CO2 Angiography in the Standard and Complex Endovascular Repair of the Abdominal Aorta—A Narrative Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. CO2-DSA in EVAR: Techniques and Search of the Literature
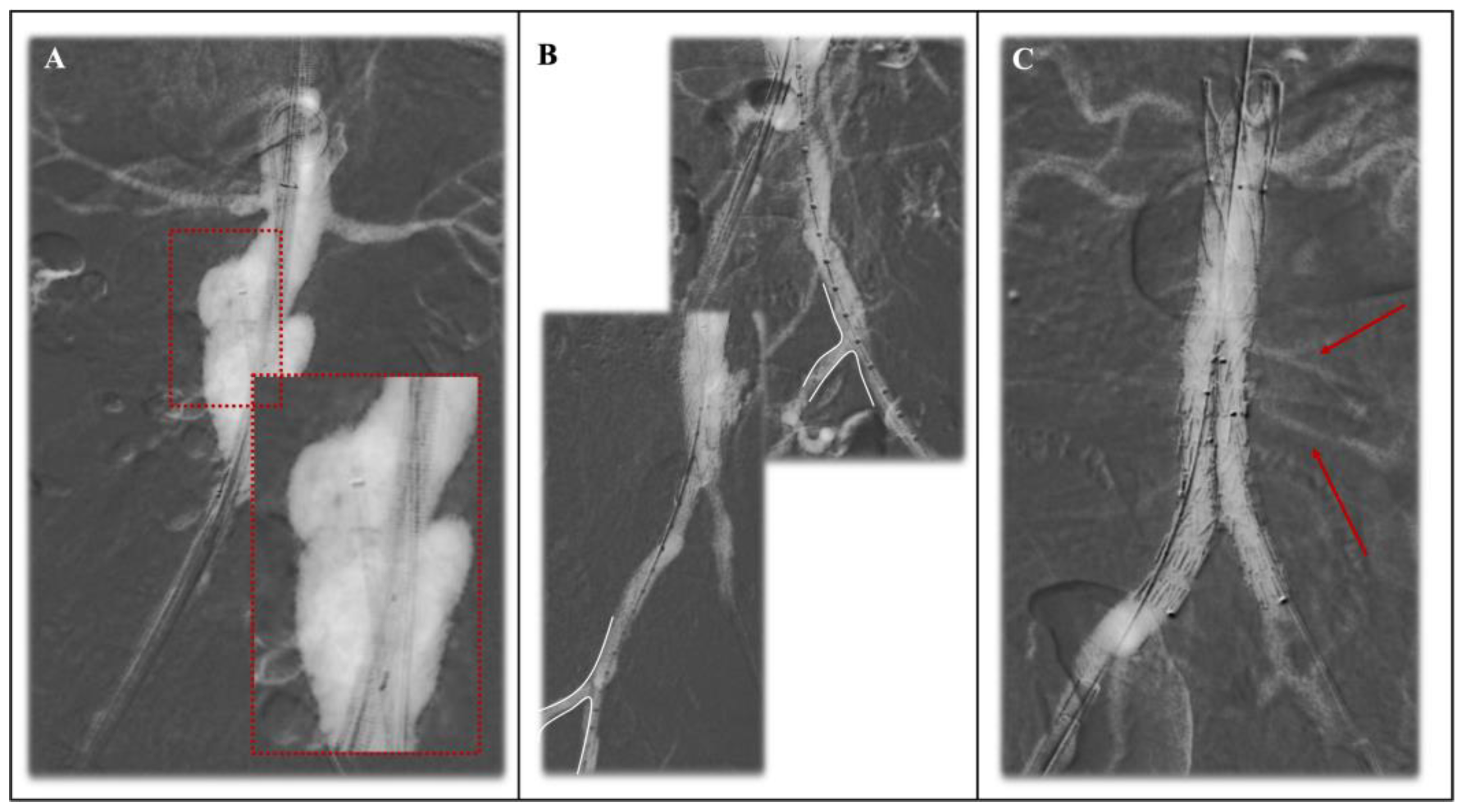
3.1.1. Primary Endpoints
3.1.2. Secondary Endpoints
3.2. CO2-DSA in Fenestrated and Branched EVAR: Techniques and Search of the Literature
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Powell, J.T.; Sweeting, M.J.; Ulug, P.; Blankensteijn, J.D.; Lederle, F.A.; Becquemin, J.-P.; Greenhalgh, R.M. EVAR-1, DREAM, OVER and ACE Trialists Meta-Analysis of Individual-Patient Data from EVAR-1, DREAM, OVER and ACE Trials Comparing Outcomes of Endovascular or Open Repair for Abdominal Aortic Aneurysm over 5 Years. Br J. Surg. 2017, 104, 166–178. [Google Scholar] [CrossRef]
- Greenhalgh, R.M.; Brown, L.C.; Kwong, G.P.S.; Powell, J.T.; Thompson, S.G. EVAR trial participants Comparison of Endovascular Aneurysm Repair with Open Repair in Patients with Abdominal Aortic Aneurysm (EVAR Trial 1), 30-Day Operative Mortality Results: Randomised Controlled Trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Saratzis, A.; Melas, N.; Mahmood, A.; Sarafidis, P. Incidence of Acute Kidney Injury (AKI) after Endovascular Abdominal Aortic Aneurysm Repair (EVAR) and Impact on Outcome. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 534–540. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Xin, S.; Liu, J.; Sun, G.; Chen, S.; Cen, X.; Dai, X.; He, Y.; Song, F.; et al. Risk Factors for Contrast-Induced Acute Kidney Injury (CI-AKI): Protocol for Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e030048. [Google Scholar] [CrossRef]
- Mun, J.-H.; Kwon, S.; Park, J.; Chu, W.; Kim, D.H.; Jung, H.J.; Lee, S.S. Renal Function-Adjusted Contrast Medium Volume Is a Major Risk Factor in the Occurrence of Acute Kidney Injury after Endovascular Aneurysm Repair. Medicine 2021, 100, e25381. [Google Scholar] [CrossRef]
- Cheng, E.L.; Hong, Q.; Yong, E.; Chandrasekar, S.; Tan, G.W.L.; Lo, Z.J. Validating the Use of Contrast-Induced Nephropathy Prediction Models in Endovascular Aneurysm Repairs. J. Vasc. Surg. 2020, 71, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Van Der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.-F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.W.; et al. Post-Contrast Acute Kidney Injury—Part 1: Definition, Clinical Features, Incidence, Role of Contrast Medium and Risk Factors: Recommendations for Updated ESUR Contrast Medium Safety Committee Guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.B.; Hurrell, C.; Costa, A.F.; McInnes, M.D.F.; O’Malley, M.E.; Barrett, B.; Brown, P.A.; Clark, E.G.; Hadjivassiliou, A.; Kirkpatrick, I.D.C.; et al. Canadian Association of Radiologists Guidance on Contrast Associated Acute Kidney Injury. Can. J. Kidney Health Dis. 2022, 73, 499–514. [Google Scholar] [CrossRef]
- Cho, K.J. Carbon Dioxide Angiography: Scientific Principles and Practice. Vasc. Spec. Int. 2015, 31, 67–80. [Google Scholar] [CrossRef]
- Rudnick, M.R.; Leonberg-Yoo, A.K.; Litt, H.I.; Cohen, R.M.; Hilton, S.; Reese, P.P. The Controversy of Contrast-Induced Nephropathy with Intravenous Contrast: What Is the Risk? Am. J. Kidney Dis. 2020, 75, 105–113. [Google Scholar] [CrossRef]
- Busutti, M.; Sensoni, A.; Vacirca, A.; Abenavoli, C.; Donadei, C.; Croci Chiocchini, A.L.; Righini, M.; Comai, G.; Pini, A.; Faggioli, G.; et al. Renal Benefits of CO2 as a Contrast Media for EVAR Procedures: New Perspectives on 1 Year Outcomes. J. Endovasc. Ther. 2023; ahead of print. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Marjan, A.E. Current Status of Carbon Dioxide Angiography. J. Vasc. Surg. 2017, 66, 618–637. [Google Scholar] [CrossRef]
- Ghumman, S.S.; Weinerman, J.; Khan, A.; Cheema, M.S.; Garcia, M.; Levin, D.; Suri, R.; Prasad, A. Contrast Induced-acute Kidney Injury Following Peripheral Angiography with Carbon Dioxide versus Iodinated Contrast Media: A Meta-analysis and Systematic Review of Current Literature. Catheter. Cardiovasc. Interv. 2017, 90, 437–448. [Google Scholar] [CrossRef]
- Mascoli, C.; Faggioli, G.; Gallitto, E.; Vento, V.; Pini, R.; Vacirca, A.; Indelicato, G.; Gargiulo, M.; Stella, A. Standardization of a Carbon Dioxide Automated System for Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2018, 51, 160–169. [Google Scholar] [CrossRef]
- Vacirca, A.; Faggioli, G.; Mascoli, C.; Gallitto, E.; Pini, R.; Spath, P.; Logiacco, A.; Palermo, S.; Gargiulo, M. CO2 Automated Angiography in Endovascular Aortic Repair Preserves Renal Function to a Greater Extent Compared with Iodinated Contrast Medium. Analysis of Technical and Anatomical Details. Ann. Vasc. Surg. 2022, 81, 79–88. [Google Scholar] [CrossRef]
- Lee, A.D.; Hall, R.G. An Evaluation of the Use of Carbon Dioxide Angiography in Endovascular Aortic Aneurysm Repair. Vasc. Endovasc. Surg. 2010, 44, 341–344. [Google Scholar] [CrossRef]
- Chao, A.; Major, K.; Kumar, S.R.; Patel, K.; Trujillo, I.; Hood, D.B.; Rowe, V.L.; Weaver, F.A. Carbon Dioxide Digital Subtraction Angiography–Assisted Endovascular Aortic Aneurysm Repair in the Azotemic Patient. J. Vasc. Surg. 2007, 45, 451–460. [Google Scholar] [CrossRef]
- Unal, E.U.; Iscan, H.Z.; Erol, M.E.; Naim Boran, T.; Mola, S.; Cetinkaya, F.; Hasanzade, S.; Gazioglu, Ö.; Levent, M. Carbon Dioxide Guided Endovascular Aortic Aneurysm Repair in Impaired Renal Function: Propensity Score Matched Study. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 521–529. [Google Scholar] [CrossRef]
- De Almeida Mendes, C.; De Arruda Martins, A.; Teivelis, M.P.; Kuzniec, S.; Varella, A.Y.M.; Wolosker, N. Carbon Dioxide as Contrast Medium to Guide Endovascular Aortic Aneurysm Repair. Ann. Vasc. Surg. 2017, 39, 67–73. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Morikage, N.; Matsuno, Y.; Nakamura, T.; Samura, M.; Ueda, K.; Harada, T.; Ikeda, Y.; Suehiro, K.; Ito, H.; et al. Midterm Outcomes of Endovascular Aortic Aneurysm Repair with Carbon Dioxide–Guided Angiography. Ann. Vasc. Surg. 2018, 51, 170–176. [Google Scholar] [CrossRef]
- Quaglino, S.; Ferrero, E.; Ferri, M.; Manzo, P.; Viazzo, A.; Lanza, M.; Ricotti, A.; Gaggiano, A. Safety, Effectiveness and Pitfalls of Carbon Dioxide Routine Use as a Contrast Agent for Endovascular Abdominal Aortic Repair. Ann. Vasc. Surg. 2024, 101, 120–126. [Google Scholar] [CrossRef]
- Criado, E.; Upchurch, G.R.; Young, K.; Rectenwald, J.E.; Coleman, D.M.; Eliason, J.L.; Escobar, G.A. Endovascular Aortic Aneurysm Repair with Carbon Dioxide-Guided Angiography in Patients with Renal Insufficiency. J. Vasc. Surg. 2012, 55, 1570–1575. [Google Scholar] [CrossRef]
- De Angelis, C.; Sardanelli, F.; Perego, M.; Alì, M.; Casilli, F.; Inglese, L.; Mauri, G. Carbon Dioxide (CO2) Angiography as an Option for Endovascular Abdominal Aortic Aneurysm Repair (EVAR) in Patients with Chronic Kidney Disease (CKD). Int. J. Cardiovasc. Imaging 2017, 33, 1655–1662. [Google Scholar] [CrossRef]
- Knipp, B.S.; Escobar, G.A.; English, S.; Upchurch, G.R.; Criado, E. Endovascular Repair of Ruptured Aortic Aneurysms Using Carbon Dioxide Contrast Angiography. Ann. Vasc. Surg. 2010, 24, 845–850. [Google Scholar] [CrossRef]
- Sueyoshi, E.; Nagayama, H.; Sakamoto, I.; Uetani, M. Carbon Dioxide Digital Subtraction Angiography as an Option for Detection of Endoleaks in Endovascular Abdominal Aortic Aneurysm Repair Procedure. J. Vasc. Surg. 2015, 61, 298–303. [Google Scholar] [CrossRef]
- Huang, S.G.; Woo, K.; Moos, J.M.; Han, S.; Lew, W.K.; Chao, A.; Hamilton, A.; Ochoa, C.; Hood, D.B.; Rowe, V.L.; et al. A Prospective Study of Carbon Dioxide Digital Subtraction versus Standard Contrast Arteriography in the Detection of Endoleaks in Endovascular Abdominal Aortic Aneurysm Repairs. Ann. Vasc. Surg. 2013, 27, 38–44. [Google Scholar] [CrossRef]
- Vacirca, A.; Faggioli, G.; Vaccarino, R.; Dias, N.; Austermann, M.; Usai, M.V.; Oberhuber, A.; Schäfers, J.F.; Bisdas, T.; Patelis, N.; et al. The Optimal Operative Protocol to Accomplish CO2-EVAR Resulting from a Prospective Interventional Multicenter Study. J. Vasc. Surg. 2023, 77, 1405–1412.e1. [Google Scholar] [CrossRef]
- Mascoli, C.; Faggioli, G.; Gallitto, E.; Vento, V.; Indelicato, G.; Pini, R.; Vacirca, A.; Stella, A.; Gargiulo, M. The Assessment of Carbon Dioxide Automated Angiography in Type II Endoleaks Detection: Comparison with Contrast-Enhanced Ultrasound. Contrast Media Mol. Imaging 2018, 2018, 7647165. [Google Scholar] [CrossRef]
- Esposito, D.; Fargion, A.T.; Dorigo, W.; Speziali, S.; Di Domenico, R.; Capone, A.; Calugi, G.; Piscitello, E.; Pratesi, C.; Pulli, R. Total Iodine Contrast-Free Strategy for the Endovascular Management of Abdominal Aortic Aneurysms in Chronic Kidney Disease Patients: A Pilot Study. Ann. Vasc. Surg. 2023, 93, 92–102. [Google Scholar] [CrossRef]
- Gallitto, E.; Faggioli, G.; Vacirca, A.; Pini, R.; Mascoli, C.; Fenelli, C.; Logiacco, A.; Abualhin, M.; Gargiulo, M. The Benefit of Combined Carbon Dioxide Automated Angiography and Fusion Imaging in Preserving Perioperative Renal Function in Fenestrated Endografting. J. Vasc. Surg. 2020, 72, 1906–1916. [Google Scholar] [CrossRef]
- Stacul, F.; van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.-F.; Clement, O.; et al. Contrast Induced Nephropathy: Updated ESUR Contrast Media Safety Committee Guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Kawasaki, D.; Shintani, Y.; Fukunaga, M.; Nakama, T.; Koshida, R.; Higashimori, A.; Yokoi, Y.; on behalf of the CO Angiography Registry Investigators. Endovascular Therapy by CO2 Angiography to Prevent Contrast-induced Nephropathy in Patients with Chronic Kidney Disease: A Prospective Multicenter Trial of CO2 Angiography Registry. Catheter. Cardiovasc. Interv. 2015, 85, 870–877. [Google Scholar] [CrossRef]
- Dias-Neto, M.; Vacirca, A.; Huang, Y.; Baghbani-Oskouei, A.; Jakimowicz, T.; Mendes, B.C.; Kolbel, T.; Sobocinski, J.; Bertoglio, L.; Mees, B.; et al. Outcomes of Elective and Non-Elective Fenestrated-Branched Endovascular Aortic Repair for Treatment of Thoracoabdominal Aortic Aneurysms. Ann. Surg. 2023, 278, 568–577. [Google Scholar] [CrossRef]
- Spath, P.; Tsilimparis, N.; Gallitto, E.; Becker, D.; Vacirca, A.; Berekoven, B.; Panuccio, G.; Karelis, A.; Kahlberg, A.; Melissano, G.; et al. Endovascular Repair of One-Hundred Urgent and Emergent Free or Contained Thoraco-Abdominal Aortic Aneurysms Ruptures. An International Multi-Center Trans-Atlantic Experience. Ann. Surg. 2024; ahead of print. [Google Scholar] [CrossRef]
- Corazza, I.; Sapignoli, S.; Cercenelli, L.; Marcelli, E.; Faggioli, G.; Gargiulo, M.; Stella, A.; Diemberger, I.; Rossi, P.L.; Zannoli, R. Automated CO2 Angiography: Injection Pressure and Volume Settings. Med. Eng. Phys. 2020, 80, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.V.; Gossler, A.A.; Fick, L.J.; Barnhart, W.; Lacey, D.L. Carbon Dioxide Angiography: Effect of Injection Parameters on Bolus Configuration. J. Vasc. Interv. Radiol. 1999, 10, 41–49. [Google Scholar] [CrossRef]
- Ahmad, W.; Hasselmann, H.-C.; Galas, N.; Majd, P.; Brunkwall, S.; Brunkwall, J.S. Image Fusion Using the Two-Dimensional-Three-Dimensional Registration Method Helps Reduce Contrast Medium Volume, Fluoroscopy Time, and Procedure Time in Hybrid Thoracic Endovascular Aortic Repairs. J. Vasc. Surg. 2019, 69, 1003–1010. [Google Scholar] [CrossRef]
- Asciutto, G.; Ibrahim, A.; Leone, N.; Gennai, S.; Piazza, M.; Antonello, M.; Wanhainen, A.; Mani, K.; Lindström, D.; Struk, L.; et al. Intravascular Ultrasound in the Detection of Bridging Stent Graft Instability During Fenestrated and Branched Endovascular Aneurysm Repair Procedures: A Multicentre Study on 274 Target Vessels. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 99–104. [Google Scholar] [CrossRef]
- Corazza, I.; Rossi, P.L.; Feliciani, G.; Pisani, L.; Zannoli, S.; Zannoli, R. Mechanical Aspects of CO2 Angiography. Phys. Med. 2013, 29, 33–38. [Google Scholar] [CrossRef] [PubMed]




| Iodinate Contrast Media | Carbon Dioxide Contrast Media | |
|---|---|---|
| Physical characteristics | High blood miscibility | High buoyancy Low viscosity |
| Advantages | Good visualization of all visceral vessels | No nephrotoxicity No hepatotoxicity Nonallergic |
| Disadvantages | Nephrotoxic Hepatotoxic Allergenic | Prohibited for use above the diaphragm Difficult visualization of visceral vessels originating from the posterior aortic wall Higher radiation exposure |
| Injector system | Manual Automated injectors | Manual Dedicated automated injectors |
| Author et al. | Year | Study Design | Setting | No. of CO2 Patients | Comparative Study | No. Control ICM Patients | Zero-Contrast Cases, No. | Article Focus |
|---|---|---|---|---|---|---|---|---|
| Chao et al. [20] | 2007 | R | Los Angeles, California | 16 | yes | 84 | 3 | Endoleak detection with CO2 DSA angiography |
| AD. Lee et al. [19] | 2010 | R | Kingswood, Australia | 17 | no | 0 | 0 | Impact of CO2-DSA in renal function protection and intraoperative arterial visualization |
| Knipp et al. [27] | 2010 | R | Ann Arbor, Michigan | 4 | yes | 7 | 3 | Impact of CO2-DSA in renal function protection in ruptured AAA |
| Criado et al. [25] | 2012 | R | Ann Arbor, Michigan, USA | 114 | yes | 0 | 72 | Comparison of renal function protection between CO2 and CO2+ICM angiographies |
| Huang et al. [29] | 2012 | P | Los Angeles, CA | 76 | no | 0 | 76 | Endoleak detection with CO2 DSA angiography |
| Sueyoshi et al. [28] | 2015 | P | Sakamoto, Japan | 40 | no | 0 | 40 | Endoleak detection with CO2 DSA angiography |
| Mendes et al. [22] | 2017 | RCT | San Paolo, Brazil | 16 | yes | 16 | 6 | Impact of CO2-DSA in renal function protection |
| De Angelis et al. [26] | 2017 | R | Milano, Italy | 17 | no | 0 | 16 | Efficacy of CO2-DSA in arterial visualization and graft deployment |
| Takeuchi et al. [23] | 2018 | R | Yamaguchi, Japan | 30 | yes | 351 | 0 | Impact of CO2-DSA in renal function protection |
| Mascoli et al. [17] | 2018 | R | Bologna, Italy | 31 | no | 0 | 31 | Efficacy of CO2-DSA in arterial visualization and graft deployment |
| Mascoli et al. [31] | 2018 | R | Bologna, Italy | 21 | yes | 0 | 16 | Type II endoleak detection |
| Vacirca et al. [18] | 2022 | R | Bologna, Italy | 72 | yes | 249 | 16 | Impact of CO2-DSA in renal function protection and arterial detection |
| Unal et al. [21] | 2023 | R | Ankara, Turkey | 34 | yes | 34 | 0 | Impact of CO2-DSA in renal function protection |
| Busutti et al. [14] | 2023 | R | Bologna, Italy | 22 | yes | 22 | 5 | Impact of CO2-DSA in renal function protection |
| Quaglino et al. [24] | 2023 | R | Turin, Italy | 52 | yes | 49 | 52 | Impact of CO2-DSA in renal function protection and endoleak detection |
| Vacirca et al. [30] | 2023 | P | Multicenter | 65 | no | 0 | 19 | Comparison of arterial visualization before, during, and after graft deployment |
| Esposito et al. [32] | 2023 | R | Florence, Italy | 17 | no | 0 | 17 | Evaluation of feasibility and safety of a “zero-contrast” approach in patients with CKD. |
| TOTAL | 644 | 812 | 372 |
| Author et al., Year | No. of CO2 Patients | No. of Control ICM Patients | Matched Cohorts | Adjunctive ICM Used for CO2 (SD/Range) mL | PO-RFW n (%)/in CO2-EVAR | PO-RFW n (%) ICM-EVAR | p Value* | Definition of PO-RFW | ΔsCr in CO2-EVAR (SD/Range) micrommol/L | ΔsCr in ICM-EVAR (SD/Range) micrommol/L | p Value° |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Knipp et al., 2010 [27] | 4 | 7 | no | 0 | - | - | - | - | 0.25 ± 0.19 | 0.58 ± 0.25 | 0.066 |
| Criado et al., 2012 [25] | 114 | 0 | - | 37 (3.7) | - | - | - | - | - | - | - |
| Mendes et al., 2017 [22] | 16 | 16 | yes | 5.5 (0–15) | - | - | - | - | 11.1 (3.1–22.6) | 11.7 (5.1–19.5) | 0.80 |
| Takeuchi et al., 2018 [23] | 30 | 351 | no | 18 (15) | 10 (1) | 1 (3.3) | 0.93 | RIFLE classification | - | - | - |
| Vacirca et al., 2022 [18] | 72 | 249 | no | 52.8 (6.1) | - | - | - | - | 0.08 ± 0.04 | 0.17 ± 0.09 | 0.01 |
| Quaglino et al., 2023 [24] | 52 | 49 | no | 0 | - | - | - | - | 1.1 (0.8–1.3) | 0.98 (0.85–1.2) | 0.401 |
| Unal et al., 2023 [21] | 34 | 34 | yes | 4 (8) | 2.8 | 23.5 | 0.027 | 25% increase in sCr or a 0.5 mg/dL increase sCr within 48 h | - | - | - |
| Busutti et al., 2023 [14] | 22 | 22 | yes | 49.5 (35) | 9 | 27 | <0.05 | KDIGO | - | - | - |
| Author et al., Year | n Patients | Both Renal Arteries n (%) | Aortic Bifurcation n (%) | Hypogastric Arteries n (%) | Ipsilateral Iliac Artery n (%) | Contralateral Iliac Artery n (%) |
|---|---|---|---|---|---|---|
| AD. Lee et al., 2010 [19] | 17 | 9 (53) | 17 (100) | - | 17 (100) | 17 (100) |
| Mendes et al., 2017 [22] | 16 | 16 (100) | 16 (100) | 15 (94) | 16 (100) | 16 (100) |
| De Angelis et al. 2017 [26] | 17 | 17 (100) | 17 (100) | - | - | - |
| Mascoli et al., 2018 [17] | 31 | 19 (61) | - | 31 (100) | - | - |
| Vacirca et al., 2022 [18] | 72 | 50 (69) | - | 72 (100) | - | - |
| Author et al., Year | Endoleak Detection with CO2-DSA: Main Findings |
|---|---|
| Chao et al., 2007 [20] | No difference in endoleak detection between CO2 and ICM angiographies |
| AD. Lee et al., 2010 [19] | No difference in endoleak detection between CO2 and ICM angiographies |
| Huang et al., 2012 [29] | CO2-DSA has poor sensitivity and poor positive predictive value in the detection of ELII. |
| Sueyoshi et al., 2015 [28] | Lower endoleak detection with CO2-DSA (40%; 16/40) compared to ICM-DSA (68%;27/40). No difference in detection of type I–III EL. Among the type II EL detected on ICM-DSA but not on CO2-DSA, none progressed to persistent type II EL. |
| Mascoli et al., 2018 [31] | Type II EL detection with CO2-DSA has a higher agreement with CEUS detection if compared to ICM-DSA |
| Quaglino et al., 2023 [24] | Lower intraoperative type 2 EL detection rate (14.3%; 7/49) in the ICM group compared to CO2 group (25%; 13/52) (p = 0.2). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spath, P.; Caputo, S.; Campana, F.; Gallitto, E.; Pini, R.; Mascoli, C.; Vacirca, A.; Faggioli, G.; Gargiulo, M. CO2 Angiography in the Standard and Complex Endovascular Repair of the Abdominal Aorta—A Narrative Review of the Literature. J. Clin. Med. 2024, 13, 4634. https://doi.org/10.3390/jcm13164634
Spath P, Caputo S, Campana F, Gallitto E, Pini R, Mascoli C, Vacirca A, Faggioli G, Gargiulo M. CO2 Angiography in the Standard and Complex Endovascular Repair of the Abdominal Aorta—A Narrative Review of the Literature. Journal of Clinical Medicine. 2024; 13(16):4634. https://doi.org/10.3390/jcm13164634
Chicago/Turabian StyleSpath, Paolo, Stefania Caputo, Federica Campana, Enrico Gallitto, Rodolfo Pini, Chiara Mascoli, Andrea Vacirca, Gianluca Faggioli, and Mauro Gargiulo. 2024. "CO2 Angiography in the Standard and Complex Endovascular Repair of the Abdominal Aorta—A Narrative Review of the Literature" Journal of Clinical Medicine 13, no. 16: 4634. https://doi.org/10.3390/jcm13164634
APA StyleSpath, P., Caputo, S., Campana, F., Gallitto, E., Pini, R., Mascoli, C., Vacirca, A., Faggioli, G., & Gargiulo, M. (2024). CO2 Angiography in the Standard and Complex Endovascular Repair of the Abdominal Aorta—A Narrative Review of the Literature. Journal of Clinical Medicine, 13(16), 4634. https://doi.org/10.3390/jcm13164634






