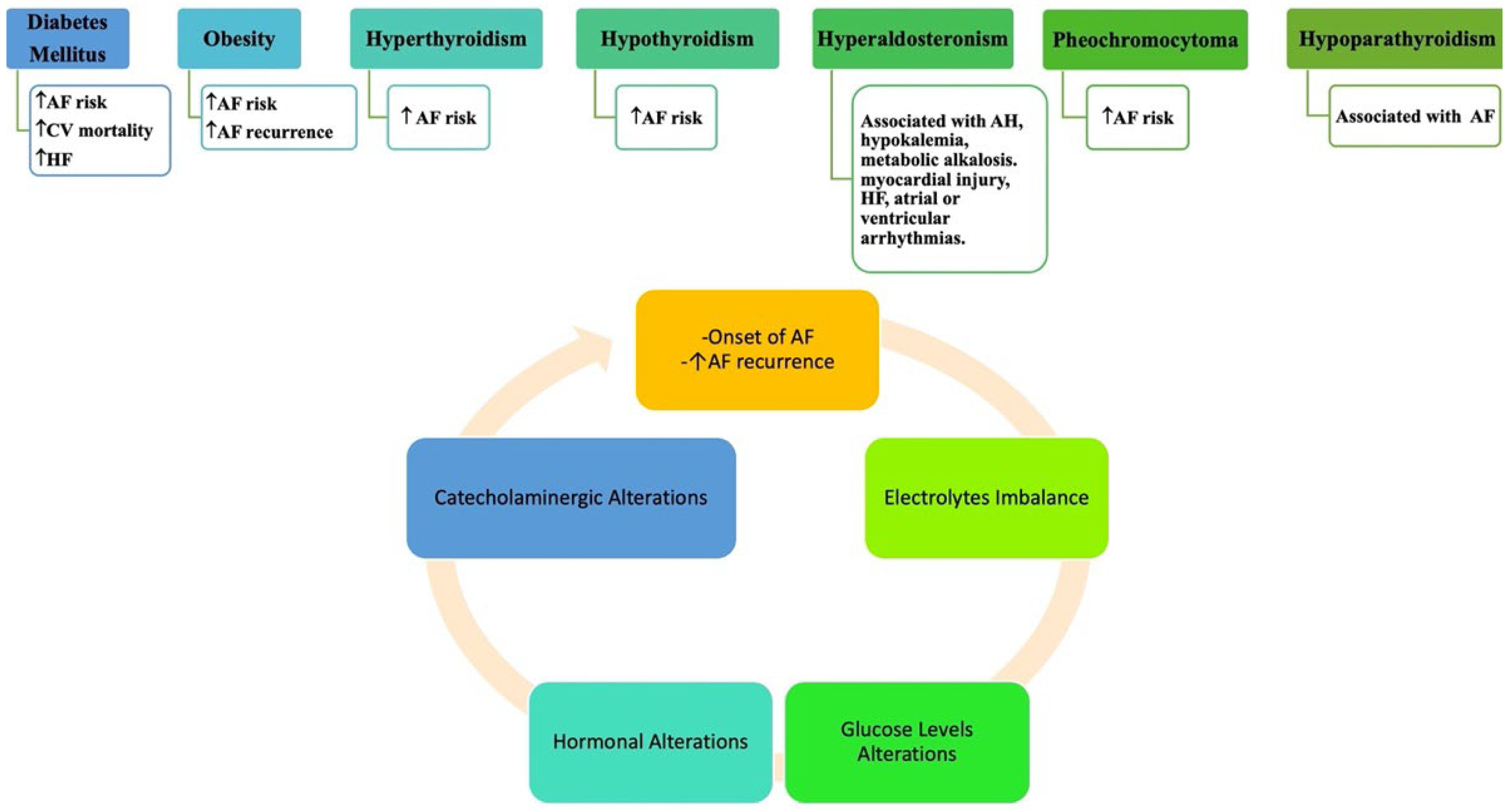
- Takawale, A.; Aguilar, M.; Bouchrit, Y.; Hiram, R. Mechanisms and Management of Thyroid Disease and Atrial Fibrillation: Impact of Atrial Electrical Remodeling and Cardiac Fibrosis. Cells 2022, 11, 4047. [Google Scholar] [CrossRef] [PubMed]
- Gorenek, B.; Boriani, G.; Dan, G.A.; Fauchier, L.; Fenelon, G.; Huang, H.; Kudaiberdieva, G.; Lip, G.Y.H.; Mahajan, R.; Potpara, T.; et al. European Heart Rhythm Association (EHRA) position paper on arrhythmia management and device therapies in endocrine disorders, endorsed by Asia Pacific Heart Rhythm Society (APHRS) and Latin American Heart Rhythm Society (LAHRS). Europace 2018, 20, 895–896. [Google Scholar] [CrossRef]
- Yue, X.; Zhou, L.; Li, Y.; Zhao, C. Multidisciplinary management strategies for atrial fibrillation. Curr. Probl. Cardiol. 2024, 49, 102514. [Google Scholar] [CrossRef]
- Proietti, M.; Romiti, G.F.; Olshansky, B.; Lane, D.A.; Lip, G.Y.H. Comprehensive Management with the ABC (Atrial Fibrillation Better Care) Pathway in Clinically Complex Patients with Atrial Fibrillation: A Post Hoc Ancillary Analysis from the AFFIRM Trial. J. Am. Heart Assoc. 2020, 9, e014932. [Google Scholar] [CrossRef] [PubMed]
- Qvist, I.; Hendriks, J.M.; Møller, D.S.; Albertsen, A.E.; Mogensen, H.M.; Oddershede, G.D.; Odgaard, A.; Mortensen, L.S.; Johnsen, S.P.; Frost, L. Effectiveness of structured, hospital-based, nurse-led atrial fibrillation clinics: A comparison between a real-world population and a clinical trial population. Open Heart 2016, 3, e000335. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.M.; de Wit, R.; Crijns, H.J.; Vrijhoef, H.J.; Prins, M.H.; Pisters, R.; Pison, L.A.; Blaauw, Y.; Tieleman, R.G. Nurse-led care vs. usual care for patients with atrial fibrillation: Results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur. Heart J. 2012, 33, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Wijtvliet, E.; Tieleman, R.G.; van Gelder, I.C.; Pluymaekers, N.; Rienstra, M.; Folkeringa, R.J.; Bronzwaer, P.; Elvan, A.; Elders, J.; Tukkie, R.; et al. Nurse-led vs. usual-care for atrial fibrillation. Eur. Heart J. 2020, 41, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Choi, E.K.; Lane, D.A.; Joung, B.; Lip, G.Y.H. Atrial fibrillation: Comorbidities, lifestyle, and patient factors. Lancet Reg. Health Eur. 2024, 37, 100784. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.J.; DeVon, H.A. Home-based management of patients with atrial fibrillation. Lancet 2015, 385, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Du, Y.X.; Wu, F.Q.; Lu, X.Y.; Chen, R.M.; Zhang, Y. Effects of nurse-led multidisciplinary team management on cardiovascular hospitalization and quality of life in patients with atrial fibrillation: Randomized controlled trial. Int. J. Nurs. Stud. 2022, 127, 104159. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, R.F.; Heeringa, J.; Wolters, F.J.; Franco, O.H.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Association Between Atrial Fibrillation and Dementia in the General Population. JAMA Neurol. 2015, 72, 1288–1294. [Google Scholar] [CrossRef]
- Kwok, C.S.; Loke, Y.K.; Hale, R.; Potter, J.F.; Myint, P.K. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology 2011, 76, 914–922. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Bai, R.; Mohanty, S.; Pump, A.; Cereceda Brantes, M.; Horton, R.; Burkhardt, J.D.; Lakkireddy, D.; Reddy, Y.M.; et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm 2012, 9, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Agarwal, S.K.; Norby, F.L.; Gottesman, R.F.; Loehr, L.R.; Soliman, E.Z.; Mosley, T.H.; Folsom, A.R.; Coresh, J.; Alonso, A. Persistent but not Paroxysmal Atrial Fibrillation Is Independently Associated with Lower Cognitive Function: ARIC Study. J. Am. Coll. Cardiol. 2016, 67, 1379–1380. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.J.; Soliman, E.Z.; McClure, L.A.; Howard, G.; Howard, V.J.; Judd, S.E.; Unverzagt, F.W.; Wadley, V.; Sachs, B.C.; Hughes, T.M. Relation of Atrial Fibrillation to Cognitive Decline (from the REasons for Geographic and Racial Differences in Stroke [REGARDS] Study). Am. J. Cardiol. 2021, 148, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.X.; Ang, E.; Lim, X.T.; Arain, S.J. Association of Risk of Dementia with Direct Oral Anticoagulants Versus Warfarin Use in Patients with Non-valvular Atrial Fibrillation: A Systematic Review and Meta-analysis. J. Cardiovasc. Pharmacol. 2021, 77, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Rodondi, N.; Mueller, A.; Beer, J.; Auricchio, A.; Ammann, P.; Hayoz, D.; Kobza, R.; Moschovitis, G.; Shah, D.; et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF): Structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med. Wkly. 2017, 147, w14467. [Google Scholar] [PubMed]
- Graff-Radford, J.; Madhavan, M.; Vemuri, P.; Rabinstein, A.A.; Cha, R.H.; Mielke, M.M.; Kantarci, K.; Lowe, V.; Senjem, M.L.; Gunter, J.L.; et al. Atrial fibrillation, cognitive impairment, and neuroimaging. Alzheimers Dement. 2016, 12, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Lopez, F.L.; Gottesman, R.F.; Huxley, R.R.; Agarwal, S.K.; Loehr, L.; Mosley, T.; Alonso, A. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: The atherosclerosis risk in communities study. Stroke 2014, 45, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Gardarsdottir, M.; Sigurdsson, S.; Aspelund, T.; Rokita, H.; Launer, L.J.; Gudnason, V.; Arnar, D.O. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2018, 20, 1252–1258. [Google Scholar] [CrossRef]
- Madhavan, M.; Hu, T.Y.; Gersh, B.J.; Roger, V.L.; Killian, J.; Weston, S.A.; Graff-Radford, J.; Asirvatham, S.J.; Chamberlain, A.M. Efficacy of Warfarin Anticoagulation and Incident Dementia in a Community-Based Cohort of Atrial Fibrillation. Mayo Clin. Proc. 2018, 93, 145–154. [Google Scholar] [CrossRef]
- Charidimou, A.; Kakar, P.; Fox, Z.; Werring, D.J. Cerebral microbleeds and recurrent stroke risk: Systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 2013, 44, 995–1001. [Google Scholar] [CrossRef]
- Bokura, H.; Saika, R.; Yamaguchi, T.; Nagai, A.; Oguro, H.; Kobayashi, S.; Yamaguchi, S. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke 2011, 42, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Thijs, V.; Lemmens, R.; Schoofs, C.; Görner, A.; Van Damme, P.; Schrooten, M.; Demaerel, P. Microbleeds and the risk of recurrent stroke. Stroke 2010, 41, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Colivicchi, F.; Oliva, F.; Abrignani, M.; Caretta, G.; Di Fusco, S.A.; Giubilato, S.; Cornara, S.; Di Nora, C.; Pozzi, A.; et al. Management of oral anticoagulant therapy after intracranial hemorrhage in patients with atrial fibrillation. Front. Cardiovasc. Med. 2023, 10, 1061618. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Kim, J.; Lee, H.; Nam, C.; Nam, H.; Heo, J.; Kim, Y.D. The frequency of cerebral microbleeds increases with CHADS 2 scores in stroke patients with non-valvular atrial fibrillation. Eur. J. Neurol. 2013, 20, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.; Planchard, R.; Zubair, A.; Graff-Radford, J.; Rydberg, C.; Brown, R.D.; Flemming, K.D. The clinical relevance of cerebral microbleeds in patients with cerebral ischemia and atrial fibrillation. J. Neurol. 2016, 263, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Ryu, S.Y.; Shim, Y.S.; Yang, D.W.; Cho, A.H. Coexistence of Cerebral Microbleeds and Amyloid Pathology in Patients with Cognitive Complaints. J. Clin. Neurol. 2020, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Greer, D.M. Clinical associations of cerebral microbleeds on magnetic resonance neuroimaging. J. Stroke Cerebrovasc. Dis. 2014, 23, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.R.; Preis, S.R.; Beiser, A.; DeCarli, C.; Viswanathan, A.; Martinez-Ramirez, S.; Kase, C.S.; Wolf, P.A.; Seshadri, S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 2014, 45, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Poels, M.M.; Vernooij, M.W.; Ikram, M.A.; Hofman, A.; Krestin, G.P.; van der Lugt, A.; Breteler, M.M.B. Prevalence and risk factors of cerebral microbleeds: An update of the Rotterdam scan study. Stroke 2010, 41 (Suppl. S10), S103–S106. [Google Scholar] [CrossRef]
- Van Es, A.; Van Der Grond, J.; De Craen, A.; Westendorp, R.; Bollen, E.; Blauw, G.; Greenberg, S.; van Buchem, M.; For the PROSPER Study Group. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology 2011, 77, 1446–1452. [Google Scholar] [CrossRef]
- Karayiannis, C.; Soufan, C.; Chandra, R.V.; Phan, T.G.; Wong, K.; Singhal, S.; Slater, L.-A.; Ly, J.; Moran, C.; Srikanth, V. Prevalence of brain MRI markers of hemorrhagic risk in patients with stroke and atrial fibrillation. Front. Neurol. 2016, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Soo, Y.O.; Yang, S.R.; Lam, W.W.; Wong, A.; Fan, Y.H.; Leung, H.H.; Chan, A.Y.Y.; Leung, C.; Leung, T.W.H.; Wong, L.K.S. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J. Neurol. 2008, 255, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, E.S.; Auriel, E.; Haley, K.E.; Ayres, A.M.; Vashkevich, A.; Schwab, K.M.; Rosand, J.; Viswanathan, A.; Greenberg, S.M.; Gurol, M.E. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014, 45, 2280–2285. [Google Scholar] [CrossRef]
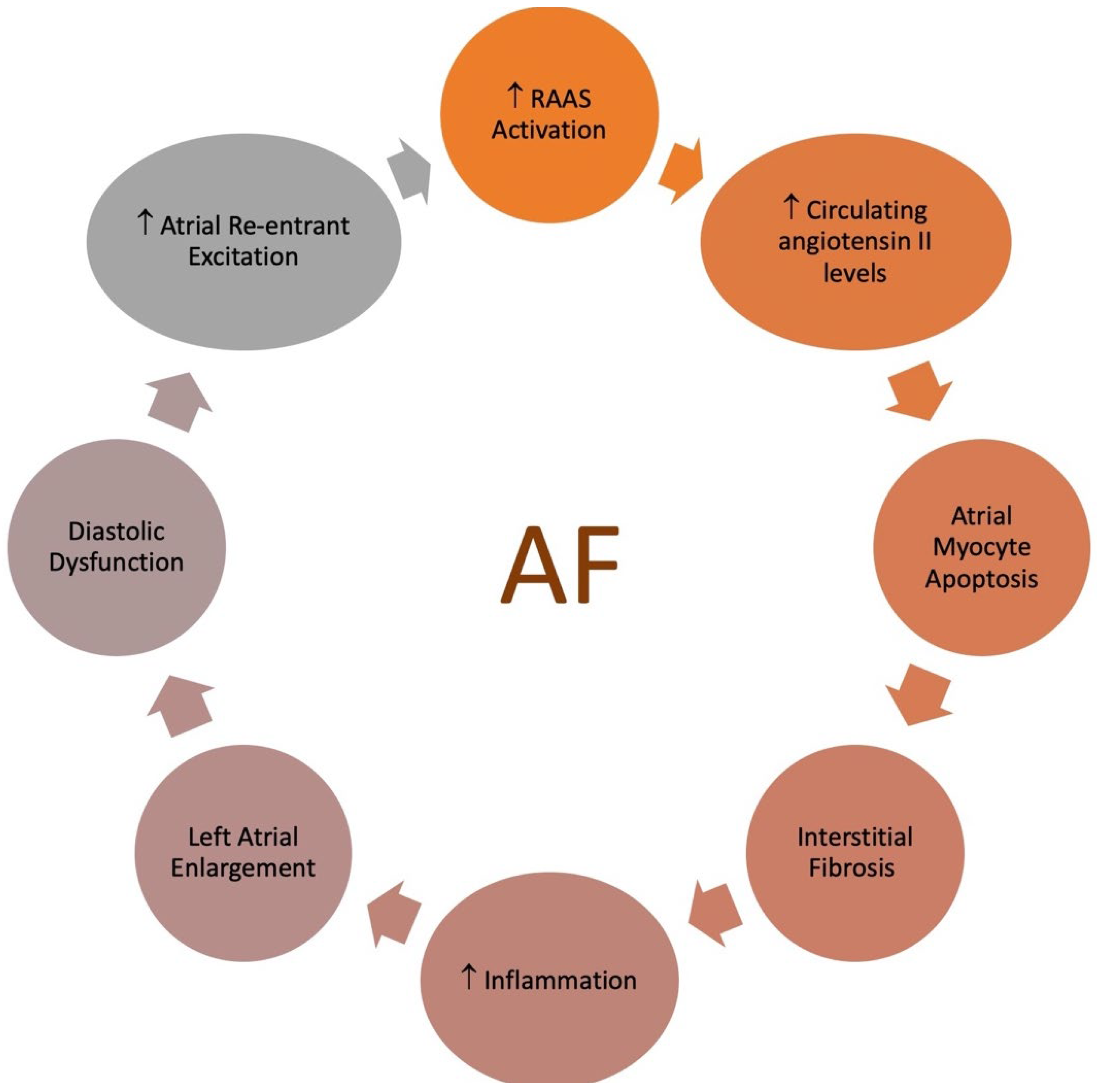
- Conway, D.S.; Lip, G.Y. Inflammation, arrhythmia burden and the thrombotic consequences of atrial fibrillation. Eur. Heart J. 2004, 25, 1761. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Miranda, C.M.; Liu, T.; Tse, G.; Roever, L. Atrial Fibrillation and Risk of Dementia: Epidemiology, Mechanisms, and Effect of Anticoagulation. Front. Neurosci. 2019, 13, 18. [Google Scholar] [CrossRef]
- Lappegård, K.T.; Pop-Purceleanu, M.; van Heerde, W.; Sexton, J.; Tendolkar, I.; Pop, G. Improved neurocognitive functions correlate with reduced inflammatory burden in atrial fibrillation patients treated with intensive cholesterol lowering therapy. J. Neuroinflamm. 2013, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Rollo, J.; Knight, S.; May, H.T.; Anderson, J.L.; Muhlestein, J.B.; Bunch, T.J.; Carlquist, J. Incidence of dementia in relation to genetic variants at PITX2, ZFHX3, and ApoE ε4 in atrial fibrillation patients. Pacing Clin. Electrophysiol. 2015, 38, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dublin, S.; Anderson, M.L.; Heckbert, S.R.; Hubbard, R.A.; Sonnen, J.A.; Crane, P.K.; Montine, T.J.; Larson, E.B. Neuropathologic changes associated with atrial fibrillation in a population-based autopsy cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 609–615. [Google Scholar] [CrossRef]
- Mavaddat, N.; Roalfe, A.; Fletcher, K.; Lip, G.Y.; Hobbs, F.D.; Fitzmaurice, D.; Mant, J.; Harrison, J.L.; Sohns, C.; Linton, N.W.; et al. Warfarin versus aspirin for prevention of cognitive decline in atrial fibrillation: Randomized controlled trial (Birmingham Atrial Fibrillation Treatment of the Aged Study). Stroke 2014, 45, 1381–1386. [Google Scholar] [CrossRef]
- Park, H.; Hildreth, A.; Thomson, R.; O‘Connell, J. Non-valvular atrial fibrillation and cognitive decline: A longitudinal cohort study. Age Ageing 2007, 36, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, P.; Lane, D.A.; Park, H.; O’Connell, J.; Quinn, T.J. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: Systematic review. Age Ageing 2016, 45, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Han, W.; Zhong, J.; Wu, L. A systematic review and meta-analysis to determine the effect of oral anticoagulants on incidence of dementia in patients with atrial fibrillation. Int. J. Clin. Pract. 2021, 75, e14269. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Liu, P.P.; Liu, A.B.; Lin, S.M.; Huang, H.K.; Loh, C.H. Lower Risk of Dementia in Patients with Atrial Fibrillation Taking Non-Vitamin K Antagonist Oral Anticoagulants: A Nationwide Population-Based Cohort Study. J. Am. Heart Assoc. 2021, 10, e016437. [Google Scholar] [CrossRef] [PubMed]
- Bezabhe, W.M.; Bereznicki, L.R.; Radford, J.; Wimmer, B.C.; Salahudeen, M.S.; Garrahy, E.; Bindoff, I.; Peterson, G.M. Oral Anticoagulant Treatment and the Risk of Dementia in Patients with Atrial Fibrillation: A Population-Based Cohort Study. J. Am. Heart Assoc. 2022, 11, e023098. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.L.; Hsieh, S.W.; Chou, P.S.; Yang, Y.H. Effects of Dabigatran on Dementia Pathogenesis and Neuropsychological Function: A Review. J. Alzheimers Dis. 2022, 86, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Saglietto, A.; Ballatore, A.; Xhakupi, H.; De Ferrari, G.M.; Anselmino, M. Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies. J. Cardiovasc. Dev. Dis. 2022, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef]
- Chugh, S.S.; Blackshear, J.L.; Shen, W.K.; Hammill, S.C.; Gersh, B.J. Epidemiology and natural history of atrial fibrillation: Clinical implications. J. Am. Coll. Cardiol. 2001, 37, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, W.M.; Blackshear, J.L.; Laupacis, A.; Kronmal, R.; Hart, R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Int. Med. 1995, 155, 469–473. [Google Scholar] [CrossRef]
- Lane, D.A.; Skjøth, F.; Lip, G.Y.H.; Larsen, T.B.; Kotecha, D. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J. Am. Heart Assoc. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Marinigh, R.; Lip, G.Y.; Fiotti, N.; Giansante, C.; Lane, D.A. Age as a risk factor for stroke in atrial fibrillation patients: Implications for thromboprophylaxis. J. Am. Coll. Cardiol. 2010, 56, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Du, X.; Ma, C.S. Atrial fibrillation and frailty. J. Geriatr. Cardiol. 2020, 17, 105–109. [Google Scholar] [PubMed]
- Dalleur, O.; Maes, F.; Henrard, S.; Wouters, D.; Scavée, C.; Spinewine, A.; Boland, B. Risk factors for underuse of anticoagulation in frail elderly patients with atrial fibrillation. Int. J. Clin. Pharm. 2012, 35, 10. [Google Scholar]
- He, L.; He, R.; Huang, J.; Zou, C.; Fan, Y. Impact of frailty on all-cause mortality and major bleeding in patients with atrial fibrillation: A meta-analysis. Ageing Res. Rev. 2022, 73, 101527. [Google Scholar] [CrossRef] [PubMed]
- Induruwa, I.; Evans, N.R.; Aziz, A.; Reddy, S.; Khadjooi, K.; Romero-Ortuno, R. Clinical frailty is independently associated with non-prescription of anticoagulants in older patients with atrial fibrillation. Geriatr. Gerontol. Int. 2017, 17, 2178–2183. [Google Scholar] [CrossRef]
- Perera, V.; Bajorek, B.V.; Matthews, S.; Hilmer, S.N. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 2009, 38, 156–162. [Google Scholar] [CrossRef]
- Wilkinson, C.; Todd, O.; Clegg, A.; Gale, C.P.; Hall, M. Management of atrial fibrillation for older people with frailty: A systematic review and meta-analysis. Age Ageing 2019, 48, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zheng, Y.; Jiang, J.; Ma, J.; Zhu, W.; Cai, X. Effectiveness and Safety of DOACs vs. Warfarin in Patients with Atrial Fibrillation and Frailty: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 907197. [Google Scholar] [CrossRef] [PubMed]
- Sardar, P.; Chatterjee, S.; Chaudhari, S.; Lip, G.Y. New oral anticoagulants in elderly adults: Evidence from a meta-analysis of randomized trials. J. Am. Geriatr. Soc. 2014, 62, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guo, S.D.; Deng, H.; Shantsila, A.; Fauchier, L.; Ma, C.S.; Lip, G.Y.H. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: A systematic review and meta-regression analysis. Age Ageing 2018, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Lucerna, M.; Pecen, L.; Siller-Matula, J.M.; Cavallari, I.; Kirchhof, P.; De Caterina, R. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients with Atrial Fibrillation: A Sub-Analysis from the PREFER in AF (PREvention oF Thromboembolic Events-European Registry in Atrial Fibrillation). J. Am. Heart Assoc. 2017, 6, e005657. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Cesari, M. Describing the relationship between atrial fibrillation and frailty: Clinical implications and open research questions. Exp. Gerontol. 2021, 152, 111455. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Cornelius, V.R.; Patel, J.P.; Davies, J.G.; Molokhia, M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation 2015, 132, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Aisenberg, J.; Ansell, J.; Atar, D.; Breithardt, G.; Eikelboom, J.; Ezekowitz, M.D.; Granger, C.B.; Halperin, J.L.; Hohnloser, S.H.; et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: Part 2. Eur. Heart J. 2017, 38, 860–868. [Google Scholar] [PubMed]
- Lucà, F.; Giubilato, S.; Di Fusco, S.A.; Leone, A.; Poli, S.; Rao, C.M.; Iorio, A.; Gelsomino, S.; Gabrielli, D.; Colivicchi, F.; et al. The Combination of Oral Anticoagulant and Antiplatelet Therapies: Stay One Step Ahead. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 391–398. [Google Scholar] [CrossRef]
- Kim, D.H.; Pawar, A.; Gagne, J.J.; Bessette, L.G.; Lee, H.; Glynn, R.J.; Schneeweiss, S. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: A cohort study. Ann. Intern. Med. 2021, 174, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.P.T.; van Doorn, S.; van de Ven, P.M.; Köhlen, B.T.G.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching from a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients with Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2024, 149, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.K.; Sood, N.A.; Bunz, T.J.; Coleman, C.I. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients with Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e008643. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.P.; Brouwer, M.A.; Mulder, H.; Vinereanu, D.; Lopes, R.D.; Proietti, M.; Al-Khatib, S.M.; Hijazi, Z.; Halvorsen, S.; Hylek, E.M.; et al. Outcomes of apixaban versus warfarin in patients with atrial fibrillation and multi-morbidity: Insights from the ARISTOTLE trial. Am. Heart J. 2019, 208, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Giugliano, R.P.; Braunwald, E.; Murphy, S.A.; Mercuri, M.; Choi, Y.; Aylward, P.; White, H.; Zamorano, J.L.; Antman, E.M.; et al. Edoxaban Versus Warfarin in Atrial Fibrillation Patients at Risk of Falling: ENGAGE AF-TIMI 48 Analysis. J. Am. Coll. Cardiol. 2016, 68, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Akao, M.; Yoshida, T.; Kawata, M.; Okazaki, O.; Akashi, S.; Eshima, K.; Tanizawa, K.; Fukuzawa, M.; Hayashi, T.; et al. Low-Dose Edoxaban in Very Elderly Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Hanon, O.; Jeandel, C.; Jouanny, P.; Paccalin, M.; Puisieux, F.; Krolak-Salmon, P.; Berrut, G. Anticoagulant treatment in elderly patients with atrial fibrillation: A position paper. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 341–354. [Google Scholar] [PubMed]
- Gage, B.F.; Boechler, M.; Doggette, A.L.; Fortune, G.; Flaker, G.C.; Rich, M.W.; Radford, M.J. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke 2000, 31, 822–827. [Google Scholar] [CrossRef]
- Abrignani, M.G.; Lucà, F.; Abrignani, V.; Pelaggi, G.; Aiello, A.; Colivicchi, F.; Fattirolli, F.; Gulizia, M.M.; Nardi, F.; Pino, P.G.; et al. A Look at Primary and Secondary Prevention in the Elderly: The Two Sides of the Same Coin. J. Clin. Med. 2024, 13, 4350. [Google Scholar] [CrossRef]
- Paciullo, F.; Proietti, M.; Bianconi, V.; Nobili, A.; Pirro, M.; Mannucci, P.M.; Lip, G.Y.H.; Lupattelli, G. Choice and Outcomes of Rate Control versus Rhythm Control in Elderly Patients with Atrial Fibrillation: A Report from the REPOSI Study. Drugs Aging 2018, 35, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shariff, N.; Desai, R.V.; Patel, K.; Ahmed, M.I.; Fonarow, G.C.; Rich, M.W.; Aban, I.B.; Banach, M.; Love, T.E.; White, M.; et al. Rate-control versus rhythm-control strategies and outcomes in septuagenarians with atrial fibrillation. Am. J. Med. 2013, 126, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Heeger, C.H.; Bellmann, B.; Fink, T.; Bohnen, J.E.; Wissner, E.; Wohlmuth, P.; Rottner, L.; Sohns, C.; Tilz, R.R.; Mathew, S.; et al. Efficacy and safety of cryoballoon ablation in the elderly: A multicenter study. Int. J. Cardiol. 2019, 278, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Lioni, L.; Letsas, K.P.; Efremidis, M.; Vlachos, K.; Giannopoulos, G.; Kareliotis, V.; Deftereos, S.; Sideris, A. Catheter ablation of atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2014, 11, 291–295. [Google Scholar] [PubMed]
- Deedwania, P.C.; Lardizabal, J.A. Atrial fibrillation in heart failure: A comprehensive review. Am. J. Med. 2010, 123, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Caldwell, J.C.; Chacko, S.; Garratt, C.J.; Fath-Ordoubadi, F.; Neyses, L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Olsson, L.G.; Charlesworth, A.; Cleland, J.; Hanrath, P.; Komajda, M.; Metra, M.; Torp-Pedersen, C.; Poole-Wilson, P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: Results from COMET. Eur. Heart J. 2005, 26, 1303–1308. [Google Scholar] [CrossRef]
- Mogensen, U.M.; Jhund, P.S.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Packer, M.; Rouleau, J.L.; Solomon, S.D.; Swedberg, K.; Zile, M.R.; et al. Type of Atrial Fibrillation and Outcomes in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2490–2500. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Isnard, R.; Bauer, F.; Cohen-Solal, A.; Damy, T.; Donal, E.; Galinier, M.; Hagège, A.; Jourdain, P.; Leclercq, C.; Sabatier, R.; et al. Non-vitamin K antagonist oral anticoagulants and heart failure. Arch. Cardiovasc. Dis. 2016, 109, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Lau, Y.C.; Senoo, K.; Lane, D.A.; Hong, K.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: A systemic review and meta-analysis of randomized trials. Eur. J. Heart Fail. 2015, 17, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Gori, M.; Giubilato, S.; Ceravolo, R.; Temporelli, P.L.; Cornara, S.; Rao, C.M.; et al. Heart Failure with Preserved Ejection Fraction: How to Deal with This Chameleon. J. Clin. Med. 2024, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Giubilato, S.; Di Fusco, S.A.; Piccioni, L.; Rao, C.M.; Iorio, A.; Cipolletta, L.; D’elia, E.; Gelsomino, S.; Rossini, R.; et al. Anticoagulation in Atrial Fibrillation Cardioversion: What Is Crucial to Take into Account. J. Clin. Med. 2021, 10, 3212. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; La Meir, M.; Rao, C.M.; Parise, O.; Vasquez, L.; Carella, R.; Lorusso, R.; Daniela, B.; Maessen, J.; Gensini, G.F.; et al. Pharmacological Management of Atrial Fibrillation: One, None, One Hundred Thousand. Cardiol. Res. Pract. 2011, 2011, 874802. [Google Scholar] [CrossRef] [PubMed]
- Asad, Z.U.A.; Yousif, A.; Khan, M.S.; Al-Khatib, S.M.; Stavrakis, S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ. Arrhythm Electrophysiol. 2019, 12, e007414. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Osman, M.; Abdalla, A.; Haykal, T.; Ahmed, S.; Bachuwa, G.; Hassan, M.; Bhatt, D.L. Catheter ablation of atrial fibrillation with heart failure: An updated meta-analysis of randomized trials. Int. J. Cardiol. 2018, 269, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Sawant, A.C.; Kumar, A.; McCray, W.; Tetewsky, S.; Parone, L.; Sridhara, S.; Prakash, M.P.H.; Tse, G.; Liu, T.; Kanwar, N.; et al. Superior safety of direct oral anticoagulants compared to Warfarin in patients with atrial fibrillation and underlying cancer: A national veterans affairs database study. J. Geriatr. Cardiol. 2019, 16, 706–709. [Google Scholar]
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018, 2, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.S.; Patel, A.; Khawaja, M.; Chen, C.; Zheng, H.; Kaczmarek, J.; Gao, F.; Karimzad, K.; Song, J.; Koutroumpakis, E.; et al. Outcomes by Class of Anticoagulant Use for Nonvalvular Atrial Fibrillation in Patients with Active Cancer. JACC CardioOncol. 2022, 4, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Magnocavallo, M.; Straito, M.; Piro, A.; Severino, P.; Iannucci, G.; Chimenti, C.; Mancone, M.; Della Rocca, D.G.; Forleo, G.B.; et al. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and cancer a meta-analysis. J. Thromb. Thrombolysis 2021, 51, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Lagan, J.; Fortune, C.; Bhatt, D.L.; Vestbo, J.; Niven, R.; Chaudhuri, N.; Schelbert, E.B.; Potluri, R.; Miller, C.A. Association of Cardiovascular Disease with Respiratory Disease. J. Am. Coll. Cardiol. 2019, 73, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, I.H.; Roberts-Thomson, K.C.; Kistler, P.M.; Edwards, G.A.; Spence, S.; Sanders, P.; Kalman, J.M. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: Implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010, 7, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Terzano, C.; Romani, S.; Conti, V.; Paone, G.; Oriolo, F.; Vitarelli, A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2908–2917. [Google Scholar] [PubMed]
- Caglar, I.M.; Dasli, T.; Turhan Caglar, F.N.; Teber, M.K.; Ugurlucan, M.; Ozmen, G. Evaluation of atrial conduction features with tissue Doppler imaging in patients with chronic obstructive pulmonary disease. Clin. Res. Cardiol. 2012, 101, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Acar, G.; Kahraman, H.; Akkoyun, M.; Kilinc, M.; Zencir, C.; Yusufoglu, E.; Dirnak, I.; Sahin, H.; Olmez, S.; Akcay, A.; et al. Evaluation of atrial electromechanical delay and its relationship to inflammation and oxidative stress in patients with chronic obstructive pulmonary disease. Echocardiography 2014, 31, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Tükek, T.; Yildiz, P.; Akkaya, V.; Akif Karan, M.; Atilgan, D.; Yilmaz, V.; Korkut, F. Factors associated with the development of atrial fibrillation in COPD patients: The role of P-wave dispersion. Ann. Noninvasive Electrocardiol. 2002, 7, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Nanda, S.; Kintzer, J.S. Arrhythmias as trigger for acute exacerbations of chronic obstructive pulmonary disease. Respir. Med. 2012, 106, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Miyamoto, A.; Kawaguchi, T.; Naiki, N.; Xue, J.Q.; Matsumoto, T.; Murakami, Y.; Horie, M. P pulmonale and the development of atrial fibrillation. Circ. J. 2014, 78, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Caram, L.M.d.O.; Ferrari, R.; Naves, C.R.; Tanni, S.E.; Coelho, L.S.; Zanati, S.G.; Minicucci, M.F.; Godoy, I. Association between left ventricular diastolic dysfunction and severity of chronic obstructive pulmonary disease. Clinics 2013, 68, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Eweda, I.; Hamada, G. Concordance between Doppler and pulsed-wave Doppler tissue imaging in estimation of the degree of left ventricular dysfunction and correlating it to the degree of chronic obstructive pulmonary disease. J. Saudi Heart Assoc. 2016, 28, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; Ojeda, F.M.; Wild, P.S.; Rzayeva, N.; Zeller, T.; Sinning, C.R.; Pfeiffer, N.; Beutel, M.; Blettner, M.; Lackner, K.J.; et al. Subclinical impairment of lung function is related to mild cardiac dysfunction and manifest heart failure in the general population. Int. J. Cardiol. 2016, 218, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.-Y.; Choi, J.-I.; Lee, J.Y.; Kwak, J.-J.; Park, J.-S.; Kim, J.-B.; Lim, H.-E.; Kim, Y.-H. Catheter ablation of atrial fibrillation in patients with chronic lung disease. Circ. Arrhythmia Electrophysiol. 2011, 4, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.; Senthilselvan, A.; Sin, D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-x.; Liu, Y.; Xia, W.-f.; Tang, Y.-h.; Huang, H. Oxidative stress: A possible pathogenesis of atrial fibrillation. Med. Hypotheses 2009, 72, 466–467. [Google Scholar] [CrossRef]
- De Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J.G.M. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Goudis, C.A. Chronic obstructive pulmonary disease and atrial fibrillation: An unknown relationship. J. Cardiol. 2017, 69, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Emren, S.V.; Kocabaş, U.; Duygu, H.; Levent, F.; Şimşek, E.; Yapan Emren, Z.; Tülüce, S. The role of HATCH score in predicting the success rate of sinus rhythm following electrical cardioversion of atrial fibrillation. Kardiol. Pol. 2016, 74, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Delesie, M.; Knaepen, L.; Verbraecken, J.; Weytjens, K.; Dendale, P.; Heidbuchel, H.; Desteghe, L. Cardiorespiratory polygraphy for detection of obstructive sleep apnea in patients with atrial fibrillation. Front. Cardiovasc. Med. 2021, 8, 758548. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S.; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Moula, A.I.; Parrini, I.; Tetta, C.; Lucà, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, H.; Scherlag, B.J.; Sun, L.; Xing, S.; Xu, J.; Luo, M.; Guo, Y.; Cao, G.; Jiang, H. Atrial fibrillation in obstructive sleep apnea: Neural mechanisms and emerging therapies. Trends Cardiovasc. Med. 2021, 31, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Patel, N.; Donahue, C.; Shenoy, A.; Patel, A.; El-Sherif, N. Obstructive sleep apnea and arrhythmia: A systemic review. Int. J. Cardiol. 2017, 228, 967–970. [Google Scholar] [CrossRef]
- Anter, E.; Di Biase, L.; Contreras-Valdes, F.M.; Gianni, C.; Mohanty, S.; Tschabrunn, C.M.; Viles-Gonzalez, J.F.; Leshem, E.; Buxton, A.E.; Kulbak, G.; et al. Atrial substrate and triggers of paroxysmal atrial fibrillation in patients with obstructive sleep apnea. Circ. Arrhythmia Electrophysiol. 2017, 10, e005407. [Google Scholar] [CrossRef]
- Holmqvist, F.; Guan, N.; Zhu, Z.; Kowey, P.R.; Allen, L.A.; Fonarow, G.C.; Hylek, E.M.; Mahaffey, K.W.; Freeman, J.V.; Chang, P.; et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2015, 169, 647–654.e2. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Liu, T.; Shehata, M.; Stevens, S.; Chugh, S.S.; Wang, X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011, 108, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Mohanty, P.; Di Biase, L.; Shaheen, M.; Lewis, W.R.; Quan, K.; Cummings, J.E.; Wang, P.; Al-Ahmad, A.; Venkatraman, P.; et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: The impact of continuous positive airway pressure. Circ. Arrhythmia Electrophysiol. 2010, 3, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, A.C.; Adejumo, K.L.; Akanbi, O.; Adegbala, O.M.; Alayo, Q.A.; Fijabi, D.O.; Ogundipe, O.A.; Almaddah, N.; Pani, L.; Adeboye, A. Predictors, burden and impact of cardiac arrhythmias among patients hospitalized with end-stage liver disease. Heart Lung 2020, 49, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Käräjämäki, A.J.; Hukkanen, J.; Ukkola, O. The association of non-alcoholic fatty liver disease and atrial fibrillation: A review. Ann. Med. 2018, 50, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dauriz, M.; Sandri, D.; Bonapace, S.; Zoppini, G.; Tilg, H.; Byrne, C.D.; Targher, G. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. 2019, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Cotter, T.G.; Wazni, O.M.; Elshazly, M.B.; Kochar, A.; Wilner, B.; Patel, D.; Kanj, M.; Hussein, A.; Baranowski, B.; et al. Impact of nonalcoholic fatty liver disease on arrhythmia recurrence following atrial fibrillation ablation. Clin. Electrophysiol. 2020, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Chokesuwattanaskul, R.; Thongprayoon, C.; Bathini, T.; O’Corragain, O.A.; Sharma, K.; Preechawat, S.; Wijarnpreecha, K.; Kröner, P.T.; Ungprasert, P.; Cheungpasitporn, W. Epidemiology of atrial fibrillation in patients with cirrhosis and clinical significance: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 514–519. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, B.; Wu, Y.; Peng, L.; Li, Z.; Zhu, J.; Su, Z.; Liu, J.; Li, S.; Chong, Y. Atrial fibrillation increases inpatient and 4-year all-cause mortality in critically ill patients with liver cirrhosis. Ann. Transl. Med. 2021, 9, 1239. [Google Scholar] [CrossRef]
- Ding, W.Y.; Gupta, D.; Wong, C.F.; Lip, G.Y.H. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc. Res. 2020, 117, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Mauro, E.; Lucà, F.; Tetta, C.; Parise, O.; Parrini, I.; Parise, G.; Parrini, I.; Parise, G.; Rao, C.M.; Matteucci, F.; et al. Breast Cancer and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Gulizia, M.M.; Turazza, F.M.; Ameri, P.; Alings, M.; Collins, R.; De Luca, L.; Di Nisio, M.; Lucci, D.; Gabrielli, D.; Janssens, S.; et al. Characteristics and Management of Patients with Cancer and Atrial Fibrillation: The BLITZ-AF Cancer Registry. JACC Adv. 2024, 3, 100991. [Google Scholar] [CrossRef]
- Parrini, I.; Lucà, F.; Rao, C.M.; Parise, G.; Micali, L.R.; Musumeci, G.; La Meir, M.; Colivicchi, F.; Gulizia, M.M.; Gelsomino, S. Superiority of Direct Oral Anticoagulants over Vitamin K Antagonists in Oncological Patients with Atrial Fibrillation: Analysis of Efficacy and Safety Outcomes. J. Clin. Med. 2022, 11, 5712. [Google Scholar] [CrossRef] [PubMed]
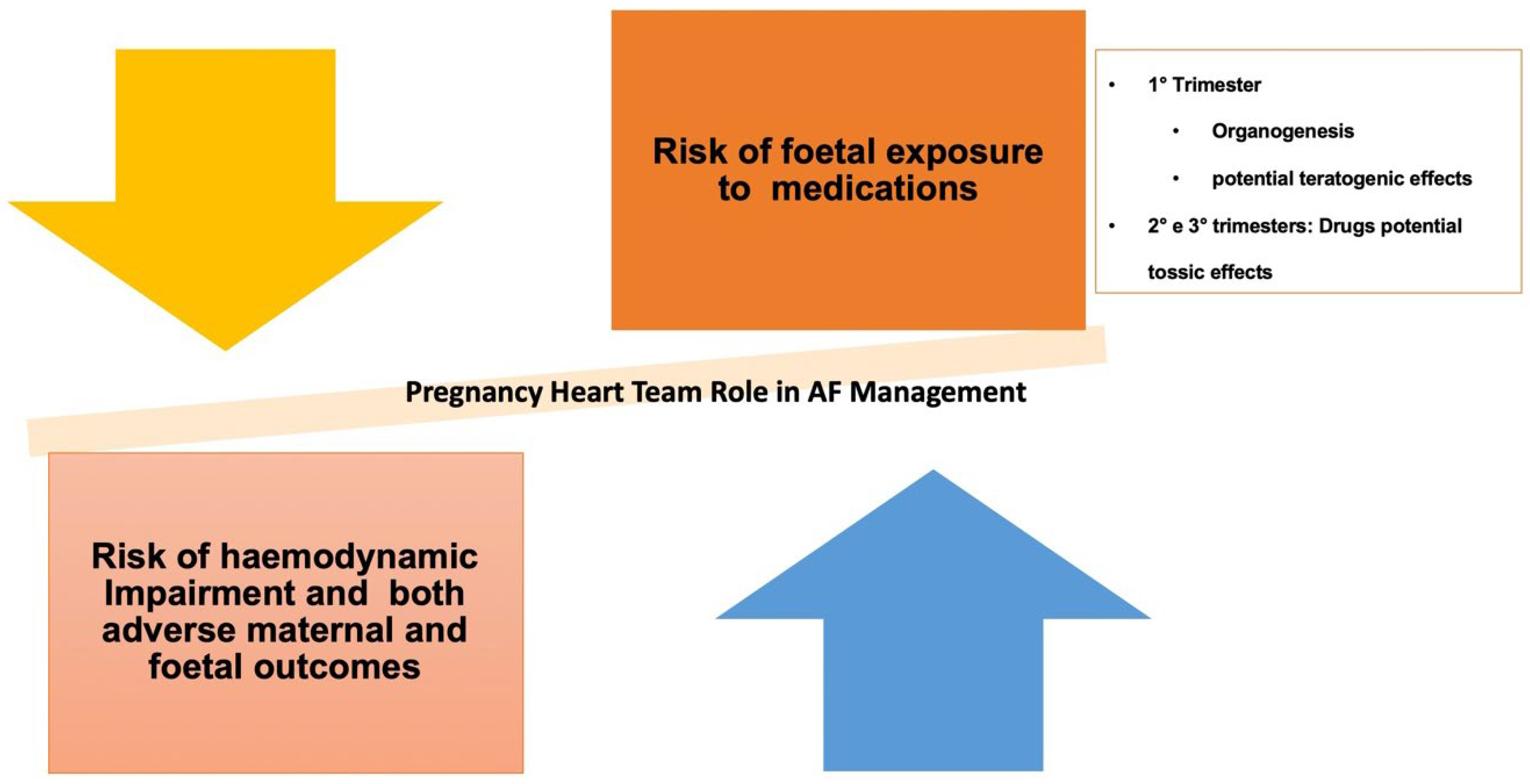
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Russo, M.G.; Parrini, I.; Cornara, S.; Ceravolo, R.; Rao, C.M.; Favilli, S.; Pozzi, A.; et al. The Challenge of Managing Atrial Fibrillation during Pregnancy. Rev. Cardiovasc. Med. 2023, 24, 279. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Arendt, K.; Bello, N.A.; Brown, H.; Briller, J.; Epps, K.; Hollier, L.; Langen, E.; Park, K.; Walsh, M.N.; et al. Team-Based Care of Women with Cardiovascular Disease from Pre-Conception through Pregnancy and Postpartum: JACC Focus Seminar 1/5. J. Am. Coll. Cardiol. 2021, 77, 1763–1777. [Google Scholar] [CrossRef]
- Lucà, F.; Colivicchi, F.; Parrini, I.; Russo, M.G.; Di Fusco, S.A.; Ceravolo, R.; Riccio, C.; Favilli, S.; Rossini, R.; Gelsomino, S.; et al. The role of the pregnancy heart team in clinical practice. Front. Cardiovasc. Med. 2023, 10, 1135294. [Google Scholar] [CrossRef]
- Parrini, I.; Luca, F.; Favilli, S.; Domenicucci, S.; Russo, M.; Sarubbi, B.; Gelsomino, S.; Colivicchi, F.; Gulizia, M.M. Gravidanza e cardiopatie: Il ruolo del Pregnancy Heart Team. G. Ital. Di Cardiol. 2022, 23, 631–644. [Google Scholar]
- Lucà, F.; Pavan, D.; Gulizia, M.M.; Manes, M.T.; Abrignani, M.G.; Benedetto, F.A.; Bisceglia, I.; Brigido, S.; Caldarola, P.; Calvanese, R.; et al. Italian Association of Hospital Cardiologists Position Paper ‘Gender discrepancy: Time to implement gender-based clinical management’. Eur. Heart J. Suppl. 2024, 26 (Suppl. S2), ii264–ii293. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Cipolletta, L.; Di Fusco, S.A.; Iorio, A.; Pozzi, A.; Rao, C.M.; Ingianni, N.; Benvenuto, M.; Madeo, A.; Fiscella, D.; et al. Remote monitoring: Doomed to let down or an attractive promise? Int. J. Cardiol. Heart Vasc. 2019, 24, 100380. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Waalen, J.; Edwards, A.M.; Ariniello, L.M.; Mehta, R.R.; Ebner, G.S.; Carter, C.; Baca-Motes, K.; Felicione, E.; Sarich, T.; et al. Effect of a Home-Based Wearable Continuous ECG Monitoring Patch on Detection of Undiagnosed Atrial Fibrillation: The mSToPS Randomized Clinical Trial. JAMA 2018, 320, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Breithardt, G.; Camm, A.J.; Lip, G.Y.; Schotten, U.; Ahlsson, A.; Arnar, D.; Atar, D.; Auricchio, A.; Bax, J.; et al. Integrating new approaches to atrial fibrillation management: The 6th AFNET/EHRA Consensus Conference. Ep Eur. 2018, 20, 395–407. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.V.; Turakhia, M.P.; Harrington, R.A.; King, A.C.; Ashley, E.A. Mobile health advances in physical activity, fitness, and atrial fibrillation: Moving hearts. J. Am. Coll. Cardiol. 2018, 71, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Ahmad, N.S.; Ali, S.; Ali, S.; George, A.; Danish, H.S.; Uppal, E.; Soo, J.; Mobasheri, M.H.; King, D.; et al. Medication adherence apps: Review and content analysis. JMIR mHealth uHealth 2018, 6, e6432. [Google Scholar] [CrossRef]
- Zeballos-Palacios, C.L.; Hargraves, I.G.; Noseworthy, P.A.; Branda, M.E.; Kunneman, M.; Burnett, B.; Gionfriddo, M.R.; McLeod, C.J.; Gorr, H.; Brito, J.P.; et al. (Eds.) Developing a conversation aid to support shared decision making: Reflections on designing anticoagulation choice. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jang, J.P.; Lin, H.T.; Chen, Y.J.; Hsieh, M.H.; Huang, Y.C. Role of Remote Monitoring in Detection of Atrial Arrhythmia, Stroke Reduction, and Use of Anticoagulation Therapy—A Systematic Review and Meta-Analysis. Circ. J. 2020, 84, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, N.; Esterman, A.; Mahajan, R.; Twomey, D.J.; Pathak, R.K.; Lau, D.H.; Roberts-Thomson, K.C.; Young, G.D.; Sanders, P.; Ganesan, A.N. Remote Monitoring of Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Clinical Outcomes. J. Am. Coll. Cardiol. 2015, 65, 2591–2600. [Google Scholar] [CrossRef]
- Ricci, R.P.; Vicentini, A.; D‘Onofrio, A.; Sagone, A.; Rovaris, G.; Padeletti, L.; Morichelli, L.; Fusco, A.; De Vivo, S.; Lombardi, L.; et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm 2017, 14, 50–57. [Google Scholar] [CrossRef]
- Zanaboni, P.; Landolina, M.; Marzegalli, M.; Lunati, M.; Perego, G.B.; Guenzati, G.; Curnis, A.; Valsecchi, S.; Borghetti, F.; Borghi, G.; et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: Randomized controlled trial. J. Med. Int. Res. 2013, 15, e106. [Google Scholar] [CrossRef] [PubMed]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Raisaro, A.; Curti, M.; Regoli, F.; Auricchio, A. Economic impact of remote patient monitoring: An integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur. J. Heart Fail. 2011, 13, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Gulizia, M.M.; Abrignani, M.G.; Benedetto, F.A.; Bisceglia, I.; Bisignani, G.; Bobbio, M.C.; Caldarola, P.; Canale, M.L.; Caretta, G.; et al. ANMCO Position paper: Choosing Wisely—ANMCO proposals for 2023. G. Ital. Cardiol. 2023, 24, 754–765. [Google Scholar]
- Khasnis, A.; Thakur, R.K. Atrial fibrillation: A historical perspective. Cardiol. Clin. 2009, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.M. Stroke in atrial fibrillation: Review of risk stratification and preventive therapy. J. Fam. Community Med. 2019, 26, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Atti, V.; Mitrani, R.D.; Viles-Gonzalez, J.F.; Goldberger, J.J. Global rising trends of atrial fibrillation: A major public health concern. Heart 2018, 104, 1989–1990. [Google Scholar] [CrossRef] [PubMed]
- Margulescu, A.D.; Mont, L. Persistent atrial fibrillation vs paroxysmal atrial fibrillation: Differences in management. Expert Rev. Cardiovasc. Ther. 2017, 15, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Cheungpasitporn, W.; Chokesuwattanaskul, A.; Thangjui, S.; Thongprayoon, C.; Bathini, T.; Vallabhajosyula, S.; Kanitsoraphan, C.; Leesutipornchai, T.; Chokesuwattanaskul, R. Diagnostic accuracy of smart gadgets/wearable devices in detecting atrial fibrillation: A systematic review and meta-analysis. Arch. Cardiovasc. Dis. 2021, 114, 4–16. [Google Scholar] [CrossRef]
- Halcox, J.P.J.; Wareham, K.; Cardew, A.; Gilmore, M.; Barry, J.P.; Phillips, C.; Gravenor, M. Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation: The REHEARSE-AF Study. Circulation 2017, 136, 1784–1794. [Google Scholar] [CrossRef]



| Author | Patients | N° of ABC Patients |
N° of Non-ABC Patients (%) | Median Follow-Up |
Results of ABC vs. Non-ABC |
|---|---|---|---|---|---|
| Romiti GF. Post hoc analysis GLORIA-AF Registry [11] | 24,608 | 23.901: −5285 (21.5%) adherent to 1 criterion; −12,112 (49.2%) adherent to 2 criteria; −6504 (26.4%) adherent to 3 criteria | 707 (2.9%) | 1 year | -↓Risk for the primary outcome (p < 0.0001) -↓Risk of mortality (p = 0.048) -↓Thromboembolism (p = 0.0078) MACE (p = 0.0071) |
| Yon M. et al. [12] | 204,842 | 10,129 (32.0%) | 66,778 (38.6%) | 6.2 ± 3.5 years | -↓All-cause death (p < 0.001) -↓Composite outcome (death, ischemic stroke, MB, and AMI) (p < 0.001) |
| Prietti M. Post hoc analysis AFFIRM [13] | 3169 | 222 (7%) | 2947(93%) | 3.7 years | -↓All study outcomes (p < 0.001) -↓Risk of all-cause death -↓Composite outcome -↓First hospitalization |
| Domek M. et al. Gulf SAFE registry [14] | 603 | 86 (14.2) | 517 (85.8) | 1 year | -↓Mortality (p = 0.0014) -↓Risk of all-cause death -↓Risk of composite outcome after 6 months and at 1 year |
| Gumprecht J. [15] | 2021 | 168 (8.3) | 1853 (91.7) | 1 year | ↓Composite outcome (p = 0.02) ↓Mortality (p = 0.033). |
| BALKAN-AF survey [16] | 2712 | 1013 (43.8%) | 1299 (56.2%) | Factors which increase ABC adherence: capital city (p = 0.02), treatment through cardiologist’s care (p = 0.01), AH (p < 0.001), DM (p = 0.01), and comorbidity (p < 0.001). Factors which decrease ABC adherence: age ≥ 80 (p < 0.001), previous MB (p= 0.001). | |
| Yang PS. [17] | 262,987 | 49,533 (15%) | 213,454 (85%) | 5.9 | -↓All-cause death (p < 0.001) -↓Beneficial effect on mortality in the high-frailty group (p < 0.001) -No statistical interaction between frailty and the composite outcome (p = 0.063) |
| Guo Y. et al. [18] | 3324 | 1646 (49%) mAFA + ABC | 1678 (51%) | 262 days (ABC)/291 days non-ABC | -↓Risk of rehospitalization (p < 0.001) -↓Risk of clinical adverse events (p < 0.001) |
| Proietti M. [19] | 6646 | 1996 (30.0%) | 4650 (70%) | 1 year | ↓Rate of TE/ACS/CV death and all-cause death (p < 0.0001) |
| Trial | Type of Evidence | Number of Patients | Drug | Summary of Evidence |
|---|---|---|---|---|
| ROCKET-AF [153] | Sub-group analysis of RCT | 640 | Rivaroxaban | No efficacy and safety differences. Increased risk of bleeding |
| ARISTOTLE [148] | Sub-group analysis of RCT | 1236 | Apixaban | Similar efficacy in preventing stroke and systemic embolism. No increase in major bleeding |
| ENGAGE AF- TIMI 48 [125] | Sub-group analysis of RCT | 1153 | Edoxaban | Similar efficacy and safety |
| Savant AC. et al. [150] | Retrospective administrative analysis | 196,521 | Various DOACs | Better safety profile than warfarin |
| Shah S. et al. [151] | Retrospective administrative analysis | 16,096 | Various DOACs | Lower or similar rates of bleeding and stroke and a lower rate of incident VTE |
| Potter AS. et al. [152] | Retrospective single-center analysis | 1133 | Various DOACs | Similar risks of cerebrovascular accident, gastrointestinal bleeding, and intracranial hemorrhage |
| Mariani MV. et al. [154] | Meta-analysis | 46,424 | Various DOACs | DOACs associated with reduction in thromboembolic events and major bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà, F.; Abrignani, M.G.; Oliva, F.; Canale, M.L.; Parrini, I.; Murrone, A.; Rao, C.M.; Nesti, M.; Cornara, S.; Di Matteo, I.; et al. Multidisciplinary Approach in Atrial Fibrillation: As Good as Gold. J. Clin. Med. 2024, 13, 4621. https://doi.org/10.3390/jcm13164621
Lucà F, Abrignani MG, Oliva F, Canale ML, Parrini I, Murrone A, Rao CM, Nesti M, Cornara S, Di Matteo I, et al. Multidisciplinary Approach in Atrial Fibrillation: As Good as Gold. Journal of Clinical Medicine. 2024; 13(16):4621. https://doi.org/10.3390/jcm13164621
Chicago/Turabian StyleLucà, Fabiana, Maurizio Giuseppe Abrignani, Fabrizio Oliva, Maria Laura Canale, Iris Parrini, Adriano Murrone, Carmelo Massimiliano Rao, Martina Nesti, Stefano Cornara, Irene Di Matteo, and et al. 2024. "Multidisciplinary Approach in Atrial Fibrillation: As Good as Gold" Journal of Clinical Medicine 13, no. 16: 4621. https://doi.org/10.3390/jcm13164621
APA StyleLucà, F., Abrignani, M. G., Oliva, F., Canale, M. L., Parrini, I., Murrone, A., Rao, C. M., Nesti, M., Cornara, S., Di Matteo, I., Barisone, M., Giubilato, S., Ceravolo, R., Pignalberi, C., Geraci, G., Riccio, C., Gelsomino, S., Colivicchi, F., Grimaldi, M., & Gulizia, M. M. (2024). Multidisciplinary Approach in Atrial Fibrillation: As Good as Gold. Journal of Clinical Medicine, 13(16), 4621. https://doi.org/10.3390/jcm13164621








