Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine
Abstract
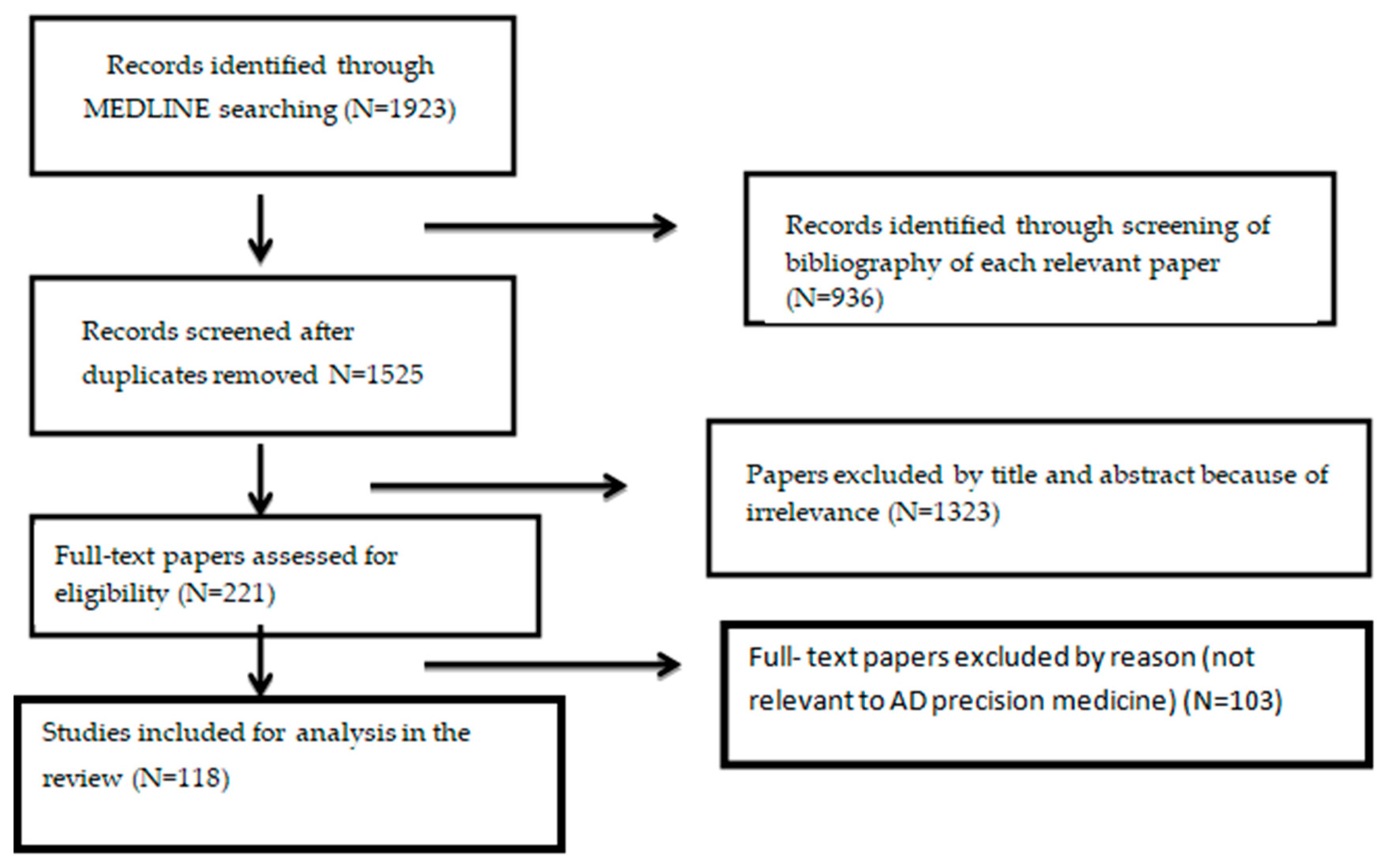
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Results
3.1. Classical Neurodegenerative Biomarkers
Overview of Fluid Biomarkers in Clinical Trials
3.2. Genetic Biomarkers
3.3. Neuroimaging Biomarkers
3.4. Proteomics
3.5. Metabolomics
3.6. Epigenomics
3.7. Exosomes
4. Discussion
5. Research Gaps
- There is a lack of certified biofluid reference methods and materials (except for cerebrospinal fluid [CSF] amyloid beta [Aβ]42, where these are available).
- The RNA and exosome isolation and downstream miRNA detection, quantification, and normalization methods varied between studies, such as enzyme-linked immunosorbent assays (ELISA), Western blotting, and mass spectrometry (S, showing conflicting results).
- No comprehensive biofluid analyses exist for CSF and blood levels of multiple inflammatory markers, along with Core 1 and 2 biomarkers.
- In order to empower cohorts for maximized therapeutic effects in clinical trials, understanding the predictive and prognostic value of omic signatures relevant to clinical trajectories is crucial.
- Despite the efforts, PET, CSF, and blood biomarkers remain less sensitive compared with neuropathologic examination for the detection of early/mild AD neuropathologic change (ADNPC). Disease staging by PET (or fluid biomarkers) is not equivalent to neuropathological staging; for example, tau PET ligand uptake in different Braak areas is not equivalent to Braak neuropathological staging. While the sensitivity limits of biomarkers could be appraised as a disadvantage, they could also be appraised as a strength because abnormal Core 1 biomarkers indicate that ADNPC more generally than just neuritic plaques alone is very likely present.
- Thoroughly studied biomarkers are not available for all relevant diseases; there is a high uncertainty of other co-pathologies in addition to AD in any individual or what the proportional disease-specific burden is among various pathologic entities.
- The proportion of the cognitive deficit observed in a single patient that is attributable to AD versus other neuropathologic pathologies is difficult to quantify. Only probabilistic rates can be calculated based on combinations of biomarker results and clinical evaluation.
6. Future Steps
- Future protocols for clinical trials should rigorously include more representative cohorts. True epidemiological and real-world data studies of biomarker properties in representative groups are crucial to determining relationships that are valid at the population level. A better understanding of the longitudinal intra-individual biological and disease-associated variability; the potential impact of clinical confounders and biological factors, including race and ethnicity, peripheral neuropathies and other neurologic diseases, BMI, and kidney disease; and the relative effects on the clinical performance of plasma Aβ42/Aβ40, p-tau, NfL, and GFAP in large cohorts is needed. In order to minimize referral bias, prospective studies in the general population would minimize the risk of overestimating the power of ApoE4.
- Longer clinical trials are needed to show the lowering rate of brain volume loss as a result of the amyloid plaque removal.
- An international consensus of standard biofluid assays, tau PET quantification methods, and cutpoints is warranted. As in other diseases, the exact thresholds for abnormality may evolve over time as additional data inform the prognostic value.
- Advanced knowledge of various post-translational modifications of tau may enhance fluid-based biological staging. The integration of genomic and epigenomic data to ascertain the influence of epigenetic mechanisms in the setting of complicated disease phenotypes may be made possible by artificial intelligence methods.
- With an improved understanding of the role of immune/inflammatory processes, microglia, and astrocyte biology in AD pathogenesis, we foresee a more notable role for biomarkers in biological characterization and prognosis, especially if brain-specific modifications can be revealed in blood.
- Keeping in mind that clinical trials target mechanisms other than anti-Aβ immunotherapy, the effects of these interventions on biomarkers and clinical outcomes should be included in future diagnostic AD criteria.
- By identifying miRNA targets, regulatory networks, and signaling pathways implicated in disease pathogenesis, researchers can develop small molecule inhibitors, antisense oligonucleotides, and gene therapies that modulate miRNA function, restore gene expression, and reverse neurodegeneration in AD.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Focus 2011, 11, 96–106. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H., Jr.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.; de Wilde, A.; Ossenkoppele, R.; Pelkmans, W.; Bouwman, F.; Groot, C.; van Maurik, I.; Zwan, M.; Yaqub, M.; Barkhof, F.; et al. Applying the ATN scheme in a memory clinic population: The ABIDE project. Neurology 2019, 93, e1635–e1646. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Mendez, M.F.; Mastri, A.R.; Sung, J.H.; Frey, W.H. Clinically diagnosed Alzheimer disease: Neuropathologic findings in 650 cases. Alzheimer Dis. Assoc. Disord. 1992, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Hansen, L.A.; Katzman, R.; Wiederholt, W.; Masliah, E.; Terry, R.; Hill, L.R.; Lessin, P.; Thal, L.J. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch. Neurol. 1994, 51, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Head, E.; Schmitt, F.A.; Davis, P.R.; Neltner, J.H.; Jicha, G.A.; Abner, E.L.; Smith, C.D.; Van Eldik, L.J.; Kryscio, R.J.; et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011, 121, 571–587. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Jeromin, A.; Bowser, R. Biomarkers in Neurodegenerative Diseases. Adv. Neurobiol. 2017, 15, 491–528. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S., Jr.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Nat. Med. 2024, 1, 1–4. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Karikari, T.K.; Ashton, N.J.; Lantero Rodríguez, J.; Milà-Alomà, M.; Gispert, J.D.; Salvadó, G.; Minguillon, C.; Fauria, K.; Shekari, M.; et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Aβ pathology are detected. EMBO Mol. Med. 2020, 12, e12921. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Edelmayer, R.M.; Boxer, A.L.; Carrillo, M.C.; Mielke, M.M.; Rabinovici, G.D.; Salloway, S.; Sperling, R.; Zetterberg, H.; Teunissen, C.E. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 2669–2686. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef]
- Forsberg, A.; Engler, H.; Almkvist, O.; Blomquist, G.; Hagman, G.; Wall, A.; Ringheim, A.; Långström, B.; Nordberg, A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol. Aging 2008, 29, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, C.L.; Mosconi, L.; Scheyer, O.; Rahman, A.; Hristov, H.; Isaacson, R.S. Precision Medicine for Alzheimer’s Disease Prevention. Healthcare 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Maes, O.C.; Schipper, H.M.; Chertkow, H.M.; Wang, E. Methodology for discovery of Alzheimer’s disease blood-based biomarkers. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 636–645. [Google Scholar] [CrossRef]
- Zetterberg, H. Applying fluid biomarkers to Alzheimer’s disease. Am. J. Physiol. Cell Physiol. 2017, 313, C3–C10. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.H.; Small, G.W.; Chang, C.Y.; Lu, C.S.; Kung De Aburto, M.A.; Chen, W.; Czernin, J.; Rapoport, S.I.; Pietrini, P.; Alexander, G.E.; et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. Jama 2001, 286, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Cruts, M.; Hendriks, L.; Van Broeckhoven, C. The presenilin genes: A new gene family involved in Alzheimer disease pathology. Hum. Mol. Genet. 1996, 5 (Suppl. S1), 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.; Pegueroles, J.; Alcolea, D.; Belbin, O.; Dols-Icardo, O.; Vaqué-Alcázar, L.; Videla, L.; Gispert, J.D.; Suárez-Calvet, M.; Johnson, S.C.; et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 2024, 30, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Belloy, M.E.; Andrews, S.J.; Le Guen, Y.; Cuccaro, M.; Farrer, L.A.; Napolioni, V.; Greicius, M.D. APOE Genotype and Alzheimer Disease Risk Across Age, Sex, and Population Ancestry. JAMA Neurol. 2023, 80, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A., Jr.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Sjögren, M.; Minthon, L.; Davidsson, P.; Granérus, A.K.; Clarberg, A.; Vanderstichele, H.; Vanmechelen, E.; Wallin, A.; Blennow, K. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J. Neural Transm. 2000, 107, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, H.; De Vreese, K.; Blennow, K.; Andreasen, N.; Sindic, C.; Ivanoiu, A.; Hampel, H.; Bürger, K.; Parnetti, L.; Lanari, A.; et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 2006, 44, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Wallin, A.; Agren, H.; Spenger, C.; Siegfried, J.; Vanmechelen, E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol. Chem. Neuropathol. 1995, 26, 231–245. [Google Scholar] [CrossRef]
- Niemantsverdriet, E.; Ottoy, J.; Somers, C.; De Roeck, E.; Struyfs, H.; Soetewey, F.; Verhaeghe, J.; Van den Bossche, T.; Van Mossevelde, S.; Goeman, J.; et al. The Cerebrospinal Fluid Aβ1-42/Aβ1-40 Ratio Improves Concordance with Amyloid-PET for Diagnosing Alzheimer’s Disease in a Clinical Setting. J. Alzheimer’s Dis. 2017, 60, 561–576. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Stomrud, E.; Zetterberg, H.; Karl, J.; Zink, K.; Bittner, T.; Mattsson, N.; Eichenlaub, U.; Blennow, K.; et al. Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease-Related β-Amyloid Status. JAMA Neurol. 2019, 76, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Benedet, A.L.; Ashton, N.J.; Lantero Rodriguez, J.; Snellman, A.; Suárez-Calvet, M.; Saha-Chaudhuri, P.; Lussier, F.; Kvartsberg, H.; Rial, A.M.; et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol. Psychiatry 2021, 26, 429–442. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Janelidze, S.; Palmqvist, S.; Cullen, N.; Svenningsson, A.L.; Strandberg, O.; Mengel, D.; Walsh, D.M.; Stomrud, E.; Dage, J.L.; et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 2020, 143, 3234–3241. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M. CSF p-tau231: A biomarker for early preclinical Alzheimer? EBioMedicine 2022, 77, 103936. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Kasselimis, D.; Kourtidou, E.; Constantinides, V.; Bougea, A.; Potagas, C.; Evdokimidis, I.; Kapaki, E. Cerebrospinal Fluid Biomarkers as a Diagnostic Tool of the Underlying Pathology of Primary Progressive Aphasia. J. Alzheimer’s Dis. 2017, 55, 1453–1461. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Paraskevas, G.P.; Emmanouilidou, E.; Petropoulou, O.; Bougea, A.; Vekrellis, K.; Evdokimidis, I.; Stamboulis, E.; Kapaki, E. CSF biomarkers β-amyloid, tau proteins and a-synuclein in the differential diagnosis of Parkinson-plus syndromes. J. Neurol. Sci. 2017, 382, 91–95. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Bougea, A.; Constantinides, V.C.; Bourbouli, M.; Petropoulou, O.; Kapaki, E. In vivo Prevalence of Alzheimer Biomarkers in Dementia with Lewy Bodies. Dement. Geriatr. Cogn. Disord. 2019, 47, 289–296. [Google Scholar] [CrossRef]
- Bjerke, M.; Engelborghs, S. Cerebrospinal Fluid Biomarkers for Early and Differential Alzheimer’s Disease Diagnosis. J. Alzheimer’s Dis. 2018, 62, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, G.P.; Constantinides, V.C.; Boufidou, F.; Tsantzali, I.; Pyrgelis, E.S.; Liakakis, G.; Kapaki, E. Recognizing Atypical Presentations of Alzheimer’s Disease: The Importance of CSF Biomarkers in Clinical Practice. Diagnostics 2022, 12, 3011. [Google Scholar] [CrossRef]
- Edgar, C.J.; Vradenburg, G.; Hassenstab, J. The 2018 Revised FDA Guidance for Early Alzheimer’s Disease: Establishing the Meaningfulness of Treatment Effects. J. Prev. Alzheimer’s Dis. 2019, 6, 223–227. [Google Scholar] [CrossRef]
- Fang, C.; Hernandez, P.; Liow, K.; Damiano, E.; Zetterberg, H.; Blennow, K.; Feng, D.; Chen, M.; Maccecchini, M. Buntanetap, a Novel Translational Inhibitor of Multiple Neurotoxic Proteins, Proves to Be Safe and Promising in Both Alzheimer’s and Parkinson’s Patients. J. Prev. Alzheimer’s Dis. 2023, 10, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; van Dyck, C.H.; et al. Evaluating the Safety and Efficacy of Crenezumab vs. Placebo in Adults With Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Pontecorvo, M.J.; Lu, M.; Burnham, S.C.; Schade, A.E.; Dage, J.L.; Shcherbinin, S.; Collins, E.C.; Sims, J.R.; Mintun, M.A. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1250–1259. [Google Scholar] [CrossRef]
- Bateman, R.J.; Smith, J.; Donohue, M.C.; Delmar, P.; Abbas, R.; Salloway, S.; Wojtowicz, J.; Blennow, K.; Bittner, T.; Black, S.E.; et al. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1862–1876. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Lerner, A.J.; Arnold, S.E.; Maxfield, E.; Koenig, A.; Toth, M.E.; Fortin, B.; Mast, N.; Trombetta, B.A.; Denker, J.; Pieper, A.A.; et al. CYP46A1 activation by low-dose efavirenz enhances brain cholesterol metabolism in subjects with early Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Mahnken, J.D.; Welch, P.; Bothwell, R.; Koppel, S.; Jackson, R.L.; Burns, J.M.; Swerdlow, R.H. A Mitochondrial Biomarker-Based Study of S-Equol in Alzheimer’s Disease Subjects: Results of a Single-Arm, Pilot Trial. J. Alzheimer’s Dis. 2017, 59, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.F.J.M.; Heuberger, J.A.A.C.; Groeneveld, G.J.; Oude Nijhuis, J.; De Deyn, P.P.; Hadi, S.; Harris, J.; Tsai, R.M.; Cruz-Herranz, A.; Huang, F.; et al. Safety, pharmacokinetics and target engagement of novel RIPK1 inhibitor SAR443060 (DNL747) for neurodegenerative disorders: Randomized, placebo-controlled, double-blind phase I/Ib studies in healthy subjects and patients. Clin. Transl. Sci. 2022, 15, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Harrison, J.E.; Chu, H.-M.; Blackburn, K.; Alam, J.J.; Scheltens, P. A phase 2 double-blind placebo-controlled 24-week treatment clinical study of the p38 alpha kinase inhibitor neflamapimod in mild Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.C.; Zuniga, G.; Ramirez, P.; Fernandez, R.; Wang, C.P.; Li, J.; Davila, L.; Pelton, K.; Gomez, S.; Sohn, C.; et al. A pilot study to investigate the safety and feasibility of antiretroviral therapy for Alzheimer’s disease (ART-AD). medRxiv 2024. [Google Scholar] [CrossRef]
- LaBarbera, K.M.; Sheline, Y.I.; Izzo, N.J.; Yuede, C.M.; Waybright, L.; Yurko, R.; Edwards, H.M.; Gardiner, W.D.; Blennow, K.; Zetterberg, H.; et al. A phase 1b randomized clinical trial of CT1812 to measure Aβ oligomer displacement in Alzheimer’s disease using an indwelling CSF catheter. Transl. Neurodegener. 2023, 12, 24. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Mecca, A.P.; O’Dell, R.S.; Bartlett, H.H.; Diepenbrock, N.G.; Huang, Y.; Hamby, M.E.; Grundman, M.; Catalano, S.M.; Caggiano, A.O.; et al. A pilot study to evaluate the effect of CT1812 treatment on synaptic density and other biomarkers in Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J.; et al. TANGO: A placebo-controlled randomized phase 2 study of efficacy and safety of the anti-tau monoclonal antibody gosuranemab in early Alzheimer’s disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Sanabria Bohorquez, S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals With Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef]
- Monteiro, C.; Toth, B.; Brunstein, F.; Bobbala, A.; Datta, S.; Ceniceros, R.; Sanabria Bohorquez, S.M.; Anania, V.G.; Wildsmith, K.R.; Schauer, S.P.; et al. Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet. Neurology 2023, 101, e1391–e1401. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.S.; Munsie, L.M.; Perahia, D.G.S.; Andersen, S.W.; Higgins, I.A.; Hauck, P.M.; Lo, A.C.; Sims, J.R.; Brys, M.; Mintun, M.; et al. Assessment of Efficacy and Safety of Zagotenemab. Neurology 2024, 102, e208061. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.A.; Lo, A.C.; Dage, J.L.; Shcherbinin, S.; Chinchen, L.; Andersen, S.W.; LaBell, E.S.; Perahia, D.G.S.; Hauck, P.M.; Lowe, S.L. Safety, Tolerability, and Pharmacokinetics of Zagotenemab in Participants with Symptomatic Alzheimer’s Disease: A Phase I Clinical Trial. J. Alzheimer’s Dis. Rep. 2023, 7, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Montufar, S.; Calero, C.; Vinueza, R.; Correa, P.; Carrera-Gonzalez, A.; Villegas, F.; Moreta, G.; Paredes, R. Association between the APOE ε4 Allele and Late-Onset Alzheimer’s Disease in an Ecuadorian Mestizo Population. Int. J. Alzheimer’s Dis. 2017, 2017, 1059678. [Google Scholar] [CrossRef]
- Spinney, L. Alzheimer’s disease: The forgetting gene. Nature 2014, 510, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997, 20, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jun, G.R.; Chung, J.; Mez, J.; Barber, R.; Beecham, G.W.; Bennett, D.A.; Buxbaum, J.D.; Byrd, G.S.; Carrasquillo, M.M.; Crane, P.K.; et al. Transethnic genome-wide scan identifies novel Alzheimer’s disease loci. Alzheimer’s Dement. 2017, 13, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Schmidt, M.; Klein, H.U.; Naj, A.C.; Hamilton-Nelson, K.L.; Larson, E.B.; Evans, D.A.; De Jager, P.L.; Crane, P.K.; Buxbaum, J.D.; et al. Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis. JAMA Neurol. 2021, 78, 102–113. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Cooper, S.; Liu, J.Z.; Barrio-Hernandez, I.; Bello, E.; Kumasaka, N.; Young, A.M.H.; Franklin, R.J.M.; Johnson, T.; Estrada, K.; et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 2021, 53, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Mok, K.Y.; Zhao, Q.; Chen, K.; Chen, Y.; Hardy, J.; Li, Y.; Fu, A.K.Y.; Guo, Q.; et al. Identification of genetic risk factors in the Chinese population implicates a role of immune system in Alzheimer’s disease pathogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Garzón Chavez, D.; Klitgaard Christensen, M.; Valle Tamayo, N.; Plana-Ripoll, O.; Rasmussen, S.A.; Roqué Figuls, M.; Alcolea, D.; Videla, L.; Barroeta, I.; et al. Association of Alzheimer Disease With Life Expectancy in People With Down Syndrome. JAMA Netw. Open 2022, 5, e2212910. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, A.; Babu, H.W.S.; Iyer, M.; Gopalakrishnan, A.V.; Vellingiri, B. Untangle the mystery behind DS-associated AD—Is APP the main protagonist? Ageing Res. Rev. 2023, 87, 101930. [Google Scholar] [CrossRef] [PubMed]
- Bejanin, A.; Iulita, M.F.; Vilaplana, E.; Carmona-Iragui, M.; Benejam, B.; Videla, L.; Barroeta, I.; Fernandez, S.; Altuna, M.; Pegueroles, J.; et al. Association of Apolipoprotein E ε4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome. JAMA Neurol. 2021, 78, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Ip, F.C.F.; Jiang, Y.; Cao, H.; Lv, G.; Zhong, H.; Chen, J.; Ye, T.; Chen, Y.; et al. Deep learning-based polygenic risk analysis for Alzheimer’s disease prediction. Commun. Med. 2023, 3, 49. [Google Scholar] [CrossRef]
- Weston, P.S.J.; Poole, T.; Nicholas, J.M.; Toussaint, N.; Simpson, I.J.A.; Modat, M.; Ryan, N.S.; Liang, Y.; Rossor, M.N.; Schott, J.M.; et al. Measuring cortical mean diffusivity to assess early microstructural cortical change in presymptomatic familial Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 112. [Google Scholar] [CrossRef]
- Stone, D.B.; Ryman, S.G.; Hartman, A.P.; Wertz, C.J.; Vakhtin, A.A. Specific White Matter Tracts and Diffusion Properties Predict Conversion From Mild Cognitive Impairment to Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 711579. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Clark, C.M.; Pontecorvo, M.J.; Beach, T.G.; Bedell, B.J.; Coleman, R.E.; Doraiswamy, P.M.; Fleisher, A.S.; Reiman, E.M.; Sabbagh, M.N.; Sadowsky, C.H.; et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. Lancet Neurol. 2012, 11, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Sabri, O.; Sabbagh, M.N.; Seibyl, J.; Barthel, H.; Akatsu, H.; Ouchi, Y.; Senda, K.; Murayama, S.; Ishii, K.; Takao, M.; et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimer’s Dement. 2015, 11, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Gamez, J.E.; Singh, U.; Sadowsky, C.H.; Villena, T.; Sabbagh, M.N.; Beach, T.G.; Duara, R.; Fleisher, A.S.; Frey, K.A.; et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015, 72, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E.; Chen, K.; Pietrini, P.; Rapoport, S.I.; Reiman, E.M. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am. J. Psychiatry 2002, 159, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K.; Ebmeier, K. Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol. 2011, 10, 667–670. [Google Scholar] [CrossRef] [PubMed]
- La Joie, R.; Ayakta, N.; Seeley, W.W.; Borys, E.; Boxer, A.L.; DeCarli, C.; Doré, V.; Grinberg, L.T.; Huang, E.; Hwang, J.H.; et al. Multisite study of the relationships between antemortem [(11)C]PIB-PET Centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimer’s Dement. 2019, 15, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lesman-Segev, O.H.; La Joie, R.; Iaccarino, L.; Lobach, I.; Rosen, H.J.; Seo, S.W.; Janabi, M.; Baker, S.L.; Edwards, L.; Pham, J.; et al. Diagnostic Accuracy of Amyloid versus (18) F-Fluorodeoxyglucose Positron Emission Tomography in Autopsy-Confirmed Dementia. Ann. Neurol. 2021, 89, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kumar, A.; Gajbhiye, A.; Santra, M.K.; Srikanth, R. Mass spectrometry-based proteomics in molecular diagnostics: Discovery of cancer biomarkers using tissue culture. BioMed Res. Int. 2013, 2013, 783131. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liao, L.; Chen, C.; Guo, Y.; Song, D.; Wang, Y.; Chen, Y.; Zhang, K.; Ying, M.; Li, S.; et al. Proteomics analysis of blood serums from Alzheimer’s disease patients using iTRAQ labeling technology. J. Alzheimer’s Dis. 2017, 56, 361–378. [Google Scholar] [CrossRef]
- Song, F.; Poljak, A.; Kochan, N.A.; Raftery, M.; Brodaty, H.; Smythe, G.A.; Sachdev, P.S. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease using iTRAQ quantitative proteomics. Proteome Sci. 2014, 12, 1–13. [Google Scholar] [CrossRef]
- Ludwig, C.; Gillet, L.; Rosenberger, G.; Amon, S.; Collins, B.C.; Aebersold, R. Data-independent acquisitionbased SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef]
- Shen, L.; Chen, C.; Yang, A.; Chen, Y.; Liu, Q.; Ni, J. Redox proteomics identification of specifically carbonylated proteins in the hippocampi of triple transgenic Alzheimer’s disease mice at its earliest pathological stage. J. Proteom. 2015, 123, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, Y.; Yang, A.; Chen, C.; Liao, L.; Li, S.; Ying, M.; Tian, J.; Liu, Q.; Ni, J. Redox proteomic profiling of specifically carbonylated proteins in the serum of triple transgenic Alzheimer’s disease mice. Int. J. Mol. Sci. 2016, 17, 469. [Google Scholar] [CrossRef]
- Baldeiras, I.; Santana, I.; Proença, M.T.; Garrucho, M.H.; Pascoal, R.; Rodrigues, A.; Duro, D.; Oliveira, C.R. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.E.; Borchers, C.H. Mass spectrometry based biomarker discovery, verification, and validation--quality assurance and control of protein biomarker assays. Mol. Oncol. 2014, 8, 840–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spellman, D.S.; Wildsmith, K.R.; Honigberg, L.A.; Tuefferd, M.; Baker, D.; Raghavan, N.; Nairn, A.C.; Croteau, P.; Schirm, M.; Allard, R.; et al. Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer’s Disease Neuroimaging Initiative (ADNI) CSF. PROTEOMICS–Clin. Appl. 2015, 9, 715–731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, J.J.; Whiteaker, J.R.; Ivey, R.G.; Burian, A.; Chowdhury, S.; Tsai, C.F.; Liu, T.; Lin, C.; Murillo, O.D.; Lundeen, R.A.; et al. Internal Standard Triggered-Parallel Reaction Monitoring Mass Spectrometry Enables Multiplexed Quantification of Candidate Biomarkers in Plasma. Anal Chem. 2022, 94, 9540–9547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell Proteomics. 2012, 11, 1475–1488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry. 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Therriault, J.; Kang, M.S.; Ng, K.P.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S.; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fransquet, P.D.; Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 2018, 58, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Piscopo, P.; Confaloni, A.; Denti, M.A. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules 2014, 19, 6891–6910. [Google Scholar] [CrossRef] [PubMed]
| Biomarkers | Screening | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| Diagnostic biomarkers of AD Low CSF Aβ42 or CSF Aβ42/t-tau ratio or Aβ42/ptau ratio or positive amyloidPET | Demographic data based such as CAIDE dementia risk score, ADAS-Cog symptomatic AD A+T+ is mandatory and exclusion of comorbidities should be conducted | |||
| Predictive biomarkers | Tau PET used to determine whether AD patients are more likely to benefit from anti-tau treatments | |||
| Prognostic biomarkers:Sort people based on likelihood of illness or include more patients in trials | Tau PET to determine which AD patients are most likely to experience cognitive decline more quickly ApoE-4 carriers in immunotherapy studies as a prognostic marker for ARIA | Tau PET to determine which AD patients are most likely to experience cognitive decline more quickly ApoE-4 carriers in immunotherapy studies as a prognostic marker for ARIA | ||
| Pharmacodynamic biomarkers: (i) Target engagement (ii) Disease modification (atrophy on MRI, hypometabolism on FDG PET, or increases in total tau in the CSF) | Phase 2′s essential result for moving on to Phase 3 | Essential outcome for the intervention to be classified as a DMT | ||
| Safety biomarkers | In immunotherapy regimens, liver function and other laboratory tests, an ECG, and an MRI are used to check for ARIA. | In immunotherapy regimens, liver function and other laboratory tests, an ECG, and an MRI are used to check for ARIA. | Liver function and other laboratory tests, ECG, MRI to monitor for ARIA in immunotherapy programs |
| Study Ref | Drug | Study Characteristics (Phase, Duration, n, Age Range) | Tools (Clinical Scales, Neuroimaging) | Biomarker Changes | Clinical/Neuropsychological Outcomes | Potential Relevance Both from Clinical and Biological Perspective |
|---|---|---|---|---|---|---|
| Fang et al. [44] | Buntanetap (Amyloid-β) | Phase 2 4 w N = 75 | CDR-SB and MMSE scores | CSF Aβ40: NS vs. placebo CSF Aβ42: NS vs. placebo CSF tTau: NS vs. placebo CSF pTau: NS vs. placebo CSF sAPPa: NS vs. placebo CSF sAPPb: NS compared to placebo CSF sTREM2: NS vs. placebo CSF GFAP: NS vs. placebo CSF YKL-40: NS compared to placebo CSF complement 3: NS vs. placebo CSF NFL: NS vs. placebo CSF NRGN: NS vs. placebo ptau: NA Study not powered to measure statistically significant differences, trends were visible. | ADAS-Cog11: Better score vs. baseline WAIS: Better score vs. baseline MMSE: NS vs. baseline CDR-SB: NS vs. baseline | Buntanetap as exploratory biomarker showing anti-inflammatory function and synaptic integrity |
| Ostrowitzki et al. [45] | Crenezumab (Amyloid-β) | Phase 3 100 w N = 805 50–85 | Amyloid PET or CSF | Discontinued due to earlier study not meeting primary endpoint | Discontinued due to earlier study not meeting primary endpoint | Crenezumab did not reduce clinical decline in early AD |
| Sims et al. [46] | Donanemab (Amyloid-β) | Phase 3 76 w N = 1800 60–85 | Gradual and progressive change in memory; Tau PET and amyloid PET | Plasma pTau217: decreased (Log10 −0.2) vs. placebo | iADRS: Better score compared to placebo | Donanemab significantly slowed clinical progression at 76 weeks in those with low/medium tau and in the combined low/medium and high tau pathology group according to PET biomarkers |
| Mintun et al., Pontecorvo et al. [47,48] | Donanemab (Amyloid-β) | Phase 2 72 w N = 266 60–85 | Gradual and progressive change in memory; positive Amyloid and Tau PET | Decreased Plasma pTau217 (Log10 −0.14) and GFAP: vs. placebo Plasma Aβ42/40, NFL: NS vs. to placebo | iADRS: Better score vs. to placebo ADAS-Cog13: Inconclusive CDR-SB/ADCS-iADL/MMSE: NS vs. placebo | Plasma biomarkers pTau217 and glial fibrillary acidic protein than placebo following donanemab might provide additional evidence of early symptomatic AD pathology change through anti-amyloid therapy. |
| Bateman et al. [49] | Gantenerumab (Amyloid-β) | Phase 3 116 w N = 1016 50–90 | CSF tau/Aβ42 or amyloid PET scan | Decreased CSF tTau, pTau181, Aβ40: vs. to placebo Increased CSF Aβ42: vs. placebo Decreased CSF NRGN and NFL vs. placebo Plasma pTau181: decreased vs. to placebo Plasma Aβ42: Increased vs. to placebo CSF pTau181: −23.8% Plasma pTau181: −24% | CDR-SB: NS compared to placebo ADAS-Cog13: NS compared to placebo ADCS-ADL: NS compared to placebo | Gantenerumab led to a lower amyloid plaque burden than placebo at 116 weeks without clinical improvement. |
| Bateman et al. [49] | Gantenerumab (Amyloid-β) | Phase 3 116 w N = 982 50–90 | CSF tau/Aβ42, amyloid PET scan | Decreased CSF tTau, pTau181, Aβ40 vs. placebo CSF Aβ42: increased compared to placebo CSF NRGN: decreased vs. placebo CSF NFL: decreased vs. placebo Plasma pTau181: decreased vs. placebo Increased plasma Aβ42 vs. placebo CSF pTau181: −23.8% Plasma pTau181: −21% | CDR-SB: NS compared to placebo ADAS-Cog13: NS compared to placebo ADCS-ADL: NS compared to placebo | Gantenerumab led to a lower amyloid plaque burden than placebo at 116 weeks without clinical improvement. |
| Van Dyck et al. [50] | Lecanemab (Amyloid-β) | Phase 3 78 w N = 1766 50–90 | Positive biomarker amyloid | Increased CSF Aβ42: vs. placebo Decreased CSF tTau and pTau181 vs. placebo Decreased CSF NRGN vs. placebo CSF Aβ40: NS vs. placebo CSF NFL: NS vs. placebo Increased Plasma Aβ42/40 vs. placebo Decreased Plasma pTau181, NFL, GFAP vs. placebo CSF pTau181: ~30 pg/mL compared to placebo −16 pg/mL compared to baseline Plasma pTau181: ~0.8 pg/mL | CDR-SB: Better score vs. placebo ADAS-Co14: Better score vs. placebo ADCOMS: Better score vs. placebo ADCS_MCI-ADL: Better score vs. placebo | Lecanemab reduced markers of amyloid in early AD and lower cognitive decline |
| Lerner et al. [51] | Efavirenz (ApoE, Lipids and Lipoprotein Receptors) | Phase 1 52 w N = 5 55–85 | MMSE CDR | Increased Plasma 24-OHC vs. baseline CSF Aβ40: NS compared to baseline CSF Aβ42: NS compared to baseline CSF tTau: NS compared to baseline CSF pTau181: NS compared to baseline | MoCA: NS compared to baseline | CYP46A1 activation by low-dose efavirenz increased brain cholesterol metabolism (as measured by high HC levels) in early AD |
| Wilkins et al. [52] | S-equol (growth factors and hormones) | Phase 2 4 w N = 40 50–90 | COX/CS | Increased COX/CS compared to baseline | MoCA: NS compared to baseline | S-equol May acts as a direct mitochondrial target engagement biomarker |
| Vissers et al. [53] | DNL747 (antiInflammatory) | Phase 1 12 w N = 16 55–85 | CSF Ab42 Amyloid PET | Decreased Plasma PBMC pRIPK1 vs. placebo | No clinical endpoints included | RIPK1 in the CNS as a potential therapeutic tool for AD |
| Prins et al. [54] | Neflamapimod (antiInflammatory) | Phase 2 24 w N = 161 55–85 | CDR, MMSE; CSF Ab1–42, p-Tau, CT, MRI compatible with AD | Decreased CSF tTau, pTau181 vs. placebo CSF NRGN: NS compared to placebo CSF NFL: NS compared to placebo CSF Aβ40: NS compared to placebo CSF Aβ42: NS compared to placebo CSF pTau181: −2.1 pg/mL | HVLT-R/WMS immediate and delayed recall/CDR-SB/MMSE: NS compared to placebo | Neflamapimod treatment lowered CSF biomarkers of synaptic dysfunction but not improve the cognitive scores |
| Sullivan et al. [55] | 3TC (lamivudine) | Phase 2 24 w N = 12 50–80 | CSF GFAP CSF Aβ42/40 CSF pTau181 Plasma Aβ42/40 CSF NFL Plasma GFAP Plasma pTau181 | CSF GFAP: decreased vs. baseline Plasma Aβ42/40: increased vs. baseline CSF NFL: NS compared to baseline CSF Aβ42/40: NS compared to baseline CSF pTau181: NS compared to baseline Plasma NFL: NS compared to baseline Plasma GFAP: NS compared to baseline Plasma pTau181: NS compared to baseline | MMSE: NS compared to baseline PACC-5: NS compared to baseline Attention, memory, naming, and EF tasks: NS compared to baseline | Decreased levels of AD and inflammatory biomarkers suggested positive effect of 3TC against MCI due AD |
| LaBarbera et al. [56] | CT1812 (Synaptic plasticity/neuroprotection) | Phase 1 1 w N = 3 50–80 | MRI and Abeta PET scan | CSF Aβ oligomers: Increased compared to baseline | No clinical endpoints were included | The degree of Aβ oligomers alteration aligned with the exposure level of CT1812 supports the use of Aβ oligomers as a biomarker of target engagement |
| Van Dyck et al. [57] | (CT1812 Synaptic plasticity/neuroprotection) | Phase 2 30 w N = 23 50–85 | Amyloid PET or Amyloid CSF | CSF Aβ40: NS compared to placebo CSF Aβ42: NS compared to placebo CSF tTau: NS compared to placebo CSF pTau: NS compared to placebo CSF NRGN: NS compared to placebo CSF synaptotagmin: NS vs. placebo CSF SNAP25: NS compared to placebo CSF NFL: NS compared to placebo | ADCS-ADL: High dose better scores compared to placebo ADAS-Cog11: NS compared to placebo MMSE: NS compared to placebo | No treatment effects relative to placebo from baseline at 24 weeks in neither SV2A nor FDG PET signal, the cognitive clinical rating scales, or in CSF biomarkers |
| Mummery et al. [58] | BIIB080 (MAPTrx) (tau) | Phase 2 61 w N = 46 50–74 | CSF biomarkers | CSF tTau: decreased compared to placebo CSF pTau181: decreased compared to placebo CSF tTau/Aβ42: decreased compared to placebo CSF NFL: NS compared to baseline CSF NFH: NS compared to baseline CSF NRGN: NS compared to baseline CSF YKL-40: NS compared to baseline CSF pTau181: Ranging from 0 to ~−55% based on dose | RBANS Total score: NS compared to baseline MMSE Total score: NS compared to baseline NPI-Q/FAQ Total score: NS compared to baseline | MAPTRx reduce tau levels in mild AD |
| Shulman et al. [59] | Gosuranemab (Tau) | Phase 2 238 w N = 654 50–80 | Positive for amyloid beta | CSF Unbound N-terminal tau: decreased in treatment compared to placebo CSF pTau181: Decreased in high dose treatment compared to placebo CSF tTau: Decreased in treatment compared to placebo CSF Aβ42: NS compared to placebo −7.1 pg/mL compared to baseline CSF pTau181: ~−25 pg/mL compared to placebo | CDR-SB/MMSE/ADCS-ADL/FAQ: NS compared to placebo group ADAS-Cog13: Significantly worse in treatment compared to placebo | No significant effects in cognitive and functional scores but reduced levels CSF Unbound N-terminal tau in gosuranemab group |
| Teng et al. [60] | Semorinemab (Tau) | Phase 2 73 w N = 457 50–80 | Amyloid PET CSF tTau and pTau181 | Plasma mid-domain tTau: increased compared to placebo CSF tTau: decreased from baseline CSF pTau181: decreased from baseline CSF pTau181 change: −9.7 pg/mL compared to placebo/−10.5 pg/mL compared to baseline | CDR-SB/ADAS-Cog13/RBANS/ ADCS-ADL/A-IADL-Q: NS compared to placebo | Semorinemab did not slow clinical AD progression |
| Monteiro et al. [61] | Semorinemab (Tau) | Phase 2 72 w N = 273 50–85 | MMSE CSF Ab42 Amyloid PET | Increased plasmatTau, pTau217 vs. placebo Decreased CSF tTau, pTau217, pTau181 vs. placebo CSF N-term Tau: NS compared to placebo Plasma pTau217: ~+88 pg/mL CSF pTau217: ~−50% CSF pTau181: ~−12% | ADAS-Cog11: Better score compared to placebo ADCS-ADL/CDR-SB/MMSE: NS compared to placebo | No treatment effects on functional scales nor on amyloid biomarkers |
| Fleiser et al. [62] | Zagotenemab (Tau) | Phase 2 104 w N = 360 60–85 | Progressive change in memory > 6 m Plasma pTau181, tTau, NFL | Increased plasma tTau, pTau181 vs. placebo Plasma NFL: NS compared to placebo Plasma pTau181: ~+15 pg/mL (low dose); ~+ 30 pg/mL (high dose) | iADRS/ADCS-iADL/ADAS-Cog13/CDR-SB/MMSE: NS compared to placebo | Zagotenemab did not slow clinical disease progression. Imaging biomarkers and plasma NfL without pharmacodynamic activity or disease progress. |
| Willis et al. [63] | Zagotenemab | Phase 1 64 w N = 24 54 | tTau | Plasma tTau: NS compared to placebo | No clinical endpoints included | The pharmacokinetics of zagotenemab were typical for a monoclonal antibody. Meaningful pharmacodynamic differences were not observed. |
| Type of Neuroimaging Biomarker | Utilityt in Research Context | Utility in Clinical Practice and Trials |
|---|---|---|
| Structural MRI | Atrophy of the hippocampus or the surrounding medial temporal lobe regions | |
| DWI | More indicative of early progressive cognitive change | |
| Functional MRI | Less connection between the medial temporal regions and the posterior cingulate cortex. | Not recommended for routine clinical usage (high cost, limited spatial resolution) |
| FDG PET | Reflective of synaptic activity and neuronal activating | Deficits in regional cerebral blood flowpredicting conversion to AD in people with MCIElevated microglial activity as an inflammatory marker to monitor the anti-inflammatory effects of AD treatments |
| Amyloid PET | Recognizing the intermediate-high neuropathologic alteration of ADThe retention time of PiB indicates the change of MCI to AD. | |
| Tau PET | Measures the fibrillar deposited form of the tau proteinto monitor in anti-tau trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougea, A.; Gourzis, P. Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. J. Clin. Med. 2024, 13, 4661. https://doi.org/10.3390/jcm13164661
Bougea A, Gourzis P. Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. Journal of Clinical Medicine. 2024; 13(16):4661. https://doi.org/10.3390/jcm13164661
Chicago/Turabian StyleBougea, Anastasia, and Philippos Gourzis. 2024. "Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine" Journal of Clinical Medicine 13, no. 16: 4661. https://doi.org/10.3390/jcm13164661
APA StyleBougea, A., & Gourzis, P. (2024). Biomarker-Based Precision Therapy for Alzheimer’s Disease: Multidimensional Evidence Leading a New Breakthrough in Personalized Medicine. Journal of Clinical Medicine, 13(16), 4661. https://doi.org/10.3390/jcm13164661






