Abstract
Background: Despite the availability of treatments such as surgery and hormonal therapy, women with endometriosis often endure chronic problems. This review aims to evaluate the effectiveness and safety of neuropelveology. Methods: In a systematic review with a meta-analysis, we searched three electronic databases: MEDLINE (PubMed), Scopus, Embase, and Web of Science (WOS). The search was conducted in January 2024 with no date or language restrictions using a carefully curated set of keywords. We conducted a comprehensive review, including all observational and clinical trials reporting data on neuropelveology approaches in the management of endometriosis, irrespective of geographical location. The studies included in our review were required to be published in peer-reviewed journals and be available in any language, with at least an abstract in English. The data of all included studies were summarized in excel (version 19) and were analyzed by Comprehensive Meta-analysis v3.3 (Biostat) and STATA (version 17). A multilevel meta-analysis was performed on studies with two arms (intervention and control) to evaluate the efficacy of neuropelveology in managing women with endometriosis. Results: After screening 476 records, 30 studies, published from 1952 to 2021, were included in this review, each employing various methodologies. The studies were divided into the following three categories: (a) efficacy of neurectomy or nerve resection (n = 20), (b) efficacy of neurolysis (nerve blocks) (n = 4), and (c) efficacy of neuromodulation (n = 6) in the management of endometriosis. Among the studies evaluating the efficacy of neurectomy or nerve resection, 10 studies (with 18 group comparisons) were included in the random-effects meta-analysis. Treatment success (not occurrence of pain) was higher with neurectomy vs. controls (RR = 0.497, 95% CI = 0.236 to 1.04, p = 0.06 (for experimental studies) and RR = 0.248, 95% CI = 0.14 to 0.43, p < 0.001 (for observational studies)), representing a 50% and 75.2% risk reduction in the recurrence of pain in experimental and observational studies, respectively. Similarly, neurolysis, particularly superior hypogastric plexus blocks and uterine nerve ethanol neurolysis, demonstrated encouraging outcomes in pain reduction and an improved quality of life for women with endometriosis. The efficacy of neuromodulation in managing endometriosis symptoms appears promising but requires further investigation. Conclusions: In conclusion, neuropelveology approaches, such as neurectomy, neurolysis, and neuromodulation, offer significant potential for pain reduction in endometriosis patients, albeit with risks of complications and high recurrence rates, necessitating careful patient selection and long-term monitoring.
1. Introduction
Endometriosis, characterized by the ectopic growth of endometrial tissues outside the uterine cavity, stands as a prevalent gynecological disorder affecting approximately 6–20% of reproductive-age women [1]. This estrogen-dependent condition carries significant morbidity and ranks among the primary causes of infertility, dyspareunia, and dysmenorrhea, with the latter being the most frequently reported complaint [2]. The associated pain can be divided into visceral pain caused by the autonomous nervous system and somatic pain caused by affection of the somatic nervous system. Most of the pain in the pelvis that is associated with endometriosis can be referred to the autonomous nervous system (sympathicus and parasympathicus) [3]. While pelvic pain predominates, women with symptomatic endometriosis may also experience pain in the lower back and lower extremities unrelated to the autonomous nervous system [4,5,6]. Notably, affected individuals face a heightened risk of enduring chronic pelvic, genital, and low lumbar pain, often accompanied by distal radiation pain due to sacral plexus or somatic nerve compression [7,8,9,10]. Despite available conservative treatments such as pain medication and hormonal therapy, along with central pain management, satisfactory outcomes remain elusive. Conversely, surgical interventions carry considerable risks of severe and potentially irreversible complications [11,12,13,14,15]. The emergence of neuropelveology as a distinct discipline dedicated to pelvic nervous system pathologies and enhanced neurologic diagnoses in chronic pelvic pain presents a promising avenue for intervention [10]. Encompassing various medical treatments and pelvic nerve surgeries, neuropelveology spans techniques from decompression and neurolysis to nerve reconstruction and resection (e.g., sciatic nerve endometriosis) to pelvic neurofunctional surgery [16]. In response to the growing demand for innovative therapeutic approaches and heightened medical community interest, the establishment of the International Society of Neuropelveology in 2014 aims to drive advancements in research, standardize diagnostic and therapeutic procedures, and facilitate ongoing medical education in neuropelveology (see www.theison.org) [10]. In light of this context, this study endeavors to review and analyze the role of neuropelveology in managing endometriosis. By assessing available data, it seeks to elucidate the efficacy and safety of neuropelveology.
2. Materials and Methods
This review adheres to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [17] and was prospectively registered at PROSPERO with the registration ID CRD42024512390. The primary objective of this review is to evaluate the efficacy and safety of neuropelveology in addressing complications associated with endometriosis.
The PICO question guiding this analysis is structured as follows:
P—population: women diagnosed with endometriosis (as per the International Classification of Diseases (ICD)-11 code GA10, confirmed histologically); I—intervention: neuropelveology interventions including (a) neurectomy or pelvic nerve resection, (b) neurolysis (nerve block), and (c) an implantation of leads for neuromodulation; C—control: conservative surgery or non-intervention; O—outcome: effectiveness and safety of the interventions; S—study designs: clinical trials and observational studies.
2.1. Search Strategy and Information Sources
We conducted a thorough search for relevant articles in three reputable databases: PubMed/MEDLINE, Scopus, and Web of Science. The search was conducted in January 2024 with no date or language restrictions, using a carefully curated set of keywords. These keywords included “neuropelveology”, “neurosurgical techniques”, “endometriosis”, “intensity of pain”, “sexual function”, “health-related quality of life”, “complications”, and “adverse effects”. To optimize the search results, we employed MeSH keywords and Boolean operators (such as AND, OR). The reference lists of relevant papers were reviewed manually by one reviewer (L.A.). For transparency and reproducibility, the search strategy and a comprehensive list of search terms can be found in Supplementary Materials.
2.2. Study Selection
The study selection process was facilitated using the EndNote software (version X9, Thomson Reuters), which aided in listing and screening studies. The selection process was divided into three distinct phases: screening, selection, and data abstraction. During the screening phase, two trained authors (L.A. and A.M.M.) independently evaluated titles and abstracts of the identified studies. A total of 221 articles were deemed potentially relevant and subsequently progressed to the full-text review stage. In the selection phase, two authors (L.A. and S.H.) independently assessed the full-text articles against the inclusion criteria using a checklist-style form. Articles meeting the inclusion criteria were included in the final analysis. To ensure consistency and resolve any discrepancies, a third expert author (I.A.) reviewed the full-text articles and addressed any inconsistencies or disagreements that arose during the selection process. This rigorous approach to study selection helped to minimize biases and ensured that only high-quality and relevant studies were included in the systematic review. We conducted a comprehensive review, including all observational and clinical trials reporting data on neuropelveology approaches in the management of endometriosis, irrespective of geographical location. Reference lists of included papers and relevant meta-analyses were manually searched. Additionally, we thoroughly assessed reports from abstracts and presentations at major gynecological meetings to mitigate the risk of a publication bias. It is worth noting that the studies included in our review were required to be published in peer-reviewed journals and be available in any language, with at least an abstract in English. Notably, we imposed no restrictions on the publication date, thereby ensuring a comprehensive consideration of relevant literature spanning diverse temporal contexts. This approach aimed to provide a robust and holistic analysis of the role of neuropelveology in addressing the challenges posed by endometriosis. Studies of an experimental nature, investigations utilizing human cadavers, letters to the editor, review articles, case reports, video articles, and those primarily focused on teaching techniques or introducing novel procedures were all excluded from our analysis. Additionally, studies lacking full-text availability and those addressing adenomyosis, myoma, or other gynecological pathologies were omitted.
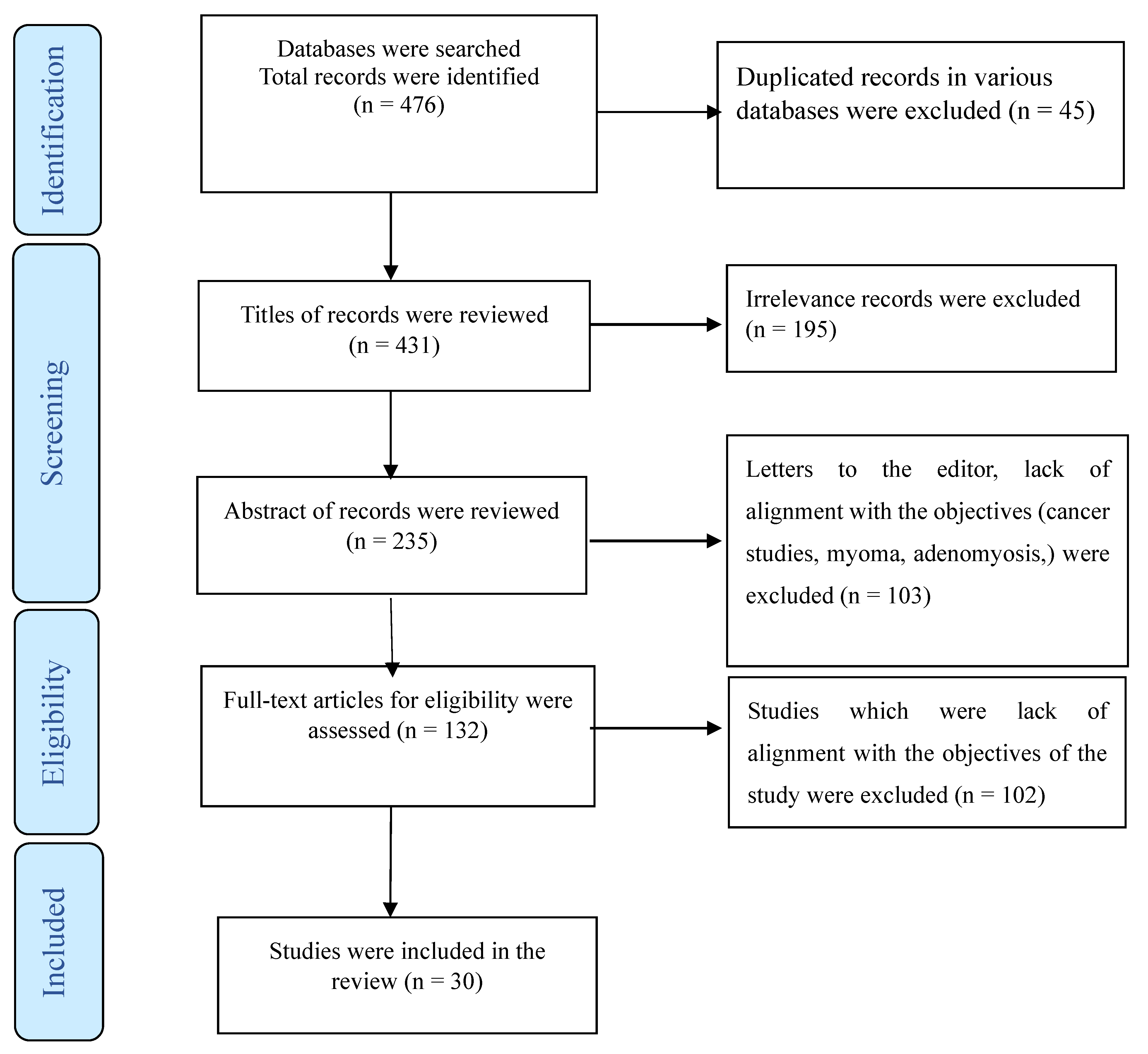
Furthermore, studies involving combined medical interventions, such as gonadotropin hormone-releasing hormone analogs, were not considered. Participants with coexisting psychiatric illnesses, including depression, were also excluded from our study cohort. To elucidate our selection process, we employed a PRISMA flowchart (Figure 1), providing a visual representation of how studies were screened and included in our review.
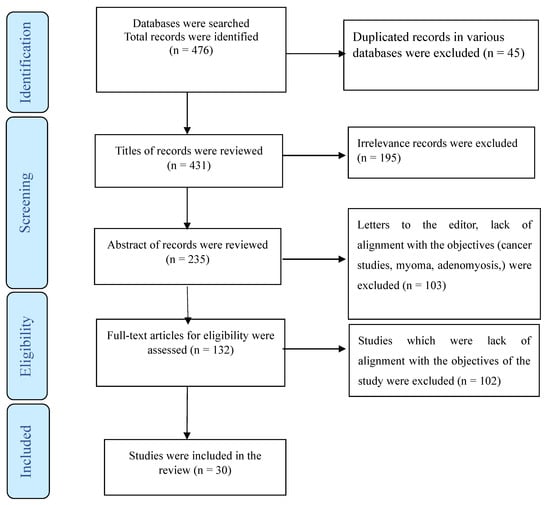
Figure 1.
The process of screening and selecting relevant studies.
2.3. Outcomes
The primary endpoint of this systematic review centered on treatment success, specifically defined as the proportion of women experiencing a resolution of pain symptoms (including dysmenorrhea, dyspareunia, pelvic pain, and dyschezia) following neuropelveology interventions. Treatment success was determined by the absence or non-recurrence of moderate-to-severe pain during the designated follow-up period.
Additionally, secondary endpoints encompassed various symptom changes post-neuropelveology procedures, such as alterations in pregnancy rates, sexual function, quality of life, subjective satisfaction reported by women, levels of anxiety, non-opioid analgesic consumption during menses, voiding symptoms at different postoperative intervals, as well as peri- and postoperative complications.
2.4. Data Synthesis and Extraction
The two reviewers independently extracted data from the included studies using a customized data extraction table created in Microsoft Excel. The data extracted from the included articles for further analysis encompassed various key elements, structured to facilitate a comprehensive understanding and comparison. These included the following:
Demographic information (title of the study, last author’s name, date of publication, and country of publication); characteristics of the sample (participants, sample size, and age distribution); study-specific parameters (details of the intervention (neuropelveology approach); details of the control group (conservative surgery); study design; agent (dosage and amount of agent for injection); outcomes measured and instruments used for assessment; surgery techniques applied (open, laparoscopic, robot-assisted laparoscopic); and duration of follow-up); and main results (categorization of results based on the type of intervention applied) and complications (intraoperative and postoperative complications). To organize and present this information effectively, tables were utilized to describe both the characteristics of the studies and the extracted data. These tables served as valuable tools for summarizing and synthesizing the findings, enabling a clear interpretation and comparison across studies. In instances where discrepancies arose between the two reviewers, a process of discussion and debate ensued until a consensus was reached. This collaborative approach ensured that any differences in data extraction were resolved, and a unified interpretation of the findings was achieved.
2.5. Assessment of Risk of Bias in Individual Studies
Depending on the type of investigation, four tools were used to assess the methodological quality of the studies:
- (a)
- The Cochrane Risk of Bias Tool (RoB 2) for randomized controlled trials does not have specific predefined cutoffs but rather assesses the risk of bias in various domains of a study. For each domain, the RoB provides options to assign a judgment of “low risk”, “high risk”, or “unclear risk” of bias [18].
- (b)
- The methodological index for non-randomized studies (MINORS) tool includes twelve questions and divides articles into two qualitative ranks (low and high risk of bias) [19].
- (c)
- The Newcastle–Ottawa Scale (NOS) for cohort studies was used. The quality of each study was rated using the following scoring algorithms: ≥7 points were considered as “good”, 2 to 6 points were considered as “fair”, and ≤1 point was considered as “poor” quality [20].
- (d)
- The National Institutes of Health’s (NIH) quality assessment tool for case series studies, last updated in July 2021, consists of nine questions and assigns three quality ratings (good, fair, and poor) [21].
Two independent reviewers (L.A., S.H.) scored each study, and any discrepancies between reviewers were resolved by a consensus that was reached after a discussion, with the possibility of consulting a third reviewer if necessary.
2.6. Data Analysis
All statistical analyses were conducted using Excel (version 19), Comprehensive Meta-analysis v3.3 (Biostat), and STATA (version 17). The meta-analysis was performed on studies with two arms (intervention and control) to evaluate the efficacy of neurectomy in managing women with endometriosis. The studies evaluated different types of pain, including dysmenorrhea, dyspareunia, pelvic pain, and dyschezia, often measured using a visual analog scale (VAS). In cases where studies reported multiple types of pain, each outcome was analyzed separately. To conduct the meta-analysis, women treated with neurectomy and conservative surgery were compared to those receiving conservative surgery alone (controls) using the risk ratio (RR) statistic. An RR > 1 indicated a higher risk of pain recurrence, while an RR < 1 indicated a lower risk of pain recurrence with neurectomy. The pooled estimate and 95% confidence interval (CI) were computed for each outcome, namely, dysmenorrhea, dyspareunia, pelvic pain, and dyschezia. A random-effects model was employed for the multilevel meta-analysis, acknowledging the distribution of true effects due to diverse study designs. The analysis encompassed three levels: (a) level 1, where effect sizes varied among individuals within the primary study (sampling error variance); (b) level 2, where effect sizes differed among various outcomes (dysmenorrhea, dyspareunia, pelvic pain, and dyschezia) within the primary studies; and (c) level 3, where effect sizes varied across different studies. Corresponding forest plots were constructed for experimental and observational studies to present individual study findings and pooled meta-analysis results, where applicable. The heterogeneity of outcomes was assessed using the I2 statistic, categorizing it as small (25%), moderate (50%), and large (75%) [22]. Significant heterogeneity was defined by a Cochran Q test, with p < 0.1 or I2 > 50% [23]. A sensitivity analysis was conducted. Publication bias was visually assessed with funnel plots and quantitatively assessed with Harbord’s test [24]. The prediction interval for true effect sizes was calculated.
3. Results
3.1. Search Results
A total of 476 publications, 45 of which were duplicate articles, were indexed in three or at least in two databases (Web of Science, PubMed, and Scopus). Following a review of titles and abstracts, 298 were excluded. Among the remaining articles, 164 were omitted due to their lack of alignment with the objectives of this study. We reached out to the authors of three studies for access to the full text or for a clarification of the reported results [25,26,27]. Furthermore, four studies were deemed eligible for inclusion in this review. However, due to a lack of data separation in the findings section, we were unable to include them in our analysis. [28,29,30,31]. Finally, the present review comprised 30 studies (Figure 1) utilizing various methodologies, including randomized or non-randomized controlled trials, prospective clinical case series, and retrospective studies. Some studies did not provide data about the study design. These studies were conducted in various countries, including the USA [32,33,34,35,36,37,38,39,40], Italy [26,41,42,43,44], Finland [45,46], Taiwan [47,48], Turkey [49,50], Australia [51], Iran [52], New Zealand [53], Germany [9], Poland [54], Switzerland [55], France [56], China [57], the UK [58], France, and Danmark [27]. The detailed characteristics of each study are summarized in Table 1, Table 2 and Table 3.

Table 1.
Overall view of studies which consider neurectomy or nerve resection in the management of endometriosis.

Table 2.
Characteristics of studies that reviewed outcomes of neurectomy or nerve resection in the management of endometriosis.

Table 3.
Overall view of studies which considered neurolysis (nerve blocks) in the management of endometriosis.
Among the reviewed studies in this systematic review, 10 studies (with 18 group comparisons) that evaluated the effectiveness of neurectomy on pain related to endometriosis were included in the meta-analysis [32,33,34,35,41,42,43,44,53,57].
3.2. Synthesis of Results
Based on the applied neuropelveology approach, studies were divided into the following three categories: (a) efficacy of neurectomy or nerve resection in the management of endometriosis (n = 20), (b) efficacy of neurolysis (nerve blocks) in the management of endometriosis (n = 4), and (c) efficacy of neuromodulation in the management of endometriosis (n = 6).
- (a)
- Efficacy of neurectomy or nerve resection in the management of endometriosis:
In total, we found 20 studies, conducted between 1952 and 2017, that evaluated the effectiveness of presacral neurectomy (PSN) (n = 16) [32,33,34,35,36,37,38,41,42,43,44,47,48,51,54,57], uterine nerve ablation (UNA) (n = 2) [53,58], and a decompression or large resection of the sciatic nerve (>30% of the nerve) (n = 2) [27,55] in the management of endometriosis. Out of the 20, 10 studies were conducted in two groups (intervention vs. control), and the remaining 10 studies were conducted without control groups. One of the studies had two types of designs [35]. In various studies, women of different average age ranges were included, spanning from 27.8 to 32.5 years old. The duration of follow-ups for patients varied significantly across studies, ranging from 2 to 96 months. An overall view of the studies which considered neurectomy or nerve resection in the management of endometriosis is shown Table 1. The risk of bias was high for 6 studies, low for 2 studies, fair for 10 studies, and poor for 2 studies and there were some concerns for 1 study (Table 2).
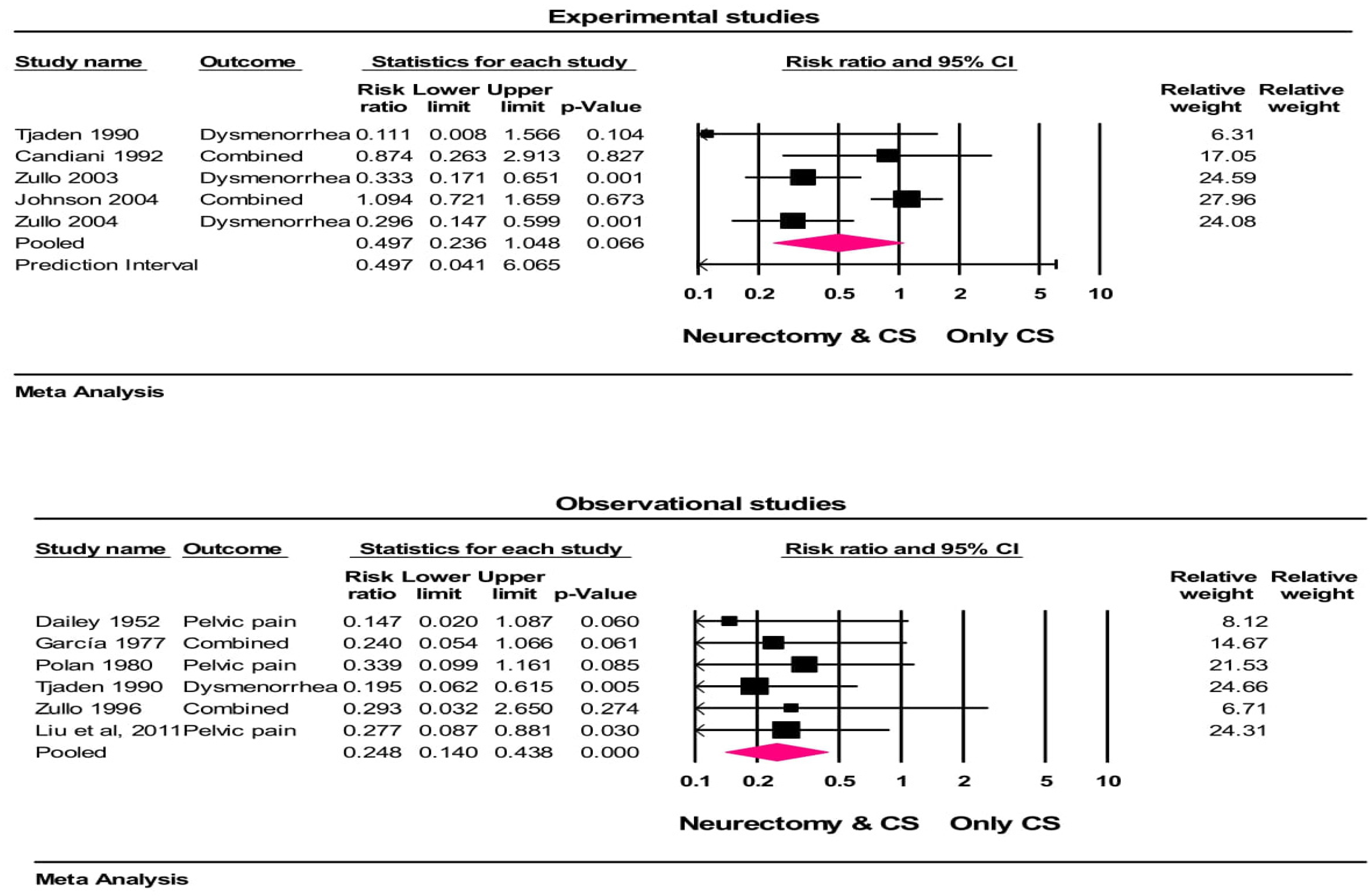
Among 10 studies with 18 group comparisons (in some studies more than one outcome had been evaluated), the crude rates of treatment were 81% (374/462) with neurectomy and 61% (327/532) with controls at follow-ups. The heterogeneity in the treatment success rate among the studies was moderate (I2 = 46.9%, p = 0.113). In the random effects meta-analysis, the treatment success (not occurrence of pain) was higher with neurectomy vs. controls (RR = 0.497, 95% CI = 0.236 to 1.04, p = 0.06 (for experimental studies) and RR = 0.248, 95% CI = 0.14 to 0.43, p < 0.001 (for observational studies)), representing a 50% and 75.2% risk reduction in the recurrence of pain in experimental and observational studies, respectively. Based on the results of the experimental studies, this risk reduction was not statistically significant (Figure 2). The results of a one-study-removed analysis suggest that the meta-analysis conclusions were not significantly influenced by any single study (all p < 0.001).
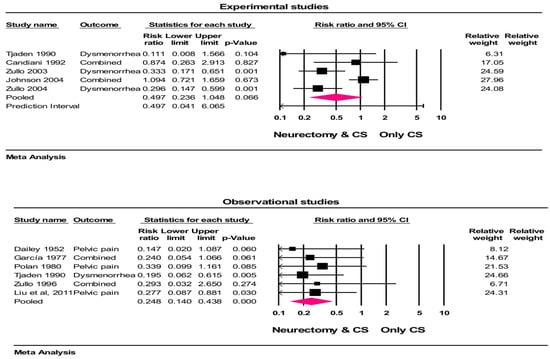
Figure 2.
Forest plot of treatment success comparing conservative surgery with or without neurectomy. The risk ratio and 95% confidence interval are plotted for each study. The pooled risk ratio (diamond apex) and 95% confidence interval (diamond width) were calculated using a random effects model. A pooled risk ratio >1 suggests a higher risk with pain recurrence. A pooled risk ratio < 1 suggests a lower risk with pain recurrence. Random effects risk ratio: neurectomy vs. controls (RR = 0.497, 95% CI = 0.236 to 1.04, p = 0.06 (for experimental studies) and RR = 0.248, 95% CI = 0.14 to 0.43, p <0.001 (for observational studies)). Box size represents study weighting. Diamond represents overall effect size and 95% CIs. CS = conservative surgery [32,33,34,35,41,42,43,44,53,57].
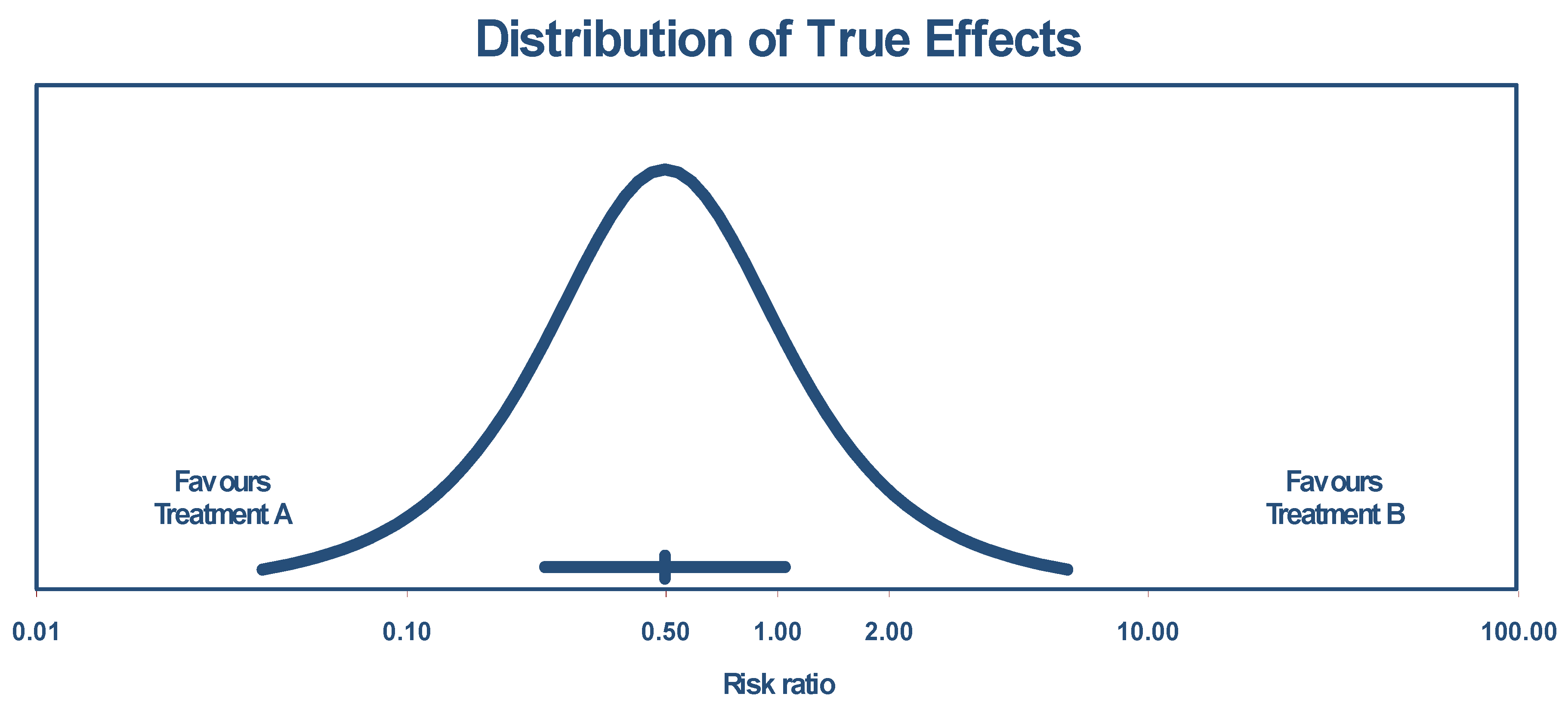
The true effect size in 95% of all comparable populations falls in the interval of 0.04 to 6.06. The distribution of the true effects (prediction interval) is shown by Figure 3.
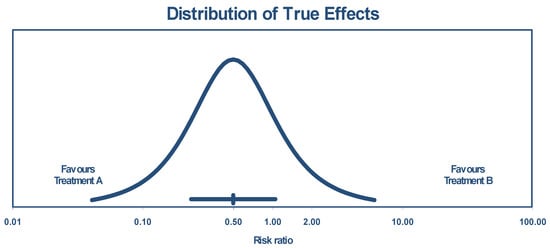
Figure 3.
Distribution of true effects size based on experimental studies.
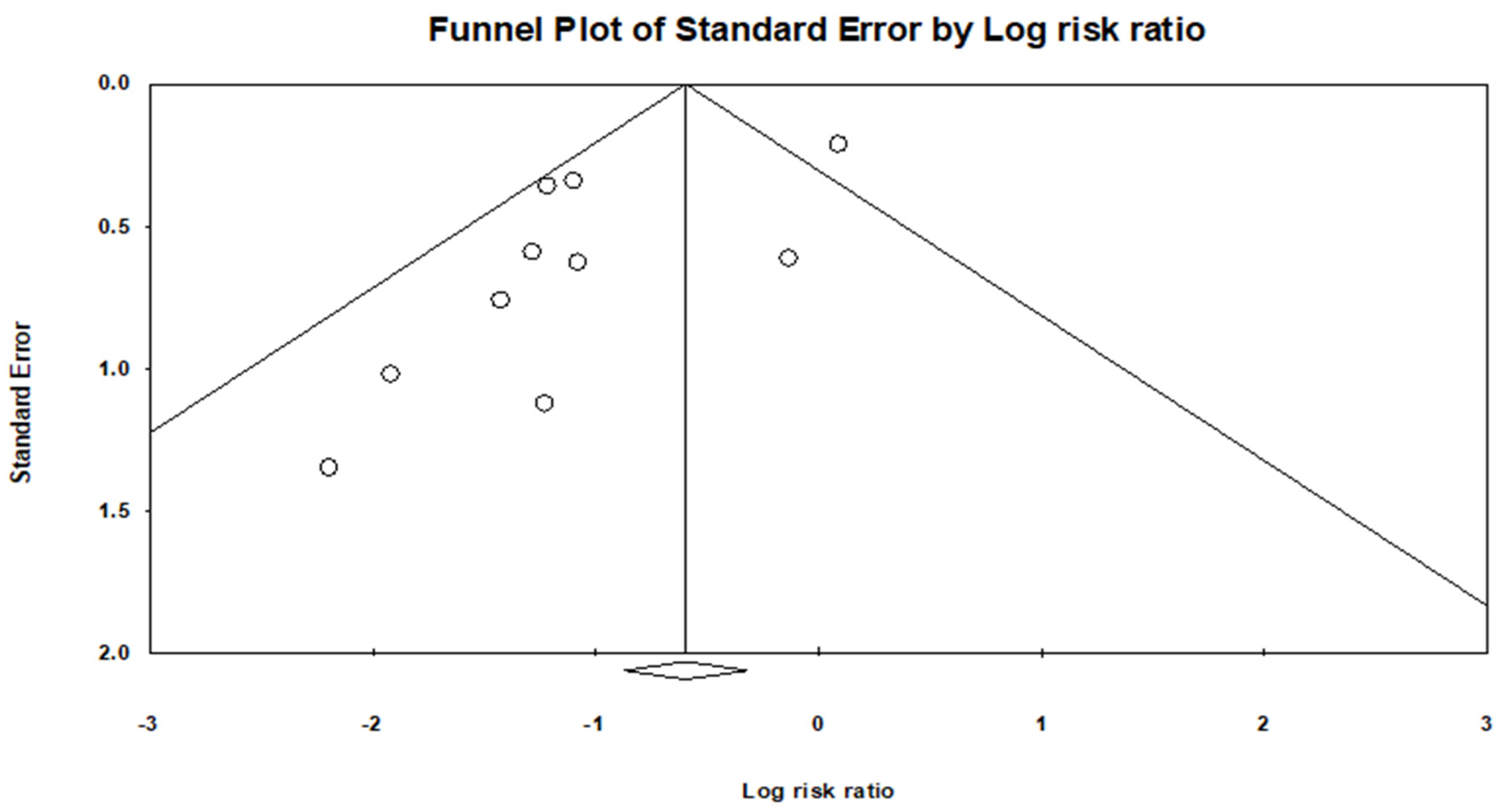
A funnel plot asymmetry was evident (Figure 4), and a quantitative assessment indicated a publication bias (p = 0.6081).
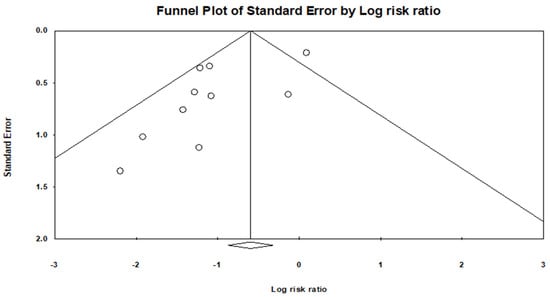
Figure 4.
Funnel plot shows there is a publication bias.
Sexual function was either unaffected or improved following neurectomy [37]. Additionally, a significant improvement in the quality of life was observed in the intervention group compared with controls [27,43,44]. Studies have indicated that neurectomy has no significant effect on pregnancy rates or infertility [33,34]. However, the probability of recurrence or symptom persistence following these procedures is a consideration. It was reported that the eight-year probability of recurrence was 81.8% (27 patients) in the laparoscopic presacral neurectomy group [48]. The results of the efficacy of neurectomy or nerve resection in the management of endometriosis are summarized in Table 2.
The safety of neurectomy or nerve resection in the management of endometriosis was evaluated in none of the studies in 1036 patients [27,32,37,41,42,44,48,51,55]. The peri- and post-complication rates were 7.33% (76 out of 1036 patients) with interventions vs. 0% with controls (p = 0.02). The most complications were related to constipation (41%, 31 out of 76 patients) and bladder impairment (31.6%, 24 out of 76 patients). Hypoesthesia, hyperesthesia, or allodynia was reported in 11.9% of the participants (9 out of 76 patients), and infections were seen in 6.6% of the participants (5 out of 76 patients). Bleeding, vaginal dryness, and re-admission for further surgery accounted for 2.6% of the participants (2 out of 76 patients) for each complication. One study only mentioned one case of failure in PSN without specifying the complication. The complications following neurectomy or nerve resection in the management of endometriosis are summarized in Table 2.
- (b)
- Efficacy of neurolysis (nerve blocks) in the management of endometriosis:
In total, we identified four studies, conducted between 1995 and 2021, that evaluated the effectiveness of neurolysis (nerve blocks) in the management of endometriosis [39,49,50,52]. A total of 45 women with endometriosis underwent neurolysis across these studies, utilizing different agents. The studies included women of different average age ranges, spanning from 31.6 to 33.4 years. The duration of follow-up varied from 6 to 12 months across the studies. Three studies performed superior hypogastric plexus blocks [39,49,52], while Sönmez et al. conducted uterine nerve ethanol neurolysis in 2016 [50]. An overall view of the studies which considered neurolysis (nerve blocks) in the management of endometriosis is shown Table 3. The risk of bias was low for one study and fair for three studies (Table 4).

Table 4.
Characteristics of studies that reviewed outcomes of neurolysis (nerve blocks) in the management of endometriosis.
Neurolysis (nerve blocks) was able to reduce the pain score of patients (dysmenorrhoea, dyspareunia, and chronic pelvic pain) by between 4.41 and 7, while CS only reduced by 2.55 scores, and this difference was statistically significant. In Wechsler’s study, 80% (4/5) of the patients had considerable or complete pain relief [39]. Superior hypogastric plexus blocks resulted in a 33.3-point and 29.7-point increase in the sexual rating scale scores and EHP-5 score and a decrease of 6.3 points of analgesic consumption during menses [49]. The efficacy of neurolysis (nerve blocks) in the management of endometriosis is summarized in Table 4.
The safety of neurolysis (nerve blocks) in the management of endometriosis was evaluated in four studies. The peri- and post-complication rates were 33.3% (15 out of 45 patients) with neurolysis [39,49,52]. The most complications were related to constipation (73.3%, 11 out of 15 patients) and bruising of the injection (13.3%, 2 out of 15 patients). Abdominal pain and retention of urine each were 6.66% (1 out of 15 patients). The complications following neurectomy or nerve resection in the management of endometriosis are summarized in Table 4.
- (c)
- Efficacy of neuromodulation in the management of endometriosis
In total, six studies, conducted between 2012 and 2023, evaluated the effectiveness of neuromodulation in managing endometriosis symptoms [9,26,40,45,46,56]. Neuromodulation was performed in a total of 74 women with endometriosis across the studies. Various methods were applied for neuromodulation, including sacral neuromodulation (SNM), a stimulation of the lumbar or sacral nerve roots by electrode implantation, and a respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) versus a non-vagal auricular stimulation (NVAS). In studies employing sacral neuromodulation, the S3 nerve root (sometimes S4) was stimulated with a low electrical current via an electrode placed through the sacral foramen under local anesthesia. These electrodes were connected to an external stimulator [26,45,46]. In a study by Kolodziej et al., a lead was laparoscopically placed in direct contact with the affected nerve and with a permanent generator [9]. Nyangoh Timoh et al. placed an electrode unilaterally next to the S3 sacral nerve root and connected it to an external pacemaker, with the patients wearing the electrode and external neurostimulator for 21 days [56]. Napadow et al. conducted a study comparing RAVANS to NVAS, where the subjects were seated in a reclined position for both sessions, and modified press-tack electrodes were inserted in the left ear during the RAVANS stimulation session. The duration, intensity, pulse frequency, and other stimulation parameters were consistent between the RAVANS and NVAS sessions [40]. An overall view of the studies which considered neuromodulation in the management of endometriosis is shown Table 5. The risk of bias was some concern for one study and fair for five studies (Table 6).

Table 5.
Overall view of studies which considered neuromodulation in the management of endometriosis.

Table 6.
Characteristics of studies that reviewed outcomes of neuromodulation in the management of endometriosis.
The age range of women in the studies varied from 34.4 to 36.3 years, and the duration of patient follow-ups ranged from 15 min to 60 months. The results of the studies show that neuromodulation reduced the frequency and intensity of pain (pelvic pain, dysmenorrhea, and dyspareunia) and anxiety, and they show an improvement in symptoms and a better quality of life, and most patients expressed a desire to continue with SNM [9,40,45,46,56]. But in a study by Agnello et al., it was seen that 30.8% of the patients experienced no relief, leading to a removal of the system [26]. The characteristics of the studies that reviewed outcomes of neuromodulation in the management of endometriosis are summarized in Table 6.
The safety of neuromodulation in the management of endometriosis was evaluated in three studies [9,46,56], and the complication rate was 15% (11 out of 74 patients) with neuromodulation. The reported complications were infection (73%, 8 out of 11 patients), and pain was reported in 23% of the participants (3 out of 11 patients). The complications following neuromodulation in the management of endometriosis are summarized in Table 6.
4. Discussion
Pain often recurs after conservative surgery for endometriosis [59], a conclusion supported by this systematic review and meta-analysis. A synthesis of the results from the studies on neuropelveology approaches for managing endometriosis provides valuable insights into three main categories: neurectomy or nerve resection, neurolysis (nerve blocks), and neuromodulation. The efficacy of neurectomy or nerve resection, as evidenced by 20 studies spanning several decades, indicates a promising treatment option for reducing pain associated with endometriosis [27,32,33,34,35,36,37,38,41,42,43,44,47,48,51,53,54,55,57,58]. Despite varying surgical techniques and durations of follow-ups, the overall success rate of neurectomy compared to conservative surgery alone suggests a significant reduction in pain symptoms (representing a 50% and 75.2% risk reduction in recurrences of pain in experimental studies [35,41,43,44,53,58] and observational studies [32,33,34,35,42,57], respectively, with notable improvements in the quality of life and sexual function [27,43,44]). Despite a trend towards a reduction in pain recurrence with neurectomy compared to controls, the findings did not reach statistical significance. This suggests that while neurectomy may offer potential benefits in alleviating endometriosis-associated pain, the evidence from experimental studies alone is not strong enough to conclusively support its efficacy in this regard. However, it is essential to acknowledge the potential for complications associated with neurectomy, including but not limited to nerve injury, sensory disturbances, and the risk of pain recurrence [27,32,37,41,42,44,48,51,55]. Notably, the analysis revealed an eight-year recurrence rate of 81.8% [48], highlighting the need for ongoing monitoring and potentially adjunctive therapies to optimize long-term outcomes while mitigating the risk of complications. Furthermore, the high risk of bias in some studies and the potential for complications underscore the importance of careful patient selection and postoperative management.
Similarly, neurolysis, particularly superior hypogastric plexus blocks and uterine nerve ethanol neurolysis, demonstrates encouraging outcomes in pain reduction and an improved quality of life for women with endometriosis [39,52]. Though the number of studies is limited, the consistent findings across different agents and follow-up durations suggest the potential for neurolysis as an adjunctive therapy or an alternative for patients with refractory symptoms. However, it is important to note the occurrence of complications associated with neurolysis, such as constipation and bruising at the injection site, underscoring the need for careful patient selection and monitoring during these interventions [39,49,52].
In contrast, the efficacy of neuromodulation in managing endometriosis symptoms appears promising but requires further investigation. With only six studies conducted in recent years, employing various neuromodulation techniques, the evidence is still evolving [9,26,40,45,46,56]. However, initial results indicate potential benefits in pain reduction and functional improvement, particularly with sacral neuromodulation [9,40,45,46,56]. Further research with larger sample sizes and longer follow-up periods is warranted to establish the long-term efficacy and safety profile of neuromodulation in this patient population.
The variability in neuromodulation outcomes underscores the complexity of treating endometriosis-related pain and the potential influence of individual patient factors on treatment responses [46]. Moreover, the analysis highlighted the need for further research to elucidate the optimal parameters and patient selection criteria for neuromodulation approaches in this context.
Overall, while each neuropelveology approach offers unique advantages and considerations, the synthesis of available evidence underscores the importance of a multidisciplinary approach in managing endometriosis, tailoring treatment strategies to individual patient needs and prioritizing long-term symptom relief and quality-of-life outcomes. Further research and clinical trials are essential to elucidate the optimal role of neuropelveology interventions in the comprehensive management of endometriosis. Future studies should adopt comprehensive strategies for the design and reporting of randomized controlled trials, with a strong emphasis on standardization and methodological rigor [60]. Additionally, ensuring an adherence to standardized protocols and proper training in surgical techniques is essential for minimizing variability and improving the reliability of outcomes [61].
5. Limitations
This meta-analysis faces limitations related to the quality of the available studies, which may impact the interpretation of the results. The heterogeneity in the study designs and surgical techniques introduces potentially confounding factors that may affect the data analysis. Consequently, a generalization of the findings should be made with caution, acknowledging these limitations and the potential variability in the data.
Additionally, due to the limited number of studies and the inseparability of some information, we were unable to adequately assess factors such as surgical access (laparoscopy vs. laparotomy) and follow-up durations. To address these issues, future research should involve multicenter trials, where all participating centers adhere to a uniform surgical technique (e.g., laparoscopic neurectomy) and standardized follow-up schedules (e.g., assessments at 6 months, 1 year, and 5 years, post-surgery). This approach could help reduce variability and enable a more accurate comparison of outcomes between interventions and control groups. Future research efforts should focus on identifying predictors of treatment responses and developing personalized treatment algorithms to maximize the efficacy and safety of neuropelveology interventions in individuals with endometriosis-associated pain. Additionally, long-term randomized clinical trial studies are warranted to evaluate the durability of pain relief and the impact on the quality of life following neuropelveology procedures.
6. Conclusions
In conclusion, the systematic review and meta-analysis underscore the potential of neuropelveology approaches in alleviating endometriosis-related pain. Neurectomy or nerve resection, neurolysis, and neuromodulation offer promising avenues for symptom relief, with varying levels of evidence supporting their efficacy. While neurectomy demonstrates significant pain reduction, it comes with potential complications and high recurrence rates, highlighting the need for careful patient selection and long-term monitoring. Neurolysis presents as a valuable adjunctive therapy, albeit with associated complications that necessitate cautious management. Neuromodulation shows promise but requires further investigation to establish its long-term efficacy and safety profile. Overall, the findings emphasize the importance of a multidisciplinary approach in managing endometriosis, tailoring interventions to individual patient needs and prioritizing long-term symptom management and quality-of-life improvements. Further research, including larger clinical trials, is essential to elucidate the optimal role of neuropelveology interventions in the comprehensive management of endometriosis and to refine treatment strategies for better patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13164676/s1.
Author Contributions
L.A., S.H., Z.M., A.M., S.ZA., H.S. and I.A. contributed to the design and implementation of the research project. L.A., F.R., A.R., S.Z.A., G.M., F.H. and H.S. conducted the analysis of results. L.A., S.H., Z.M., A.M., H.S. and I.A. prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Allaire, C.; Bedaiwy, M.A.; Yong, P.J. Diagnosis and management of endometriosis. Can. Med. Assoc. J. 2023, 195, E363–E371. [Google Scholar] [CrossRef]
- Song, S.Y.; Jung, Y.W.; Shin, W.; Park, M.; Lee, G.W.; Jeong, S.; An, S.; Kim, K.; Ko, Y.B.; Lee, K.H.; et al. Endometriosis-Related Chronic Pelvic Pain. Biomedicines 2023, 11, 2868. [Google Scholar] [CrossRef]
- Maddern, J.; Grundy, L.; Castro, J.; Brierley, S.M. Pain in Endometriosis. Front. Cell. Neurosci. 2020, 14, 590823. [Google Scholar] [CrossRef]
- Bove, G.M. A model for radiating leg pain of endometriosis. J. Bodyw. Mov. Ther. 2016, 20, 931–936. [Google Scholar] [CrossRef]
- Missmer, S.A.; Bove, G.M. A pilot study of the prevalence of leg pain among women with endometriosis. J. Bodyw. Mov. Ther. 2011, 15, 304–308. [Google Scholar] [CrossRef]
- Clarizia, R.; Manzone, M.; Roviglione, G.; Bruni, F.; Ceccarello, M.; Mautone, D.; Staffa, G.; Zorzi, C.; Ceccaroni, M. Laparoscopic Nerve Detrapment and Neurolysis of Somatic Pelvic Nerves in Deep Endometriosis: Prospective Study of 433 Patients. J. Minim. Invasive Gynecol. 2022, 29 (Suppl. S11), S34. [Google Scholar] [CrossRef]
- Nyangoh Timoh, K.; Lavoué, V.; Roman, H. Anatomical Pitfalls of Excision of Deep Endometriosis Nodules of the Sciatic Nerve: A three-dimensional Reconstruction and Surgical Educational Video. J. Obstet. Gynaecol. Res. 2023, 30, 264–265. [Google Scholar] [CrossRef]
- Kolodziej, M.; Uhl, E.; Schwarm, F. Interdisciplinary Laparoscopic Implantation of Neuromodulation Leads to the Sacral Plexus for Therapy of Chronic Pelvic Pain and Neurogenic Bladder Dysfunctions. Neuromodulat. Technol. Neural Interface 2020, 23, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Possover, M.; Forman, A.; Rabischong, B.; Lemos, N.; Chiantera, V. Neuropelveology: New Groundbreaking Discipline in Medicine. J. Minim. Invasive Gynecol. 2015, 22, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Mazidimoradi, A.; Günther, V.; Salehiniya, H.; Allahqoli, L. Total or Subtotal Hysterectomy for the Treatment of Endometriosis: A Review. J. Clin. Med. 2023, 12, 3697. [Google Scholar] [CrossRef] [PubMed]
- Freytag, D.; Peters, G.; Mettler, L.; Gitas, G.; Maass, N.; Alkatout, I. Perioperative considerations in the treatment of endometriosis. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Kashi, A.; Niakan, G.; Ebrahimpour, M.; Allahqoli, L.; Hassanlouei, B.; Gitas, G.; Alkatout, I. A randomized, double-blind, placebo-controlled pilot study of the comparative effects of dienogest and the combined oral contraceptive pill in women with endometriosis. Int. J. Gynaecol. Obstet. 2022, 156, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mettler, L.; Ruprai, R.; Alkatout, I. Impact of medical and surgical treatment of endometriosis on the cure of endometriosis and pain. Biomed. Res. Int. 2014, 2014, 264653. [Google Scholar] [CrossRef]
- Alkatout, I.; Mettler, L.; Beteta, C.; Hedderich, J.; Jonat, W.; Schollmeyer, T.; Salmassi, A. Combined surgical and hormone therapy for endometriosis is the most effective treatment: Prospective, randomized, controlled trial. J. Minim. Invasive Gynecol. 2013, 20, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Possover, M. Neuropelveology: An Emerging Discipline for the Management of Pelvic Neuropathies and Bladder Dysfunctions through to Spinal Cord Injury, Anti-Ageing and the Mars Mission. J. Clin. Med. 2020, 9, 3285. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools. July 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 19 March 2024).
- Lin, L. Comparison of four heterogeneity measures for meta-analysis. J. Eval. Clin. Pract. 2020, 26, 376–384. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Mu, B.; Zhang, Z.; Xu, H. Correlative factors analysis and effect of pelvic pain associated with endometriosis after onservative surgery. Zhonghua Fu Chan Ke Za Zhi 2014, 49, 681–684. [Google Scholar] [PubMed]
- Agnello, M.; Vottero, M.; Bertapelle, P. Sacral neuromodulation to treat voiding dysfunction in patients with previous pelvic surgery for deep infiltrating endometriosis: Our centre’s experience. Int. Urogynecol. J. 2021, 32, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Roman, H.; Dehan, L.; Merlot, B.; Berby, B.; Forestier, D.; Seyer-Hansen, M.; Abo, C.; Tuech, J.J. Postoperative Outcomes after Surgery for Deep Endometriosis of the Sacral Plexus and Sciatic Nerve: A 52-patient Consecutive Series. J. Minim. Invasive Gynecol. 2021, 28, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Puolakka, J.; Kauppila, A.; Rönnberg, L. Results in the operative treatment of pelvic endometriosis. Acta Obstet. Gynecol. Scand. 1980, 59, 429–431. [Google Scholar] [CrossRef]
- Possover, M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J. Urol. 2009, 181, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Biggerstaff, E.D.; Foster, S.N. Laparoscopic Presacral Neurectomy for Treatment of Midline Pelvic Pain. J. Am. Assoc. Gynecol. Laparosc. 1994, 2, 31–35. [Google Scholar] [CrossRef]
- Daniels, J.; Gray, R.; Hills, R.K.; Latthe, P.; Buckley, L.; Gupta, J.; Selman, T.; Adey, E.; Xiong, T.; Champaneria, R.; et al. Laparoscopic Uterosacral Nerve Ablation for Alleviating Chronic Pelvic Pain: A Randomized Controlled Trial. JAMA 2009, 302, 955–961. [Google Scholar] [CrossRef]
- Dailey, H.R.; Tafel, R.E. Superior hypogastric sympathectomy for the relief of pain associated with endometriosis. Am. J. Obstet. Gynecol. 1952, 64, 650–654. [Google Scholar] [CrossRef]
- Garcia, C.R.; David, S.S. Pelvic Endometriosis–Infertility and Pelvic Pain. Am. J. Obstet. Gynecol. 1977, 129, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Polan, M.L.; Decherney, A. Presacral Neurectomy for Pelvic Pain in Infertility. Fertil. Steril. 1980, 34, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, B.; Schlaff, W.D.; Kimball, A.; Rock, J.A. The efficacy of presacral neurectomy for the relief of midline dysmenorrhea. Obstet. Gynecol. 1990, 76, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Nezhat, F. A simplified method of laparoscopic presacral neurectomy for the treatment of central pelvic pain due to endometriosis. BJOG Int. J. Obstet. Gynaecol. 1992, 99, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.P.; Perez, J. The role for laparoscopic presacral neurectomy. J. Gynecol. Surg. 1993, 9, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.H.; Seidman, D.S.; Nezhat, F.R.; Nezhat, C.R. Long-term outcome of laparoscopic presacral neurectomy for the treatment of central pelvic pain attributed to endometriosis. Obstet. Gynecol. 1998, 91, 701–704. [Google Scholar]
- Wechsler, R.J.; Maurer, P.M.; Halpern, E.J.; Frank, E.D. Superior hypogastric plexus block for chronic pelvic pain in the presence of endometriosis: CT techniques and results. Radiology 1995, 196, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; Edwards, R.R.; Cahalan, C.M.; Mensing, G.; Greenbaum, S.; Valovska, A.; Li, A.; Kim, J.; Maeda, Y.; Park, K.; et al. Evoked Pain Analgesia in Chronic Pelvic Pain Patients Using Respiratory-Gated Auricular Vagal Afferent Nerve Stimulation. Pain Med. 2012, 13, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Candiani, G.B.; Fedele, L.; Vercellini, P.; Bianchi, S.; Dinola, G. Presacral Neurectomy for the Treatment of Pelvic Pain Associated with Endometriosis—A Controlled-Study. Am. J. Obstet. Gynecol. 1992, 167, 100–103. [Google Scholar] [CrossRef]
- Zullo, F.; Pellicano, M.; DeStefano, R.; Mastrantonio, P.; Mencaglia, L.; Stampini, A.; Zupi, E.; Busacca, M. Efficacy of laparoscopic pelvic denervation in central-type chronic pelvic pain: A multicenter study. J. Gynecol. Surg. 1996, 12, 35–40. [Google Scholar] [CrossRef]
- Zullo, F.; Palomba, S.; Zupi, E.; Russo, T.; Morelli, M.; Cappiello, F.P.M. Effectiveness of presacral neurectomy in women with severe dysmenorrhea by endometriosis who were treated with laparoscopic conservative surgery: A 1-year prospective randomized double-blind controlled trial. J. Gynecol. Obstet. Biol. Reprod. 2003, 32, 757. [Google Scholar]
- Zullo, F.; Palomba, S.; Zupi, E.; Russo, T.; Morelli, M.; Sena, T.; Pellicano, M.; Mastrantonio, P. Long-term effectiveness of presacral neurectomy for the treatment of severe dysmenorrhea due to endometriosis. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lavonius, M.; Suvitie, P.; Varpe, P.; Huhtinen, H. Sacral Neuromodulation: Foray into Chronic Pelvic Pain in End Stage Endometriosis. Case Rep. Neurol. Med. 2017, 2017, 2197831. [Google Scholar] [CrossRef] [PubMed]
- Zegrea, A.; Ojala, E.; Suvitie, P.; Varpe, P.; Huhtinen, H.; Mäkelä-Kaikkonen, J.; Rautio, T.; Härkki, P.; Salmenkylä, S.; Ukkonen, M.; et al. Sacral neuromodulation in endometriosis—A promising treatment option for chronic pelvic pain. Acta Obstet. Et Gynecol. Scand. 2023, 102, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Soong, Y.K. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet. Gynecol. 1997, 90, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.Y.; Chang, W.C.; Hung, Y.C.; Ho, M.; Yeh, L.S.; Lin, W.C. Comparison of a new modified laparoscopic presacral neurectomy and conventional laparoscopic presacral neurectomy in the treatment of midline dysmenorrhea. Int. J. Gynecol. Obstet. 2007, 99, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Soysal, M.E.; Soysal, S.; Gurses, E.; Ozer, S. Laparoscopic presacral neurolysis for endometriosis-related pelvic pain. Hum. Reprod. 2003, 18, 588–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sönmez, S.; Nayki, Ü.; Paşa, U.; Nayki, C.; Sönmez, F.; Tinar, Ş.; Yildirim, Y. Laparoscopic uterine nerve ethanol neurolysis (LUNEN) in patients with chronic pelvic pain. J. Clin. Exp. Investig. 2016, 7, 7–13. [Google Scholar] [CrossRef]
- Kwok, A.; Lam, A.; Ford, R. Laparoscopic presacral neurectomy—Retrospective series. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 195–197. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Alebouyeh, M.R.; Sadegi, K.; Mehdizadehkashi, A.; Kaveh, M.; Entezari, S.R.; Mirzaei, H.; Khaledi, M.; Khodaverdi, M. Superior hypogastric plexus block as an effective treatment method for endometriosis-related chronic pelvic pain: An open-label pilot clinical trial. J. Obstet. Gynaecol. 2021, 41, 966–971. [Google Scholar] [CrossRef]
- Johnson, N.P.; Farquhar, C.M.; Crossley, S.; Yu, Y.; Van Peperstraten, A.M.; Sprecher, M.; Suckling, J. A double-blind randomised controlled trial of laparoscopic uterine nerve ablation for women with chronic pelvic pain. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Sokalska, A.; Spaczyński, R.Z.; Duleba, A.J.; Pawelczyk, L. Effects of presacral neurectomy on pelvic pain in women with and without endometriosis. Ginekol. Pol. 2009, 80, 172–178. [Google Scholar] [PubMed]
- Possover, M. Five-Year Follow-Up After Laparoscopic Large Nerve Resection for Deep Infiltrating Sciatic Nerve Endometriosis. J. Minim. Invasive Gynecol. 2017, 24, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Nyangoh Timoh, K.; Canlorbe, G.; Verollet, D.; Peyrat, L.; Ballester, M.; Amarenco, G.; Darai, E. Contribution of sacral neuromodulation to manage persistent voiding dysfunction after surgery for deep infiltrating endometriosis with colorectal involvement: Preliminary results. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 190, 31–35. [Google Scholar] [CrossRef]
- Liu, K.J.; Cui, L.Q.; Huang, Q.; Liu, Q.; Han, N.N.; Li, P.Q.; Wang, J. Effectiveness and safety of laparoscopic presacral neurectomy in treating endometriosis-associated pain. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2011, 33, 485–488. [Google Scholar] [PubMed]
- Sutton, C.; Pooley, A.S.; Jones, K.D.; Dover, R.W.; Haines, P. A prospective, randomized, double-blind controlled trial of laparoscopic uterine nerve ablation in the treatment of pelvic pain associated with endometriosis. Gynaecol. Endosc. 2001, 10, 217–222. [Google Scholar] [CrossRef]
- Miller, L.E.; Bhattacharyya, R.; Miller, V.M. Clinical Utility of Presacral Neurectomy as an Adjunct to Conservative Endometriosis Surgery: Systematic Review and Meta-Analysis of Controlled Studies. Sci. Rep. 2020, 10, 6901. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001, 357, 1191–1194. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).