Urgent or Emergent Endovascular Aortic Repair of Infective Aortitis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Preoperative and Postoperative Management
2.4. Endpoints Definition
2.5. Statistical Analysis
3. Results
3.1. Population
3.2. Early Results
3.3. Late Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gornik, H.L.; Creager, M.A. Aortitis. Circulation 2008, 117, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Foote, E.A.; Postier, R.G.; Greenfield, R.A.; Bronze, M.S. Infectious Aortitis. Curr. Treat. Options Cardiovasc. Med. 2005, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Skeik, N.; Ostertag-Hill, C.A.; Garberich, R.F.; Alden, P.B.; Alexander, J.Q.; Cragg, A.H.; Manunga, J.M.; Stephenson, E.J.; Titus, J.M.; Sullivan, T.M. Diagnosis, Management, and Outcome of Aortitis at a Single Center. Vasc. Endovasc. Surg. 2017, 51, 470–479. [Google Scholar] [CrossRef]
- Molacek, J.; Treska, V.; Baxa, J.; Certik, B.; Houdek, K. Acute Conditions Caused by Infectious Aortitis. Aorta 2014, 2, 93–99. [Google Scholar] [CrossRef]
- Lopes, R.J.; Almeida, J.; Dias, P.J.; Pinho, P.; Maciel, M.J. Infectious thoracic aortitis: A literature review. Clin. Cardiol. 2009, 32, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Deipolyi, A.R.; Czaplicki, C.D.; Oklu, R. Inflammatory and infectious aortic diseases. Cardiovasc. Diagn. Ther. 2018, 8 (Suppl. 1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Umezawa, H.; Goshima, M.; Hattori, T.; Nakamura, T.; Umeda, T.; Shiono, M. Primary infected abdominal aortic aneurysm: Surgical procedures, early mortality rates, and a survey of the prevalence of infectious organisms over a 30-year period. Surg. Today 2011, 41, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Guerrero, M.L.; Aguado, J.M.; Arribas, A.; Lumbreras, C.; de Gorgolas, M. The spectrum of cardiovascular infections due to Salmonella enterica: A review of clinical features and factors determining outcome. Medicine 2004, 83, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.T.; Wegener, O.R.; Grabitz, K.; Pillny, M.; Thomas, L.; Sandmann, W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: Experience with anatomic and extra-anatomic repair in 33 cases. J. Vasc. Surg. 2001, 33, 106–113. [Google Scholar] [CrossRef]
- Deipolyi, A.R.; Bailin, A.; Khademhosseini, A.; Oklu, R. Imaging findings, diagnosis, and clinical outcomes in patients with mycotic aneurysms: Single center experience. Clin. Imaging 2016, 40, 512–516. [Google Scholar] [CrossRef]
- Sörelius, K.; Budtz-Lilly, J.; Mani, K.; Wanhainen, A. Systematic Review of the Management of Mycotic Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 426–435. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsu, R.B. Primary Infected Aortic Aneurysm: Clinical Presentation, Pathogen, and Outcome. Acta. Cardiol. Sin. 2014, 30, 514–521. [Google Scholar]
- Raavi, L.; Garg, P.; Hussain, M.W.A.; Wadiwala, I.J.; Mateen, N.T.; Elawady, M.S.; Alomari, M.; Alamouti-Fard, E.; Pham, S.M.; Jacob, S. Mycotic Thoracic Aortic Aneurysm: Epidemiology, Pathophysiology, Diagnosis, and Management. Cureus 2022, 14, e31010. [Google Scholar] [CrossRef]
- Zehr, K.J.; Mathur, A.; Orszulak, T.A.; Mullany, C.J.; Schaff, H.V. Surgical treatment of ascending aortic aneurysms in patients with giant cell aortitis. Ann. Thorac. Surg. 2005, 79, 1512–1517. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar]
- Engelke, C.; Sandhu, C.; Morgan, R.A.; Belli, A.M. Endovascular repair of thoracic aortic aneurysm and intramural hematoma in giant cell arteritis. J. Vasc. Interv. Radiol. 2002, 13, 625–629. [Google Scholar] [CrossRef]
- Baril, D.T.; Carroccio, A.; Palchik, E.; Ellozy, S.H.; Jacobs, T.S.; Teodorescu, V.; Marin, M.L. Endovascular treatment of complicated aortic aneurysms in patients with underlying arteriopathies. Ann. Vasc. Surg. 2006, 20, 464–471. [Google Scholar] [CrossRef]
- Lin, R.; He, H.P.; Zhao, Y.; Lv, J.B.; Peng, J.X.; Yin, H.H. Outcomes Following Different Management of Mycotic Infrarenal Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2024, 11, 15266028241253128. [Google Scholar] [CrossRef]
- Hosaka, A.; Takahashi, A.; Kumamaru, H.; Azuma, N.; Obara, H.; Miyata, T.; Obitsu, Y.; Zempo, N.; Miyata, H.; Komori, K.; et al. Prognostic factors after open and endovascular repair for infected native aneurysms of the abdominal aorta and common iliac artery. J. Vasc. Surg. 2024, 79, 1379–1389. [Google Scholar] [CrossRef]
- Oderich, G.S.; Panneton, J.M.; Bower, T.C.; Cherry, K.J., Jr.; Rowland, C.M.; Noel, A.A.; Hallett, J.W., Jr.; Gloviczki, P. Infected aortic aneurysms: Aggressive presentation, complicated early outcome, but durable results. J. Vasc. Surg. 2001, 34, 900–908. [Google Scholar] [CrossRef]
- Ferrer, C.; Cao, P.; De Rango, P.; Tshomba, Y.; Verzini, F.; Melissano, G.; Coscarella, C.; Chiesa, R. A propensity-matched comparison for endovascular and open repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2016, 63, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.; Orrico, M.; Spataro, C.; Coscarella, C.; Ronchey, S.; Marino, M.; Giudice, R.; Mangialardi, N. Outcomes of multibranched off-the-shelf stent graft in elective and urgent/emergent repair of complex aortic aneurysms with narrow internal aortic lumen. J. Vasc. Surg. 2022, 76, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Dhillan, R.; Manral, S. Endovascular salvage of tubercular aortitis presenting as descending thoracic aortic pseudoaneurysm in association with vertebral tuberculosis. BMJ Case Rep. 2022, 15, e251838. [Google Scholar] [CrossRef] [PubMed]
- Pulimamidi, S.; Caputo, F.J.; Fraimow, H.S.; Nahra, R.; Tsigrelis, C. Salmonella aortitis treated with endovascular aortic repair. Ann. Vasc. Surg. 2014, 28, 1314.e5–1314.e10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Kuo, I.J.; Fujitani, R.M.; Kabutey, N.K. Endovascular Management of Concomitant Thoracic and Abdominal Aortic Ruptures Resulting from Brucellosis Aortitis. Ann. Vasc. Surg. 2017, 38, 190.e1–190.e4. [Google Scholar] [CrossRef]
- Koeppel, T.A.; Gahlen, J.; Diehl, S.; Prosst, R.L.; Dueber, C. Mycotic aneurysm of the abdominal aorta with retroperitoneal abscess: Successful endovascular repair. J. Vasc. Surg. 2004, 40, 164–166. [Google Scholar] [CrossRef][Green Version]

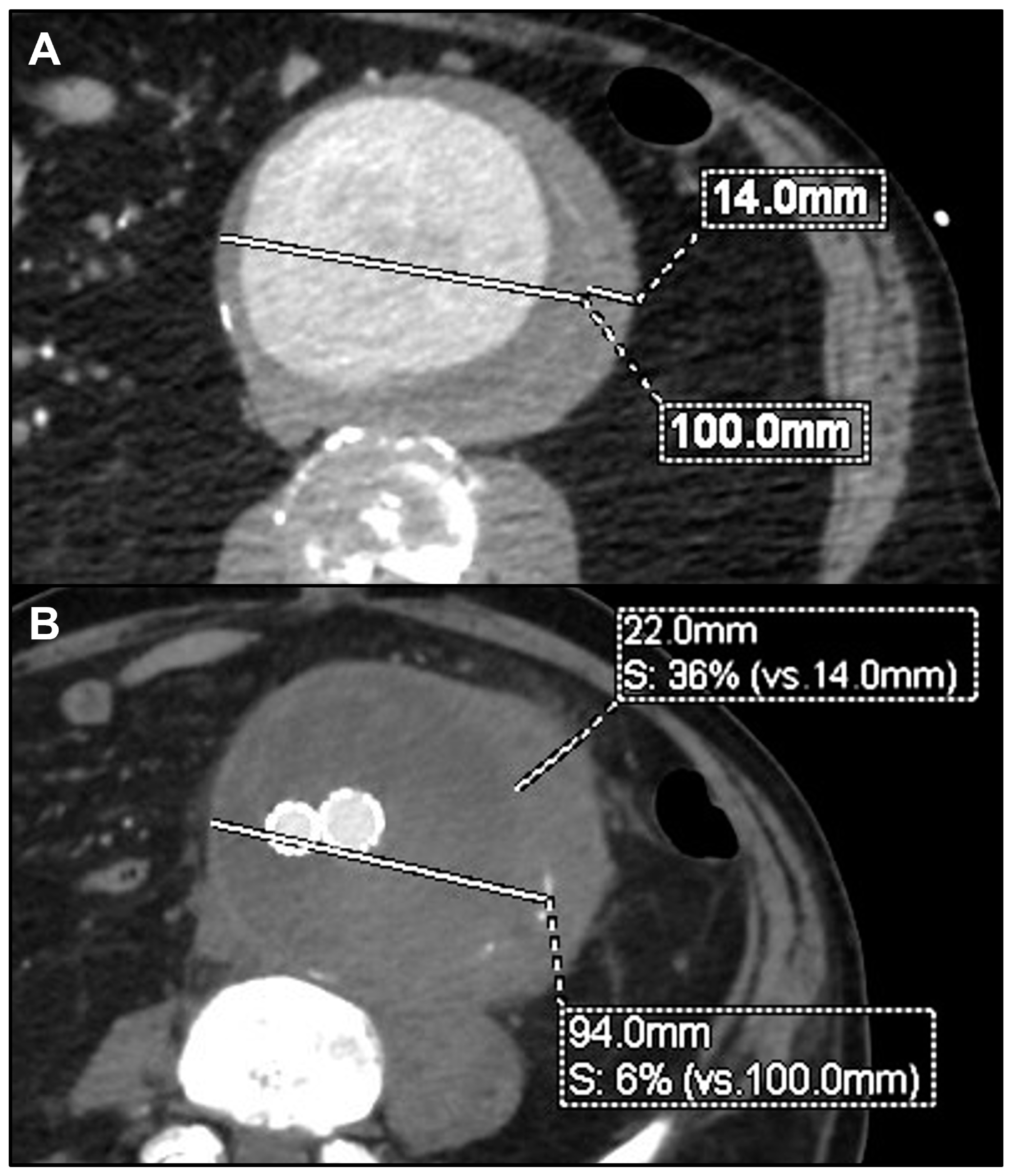
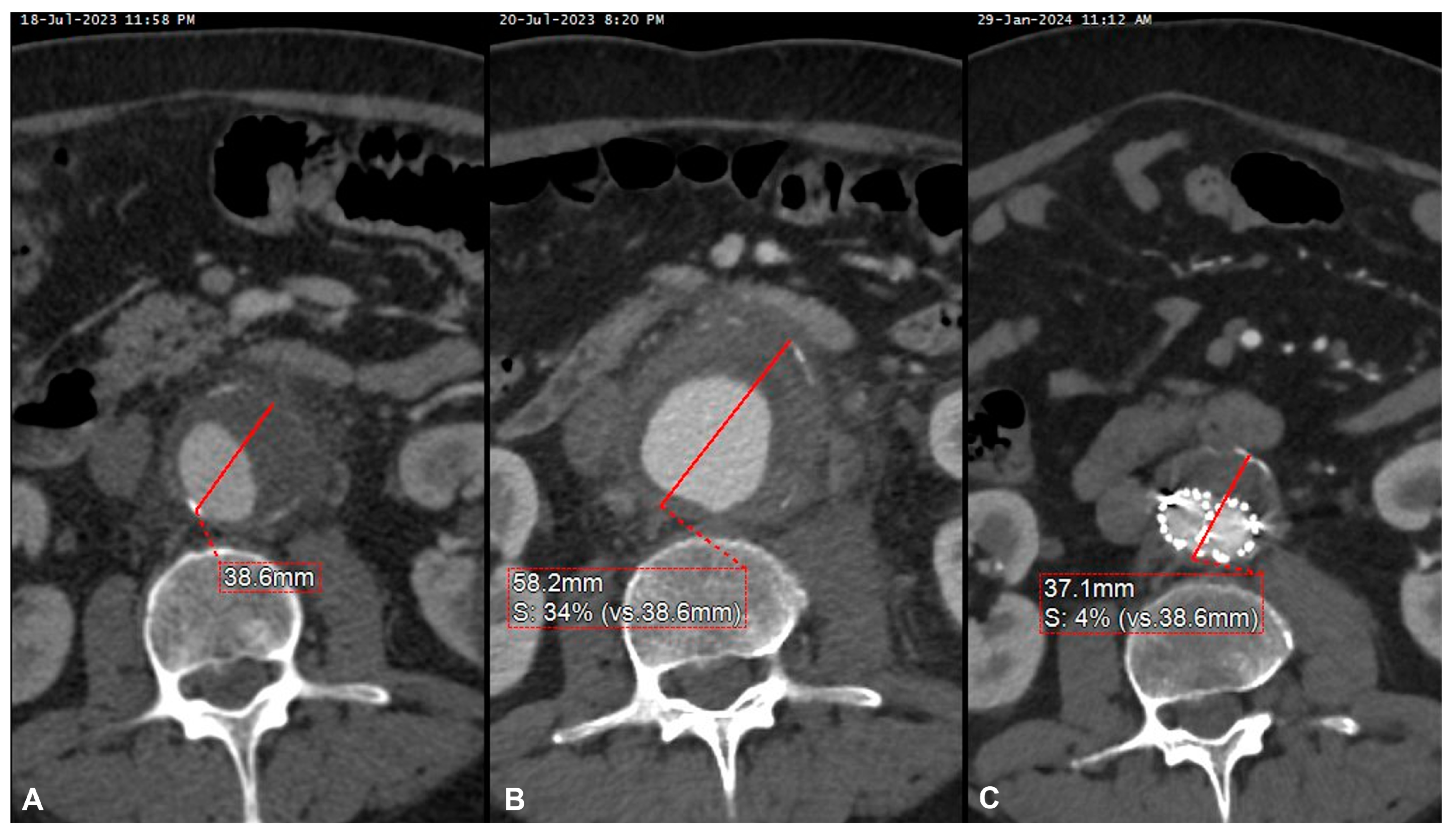

| Patients | 15 (100%) |
| Mean age (years) | 74.2 ± 8.3 (range 61–87) |
| Male | 14 (93%) |
| Female | 1 (7%) |
| Hypertension | 15 (100%) |
| Chronic kidney disease | 6 (40%) |
| Chronic obstructive pulmonary disease | 9 (60%) |
| Coronary artery disease | 8 (53%) |
| Diabetes mellitus | 7 (47%) |
| Mean aortic wall thickening (mm) | 30.3 ± 19.2 |
| Organism isolated | |
| Staphylococus Aureus | 7 (47%) |
| Salmonella spp. Non-Typhi | 3 (20%) |
| Escherichia coli | 3 (20%) |
| Brucella spp. | 1 (7%) |
| Pseudomonas and Klebsiella | 1 (7%) |
| Lesion location | |
| Thoracic aorta | 2 (13%) |
| Toracoabdominal and pararenal aorta | 5 (33%) |
| Abdominal aorta | 8 (53%) |
| Endovascular technique | |
| TEVAR | 4 (27%) |
| BEVAR | 3 (20%) |
| Ch-EVAR | 1 (7%) |
| EVAR | 7 (47%) |
| N | Sex | Age | Etiology | Organism Isolated | Fever | Lesion Characteristics | Antibiotic Therapy | Endovascular Technique | Outcome | FU (m) | FU Findings | Reintervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 85 | Endocarditis | Staphylococcus aureus | Yes | Symptomatic rapidly growing thoracic PAU | Oxacyllin | TEVAR | Alive | 48 | Proximal disease progression | Redo TEVAR ± LSA embolization |
| 2 | M | 70 | Spondylodiscitis | Staphylococcus aureus | No | Contained ruptured type I TAAA | Linezolid | TEVAR ± SMA Chimney ± RRA Periscope ± CT embolization | Alive | 52 | Stability with type II endoleak | - |
| 3 | M | 63 | Spondylodiscitis | Staphylococcus aureus | No | Contained ruptured infrarenal atheromatous plaque ± periaortic abscess | Oxacyllin | EVAR | Alive | 36 | Stability | - |
| 4 | M | 62 | Endocarditis | Staphylococcus aureus | Yes | Contained ruptured PAAA | Linezolid ± Lamivudina | BEVAR | Alive | 50 | Shrinkage | - |
| 5 | M | 68 | Sepsis in porcelain aorta | Salmonella spp. Non-Typhi | Yes | Contained ruptured infrarenal atheromatous plaque | Ampicillin ± Sulbactam | EVAR (tube graft) | Alive | 57 | Proximal and distal disease progression | Bifurcated EVAR |
| 6 | F | 75 | Endocarditis | Staphylococcus aureus | Yes | Contained ruptured supraceliac atheromatous plaque | Oxacyllin | TEVAR ± CT embolization | Alive | 20 | Shrinkage | - |
| 7 | M | 78 | Spondylodiscitis | Staphylococcus aureus | No | Symptomatic multi-lobular type IV TAAA | Oxacyllin | BEVAR | Alive | 36 | Stability | - |
| 8 | M | 72 | Sepsis in pre-existing TAAA | Escherichia coli | Yes | Symptomatic saccular type IV TAAA ± peri-aortic gas | Amoxicillin ± Clavulanate | BEVAR | Alive | 11 | Stability ± non-contiguous new arch lesion | TEVAR |
| 9 | M | 69 | Sepsis in pre-existing AAA | Salmonella spp. Non-Typhi | Yes | Symptomatic rapidly growing AAA | Cotrimoxazol | EVAR | Alive | 7 | Increase in wall thickening | - |
| 10 | M | 89 | Pneumonia | Pseudomonas ± Klebsiella | Yes | Contained ruptured saccular TAA | Piperacillin ± Tazobactam | TEVAR | Death in 15° POD | - | - | - |
| 11 | M | 87 | Pyelonephritis | Escherichia coli | No | Symptomatic rapidly growing AAA | Piperacillin ± Tazobactam | EVAR | Alive | 4 | Stability | - |
| 12 | M | 73 | Pyelonephritis | Escherichia coli | No | Ruptured rapidly growing AAA | Piperacillin ± Tazobactam | EVAR | Death in 11° POD | - | - | - |
| 13 | M | 75 | Pancreatitis | Brucella spp. | No | Ruptured juxtarenal pseudoaneurysm | Doxycycline | Ch-EVAR | Alive | 60 | Stability | - |
| 14 | M | 87 | Pancreatitis | Salmonella spp. Non-Typhi | No | Symptomatic multiple infrarenal PAU | Cotrimoxazol | EVAR | Alive | 16 | Stability | - |
| 15 | M | 61 | Endocarditis | Staphylococcus aureus | No | Symptomatic rapidly growing AAA | Oxacyllin | EVAR | Alive | 11 | Shrinkage | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orellana Davila, B.; Mancusi, C.; Coscarella, C.; Spataro, C.; Carfagna, P.; Ippoliti, A.; Giudice, R.; Ferrer, C. Urgent or Emergent Endovascular Aortic Repair of Infective Aortitis. J. Clin. Med. 2024, 13, 4669. https://doi.org/10.3390/jcm13164669
Orellana Davila B, Mancusi C, Coscarella C, Spataro C, Carfagna P, Ippoliti A, Giudice R, Ferrer C. Urgent or Emergent Endovascular Aortic Repair of Infective Aortitis. Journal of Clinical Medicine. 2024; 13(16):4669. https://doi.org/10.3390/jcm13164669
Chicago/Turabian StyleOrellana Davila, Bernardo, Carlotta Mancusi, Carlo Coscarella, Claudio Spataro, Paolo Carfagna, Arnaldo Ippoliti, Rocco Giudice, and Ciro Ferrer. 2024. "Urgent or Emergent Endovascular Aortic Repair of Infective Aortitis" Journal of Clinical Medicine 13, no. 16: 4669. https://doi.org/10.3390/jcm13164669
APA StyleOrellana Davila, B., Mancusi, C., Coscarella, C., Spataro, C., Carfagna, P., Ippoliti, A., Giudice, R., & Ferrer, C. (2024). Urgent or Emergent Endovascular Aortic Repair of Infective Aortitis. Journal of Clinical Medicine, 13(16), 4669. https://doi.org/10.3390/jcm13164669






