Abstract
Heparin-induced thrombocytopenia (HIT) is a life- and limb-threatening immune-mediated emergency classically associated with heparin therapy. This review focuses on type II HIT, characterized by the development of antibodies against platelet-factor 4 (PF4) bound to heparin after exposure, causing life-threatening thrombocytopenia, arterial thrombosis, and/or venous thrombosis. The high morbidity and mortality rates emphasize the need for early recognition and urgent intervention with discontinuation of heparin and initiation of non-heparin anticoagulation. We discuss the management of HIT with an emphasis on recent developments: (i) incorporating the phases of HIT (i.e., suspected, acute, subacute A and B, and remote) into its management, categorized according to platelet count, immunoassay, and functional assay results and (ii) direct-acting oral anticoagulants (DOACs), which are increasingly used in appropriate cases of acute HIT (off-label). In comparison to parenteral options (e.g., bivalirudin and danaparoid), they are easier to administer, are more cost-effective, and obviate the need for transition to an oral anticoagulant after platelet recovery. We also identify the knowledge gaps and suggest areas for future research.
1. Introduction
Heparin-induced thrombocytopenia (HIT) is a life- and limb-threatening immune-mediated emergency classically associated with heparin therapy. Although this has been most commonly described with unfractionated heparin (UFH), it has been seen with low-molecular-weight heparin (LMWH), and clinical syndromes with striking similarity in the absence of heparin exposure have been well reported with non-heparin polyanionic substances such as chondroitin sulfate and pentosan polysulfate (PPS) [1]. In a meta-analysis of 15 studies (n = 7287), the risks of HIT associated with UFH and LMWH were 2.6% and 0.2%, respectively [2].
HIT is characterized by the development of antibodies against platelet-factor 4 (PF4) bound to heparin after exposure, causing life-threatening thrombocytopenia, arterial thrombosis, and/or venous thrombosis. Various studies have reported high morbidity (60–90%) and mortality rates (6–30%). Even with early recognition and intervention, morbidity and mortality are 7.4% and 1.1% [3,4,5,6,7]. This emphasizes the need for early recognition and urgent intervention with discontinuation of heparin and initiation of non-heparin anticoagulation. In this review, we discuss this antibody-mediated disorder with an emphasis on management, highlighting recent developments. We also review recommendations from societal guidelines, including the American College of Chest Physicians (ACCP) published in 2012, the American Society of Hematology (ASH) published in 2018 (reviewed in 2022), the British Society of Haematology (BSH) published in 2023, the Thrombosis and Haemostasis Society of Australia and New Zealand (THANZ) published in 2019, and Thrombosis Canada published in 2023 [8,9,10,11,12].
2. Pathogenesis
HIT is categorized into types I and II. In type I HIT, there is a transient and non-immune thrombocytopenia due to heparin-induced platelet activation and aggregation. This is seen in approximately 10–20% of patients and occurs shortly after heparin exposure (usually 1–4 days). The thrombocytopenia is mild (nadir 100,000/μL), self-limiting without cessation of heparin, and not associated with thrombosis [13,14]. Type I HIT is not further discussed in this review.
In type II HIT, heparin exposure leads to the production of immunoglobulin G (IgG) antibodies against PF4 bound to heparin, as illustrated in Figure 1. These antibodies may develop as soon as 4 days post-exposure without an immunoglobulin M (IgM) response suggesting an etiological role for prior sensitization or cross-reactivity [15]. However, it is important to note that despite the detection of anti-PF4/heparin antibody complexes in some patients, they may not be functionally active and not lead to the development of HIT [15].
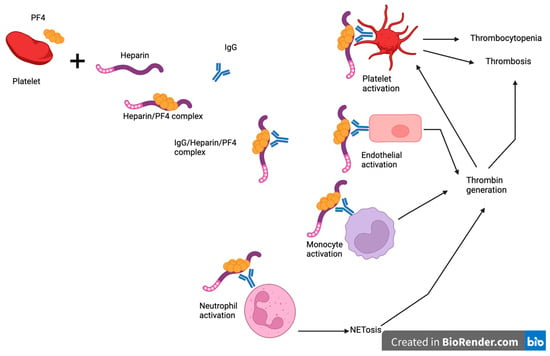
Figure 1.
Pathogenesis of heparin-induced thrombocytopenia. Heparin exposure leads to the production of immunoglobulin G (IgG) antibodies against PF4 bound to heparin. These antibodies bind to and activate platelets causing thrombosis and thrombocytopenia. They also bind to endothelium, monocytes, and neutrophils leading to thrombin generation, which promotes thrombosis and further activation of platelets. Created with BioRender.com. PF4: platelet factor 4, IgG: immunoglobulin G.
PF4 is stored in alpha granules and released upon activation of platelets. Upon binding to heparin or other polyanions, PF4 undergoes a conformational change to allow binding with HIT antibodies [16]. These immune complexes bind to platelet FcγRIIA receptors with downstream platelet activation, leading to thrombosis and consumptive thrombocytopenia [15]. More recently, the interaction of the immune complexes with neutrophil FcγRIIA receptors and subsequent NETosis has also been shown to be a major contributing factor in HIT-associated thrombosis by supporting thrombin generation. In addition, HIT antibodies also bind to PF4/glycosaminoglycan complexes on endothelial cells and monocytes with consequent expression of tissue factor and thrombin generation [17]. Thrombin promotes thrombosis and further activation.
3. Diagnosis
In patients with suspected HIT, the “4T” score is commonly used to determine the pretest likelihood of HIT. The score incorporates four clinical and laboratory components: severity of thrombocytopenia, thrombosis or other HIT-associated complications, timing of thrombocytopenia after heparin exposure, and the presence of other causes of thrombocytopenia (Table 1). A total score of 0–3, 4–5, and 6–8 points corresponds to low, intermediate, and high pretest likelihoods, respectively [18].

Table 1.
Estimating pretest likelihood of heparin-induced thrombocytopenia using the 4T score.
In patients with low pretest likelihood, further laboratory testing is not recommended except for some patients in the intensive care unit (BSH: Grade 2B), in particular those receiving extracorporeal membrane oxygenation (BSH: Grade 2C) [10]. Another exception is in cases where the pretest probability is uncertain due to missing or unclear data [9,11].
In patients with intermediate and high pretest likelihoods, an immunoassay (e.g., ELISA) is used to detect the presence of HIT antibodies. The test has a rapid turnaround time, can be semi-automated in the case of chemiluminescent platforms, and is more widely available. Functional assays (e.g., serotonin release assay, heparin-induced platelet activation, or heparin-induced platelet aggregometry) are needed to confirm the diagnosis by demonstrating heparin-dependent activation that is abrogated by a high-dose heparin step, which alters the usual stoichiometric relationship between these polyanions and anti-PF4 antibodies needed to trigger platelet activation. Functional assays are generally more time-consuming, require manual handling and expertise, and are generally reserved for major metropolitan tertiary referral centers.
4. Management
Once a presumptive diagnosis of HIT is made based on an intermediate to high pretest probability, immediate steps to mitigate the prothrombotic condition must be taken even while waiting for further laboratory testing, including suspension of all heparins and the commencement of a non-heparin anticoagulant.
HIT can be categorized into five phases, starting with suspected cases, where a presumptive clinical diagnosis has been made with pending immunoassay or functional assays. Once confirmed by laboratory testing, cases are categorized as acute HIT until recovery of platelet count (usually defined as ≥150 × 109/L; however, in some studies, the definition also includes the return to baseline if the baseline platelet count was <150 × 109/L, as shown in Table 1). In addition, acute cases without thrombosis are designated as isolated HIT. Subacute phases A and B are characterized by a normal platelet count with positive immunoassays; however, the functional assay is negative in the latter. The remote phase is indicated by seronegativity [19].
Incorporating these phases into clinical practice may improve communication between healthcare providers and streamline the patient care requirements and management of HIT. For example, as illustrated in Figure 2, the management can be categorized according to the phases of HIT. These are discussed below with a focus on newer data.
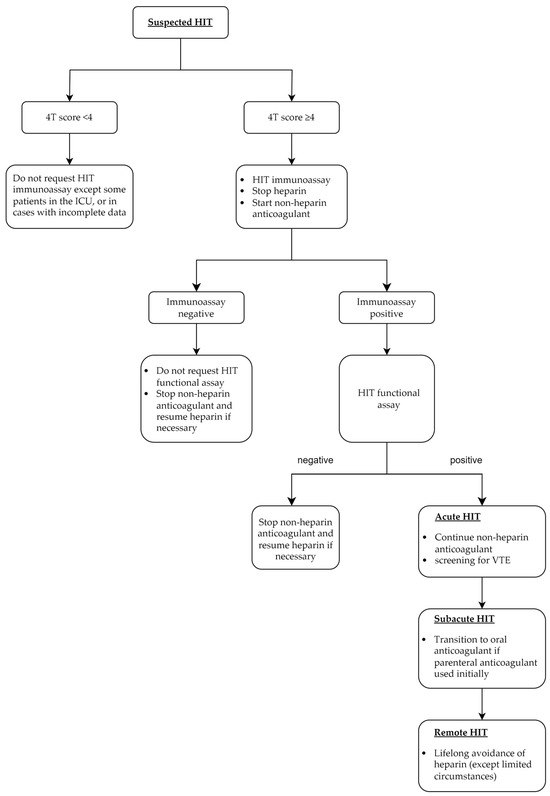
Figure 2.
Key management steps in each phase of heparin-induced thrombocytopenia. HIT: heparin-induced thrombocytopenia, ICU: intensive care unit, VTE: venous thromboembolism.
4.1. Discontinuation of Heparin
All forms of heparin, including UFH and LMWH, need to be discontinued immediately once HIT is suspected with intermediate to high pretest probability [9,10]. Hence, a thorough review is required to evaluate all potential sources, including heparin-coated intravascular catheters, heparin flushes, some prothrombin complex concentrates, peripheral blood stem cells, and total parenteral nutrition products [20]. PPS injections, occasionally used for symptomatic management of osteoarthritis (off-label), also need to be excluded.
4.2. Initiation of a Non-Heparin Anticoagulant at Therapeutic Dose
Due to the high risk of venous and/or arterial thrombosis, a non-heparin anticoagulant at therapeutic intensity needs to be commenced immediately once HIT is suspected with intermediate to high pretest probability [9,10]. However, if the bleeding risk is high, ASH suggests initiating a non-heparin anticoagulant at prophylactic intensity [9].
It is important to note that warfarin is not a suitable agent in acute HIT. This is due to the exacerbation of thrombotic risks due to a rapid depletion of protein C and protein S [21]. These anticoagulants are also reduced in the setting of acute thrombosis. For patients already receiving warfarin, reversal with vitamin K is suggested during the acute phase of HIT [8,9]. The use of warfarin after acute HIT is discussed in Section 4.4.2.
Oral and parenteral anticoagulants used in acute HIT are discussed below. Table 2 compares the agents with a dosing and monitoring guide included.
4.2.1. Direct-Acting Oral Anticoagulants (DOACs)
Traditionally, the parenteral non-heparin anticoagulants are the mainstay of therapy in acute HIT. However, there are increasing data and international acceptance for the off-label use of DOACs in suitable patients. In a recent survey of opinions from 102 international experts and practitioners, the majority agreed with the use of rivaroxaban and apixaban in acute HIT (74.5% and 73.5%, respectively), even without initial parenteral anticoagulation (39.2% and 35.3%, respectively) [22].
In addition to the parenteral anticoagulants, ASH suggests DOACs (e.g., rivaroxaban, apixaban, and dabigatran) as suitable alternatives in acute HIT. Specifically, they are suitable for patients who are clinically stable with average bleeding risk [9]. Similarly, Thrombosis Canada recommends DOACs as an option in acute HIT, with a preference for rivaroxaban [11]. In contrast, BSH and THANZ recommend parenteral agents only in acute HIT [10,12].
In comparison to parenteral anticoagulants, the advantages of DOACs include convenience and ease of administration, wide availability, lower risk of bleeding, and no requirement for routine monitoring and titration, which is needed in most parenteral anticoagulants [22]. They can also be continued to the subacute phase, hence avoiding the need to change from parenteral to oral anticoagulants. In addition, a recent pharmacoeconomic analysis favors rivaroxaban (and fondaparinux) over argatroban [23].
Due to their half-life, they may not be suitable for patients needing urgent surgery. They are also not preferred in cases with arterial thrombosis or life-threatening or limb-threatening thromboembolism due to the limited data on DOACs in this setting [9,10]. They are also not suitable for patients with mechanical heart valves, who are pregnant, or those with severe renal and/or liver dysfunction.
More data on rivaroxaban and apixaban have been published on individual DOACs. We summarized the retrospective series and prospective studies reporting the use of DOACs in acute HIT (suspected and confirmed) published in the past 10 years in Table 1.

Table 2.
Studies reporting outcomes of direct-acting oral anticoagulants in suspected or confirmed acute heparin-induced thrombocytopenia.
Table 2.
Studies reporting outcomes of direct-acting oral anticoagulants in suspected or confirmed acute heparin-induced thrombocytopenia.
| Study and Design | Agent and Number of Patients (n) | Initial Parenteral Anticoagulation | HIT with Thrombosis | Follow-Up Duration | Thrombosis and Related Outcomes During Follow-Up | Bleeding During Follow-Up | Other Findings or Outcomes During Follow-Up |
|---|---|---|---|---|---|---|---|
| Davis et al., 2022 [24] | Apixaban (n = 51) Rivaroxaban (n = 24) Dabigatran (n = 2) | 63 (81.8%) | 38 (49.4%), including 5 arterial thrombosis | 3 months from starting DOAC | Thromboembolism, gangrene, or severe limb ischemia requiring amputation: 9 (11.7%); all received initial parenteral anticoagulation, among which 7/9 (77.8%) changed to DOAC when platelet count was <150 × 109/L | Major: 5 (6.5%), CRNMB: 9 (11.7%) | Time to platelet recovery (days):
|
| Multicenter, retrospective | |||||||
| Cirbus et al., 2022 [25] | Rivaroxaban (n = 7) Apixaban (n = 5) | 10 (83.3%), 5 (36%) changed to DOAC before platelet recovery | 2 (28.5%) in rivaroxaban group, unknown in apixaban group | 6 months from discharge | 0 in 8 (67%) patients who followed-up | Not reported | |
| Single center, retrospective | |||||||
| Albuloushi et al., 2022 [26] | Apixaban (n = 21) Rivaroxaban (n = 5) | 21 (80.8%); 11 (42.3%) changed to DOAC after platelet recovery | 16 (61.5%), including 2 arterial thrombosis | 1 month | 0 | 0 | Time to platelet recovery (days):
|
| Single center, retrospective | |||||||
| Farasatinasab et al., 2022 [27] | Apixaban (n = 30) | 0 | 11 (36.7%) | 6 months | 0 | 1 (3.3%): bleeding gastric ulcer at 2 months and apixaban ceased | Time to platelet recovery (days): 5 ± 1.8 Unrelated mortality rate: 16.7% |
| Single center, prospective, open-label, single-arm | |||||||
| Wang et al., 2022 [28] | Dabigatran (n = 5) | 5 (100%), all changed to DOAC before platelet recovery ^ | 5 (100%), including 3 with arterial embolism | 3 months | 0 | 1 (20%) developed gastric bleeding | 3 (60%) recovered platelet count at end of follow-up ^ Mortality rate: 1 (20%) |
| Single center, retrospective | |||||||
| Carré et al., 2021 [29] | Rivaroxaban (n = 6) Apixaban (n = 1) | 6 (85.7%), 1 (14.3%) changed to DOAC before platelet recovery | 1 (14.3%) | 12 (4–27.5) months | 1 (14.3%), DVT after holding rivaroxaban 5 days pre-surgery; | 0 | Time to platelet recovery (days) ^: 3 (3–5) |
| Multicenter, retrospective | |||||||
| Farasatinasab et al., 2020 [30] | Rivaroxaban (n = 42) | 0 | 17 (40.5%) | 12 months from starting DOAC | 1 (2.3%) developed progressive DVT | 0 | Time to platelet recovery (days): 4.29 ± 1.78 Unrelated mortality rate: 28.6% |
| Single center, retrospective | |||||||
| Nasiripour et al., 2018 [31] | Dabigatran (n = 43) | 0 | Not reported | 12 months from starting DOAC | 1 (2.3%) developed lower-limb DVT | 0 | Time to platelet recovery: 7.4 ± 4.3 days (n = 41, 95.3%) Unrelated mortality rate: 20% |
| Single center, retrospective | |||||||
| Davis et al., 2017 [32] | Apixaban (n = 9) Rivaroxaban (n = 3) | 7 (58.3%) | 5 (41.7%), including 1 arterial embolism | 19 months | Thromboembolism, gangrene, or critical limb ischemia requiring amputation: 0 | Major: 0 | Time to platelet recovery (days): mean 7.42 days |
| Single center, retrospective | |||||||
| Kunk et al., 2016 [33] | Apixaban (n = 10) Rivaroxaban (n = 2) | 12 (100%), all changed to DOAC after platelet recovery ^ | 9 (75%), including 1 arterial thrombosis | Median 7 (range 2–39) months | 0 | Major: 2 (16.7%) ** | Time to platelet recovery (days) ^: 1–8 |
| Single center, retrospective | |||||||
| Warkentin et al., 2016 [34] | Rivaroxaban (n = 16) | 8 (50%), 2 changed to rivaroxaban when thrombocytopenic # | 6 (37.5%) | 1 month | Thrombosis or limb amputation: 0 | Major: 0 | Time to platelet recovery (days) ^†: 7 (4–12) (n = 9) Mortality rate 0% |
| Multicenter, retrospective | |||||||
| Ong et al., 2016 § [35] | Rivaroxaban (n = 9) | 0 | 9 (100%) | Not reported | Thrombosis or limb amputation due to necrosis: 0 | 0 | Time to platelet recovery (days) ^: mean 14, median 8 (range 5–41) |
| Multicenter, retrospective | |||||||
| Linkins et al., 2016 [36] | Rivaroxaban (n = 12) | 7 (58.3%) ‡ | 6 (50%), including 1 arterial thrombosis | 1 month | 1 (8.3%, 95% CI, 0.1–37.5%) developed extension of upper arm catheter-associated DVT | 1 (8.3%) major bleeding after rivaroxaban held for 9 days | Time to platelet recovery (days) ‡: mean 11, median 7. Unrelated mortality rate: 33.3% |
| Multicenter, prospective, | |||||||
| Sharifi et al., 2015 [37] | Dabigatran, rivaroxaban, and apixaban; Total 22 patients. | 22 (100%) | 7 (31.8%) | 19 ± 3 months | Recurrent venous thromboembolism or limb loss: 0 | 0 | Unrelated mortality rate: 27% |
| Single center, retrospective |
Unless otherwise indicated, data are presented as n (%), mean ± standard deviation and median (interquartile range). ^ Platelet recovery is defined as: (i) platelet count ≥150 × 109/L or (ii) if <150 × 109/L at baseline, return to baseline platelet count except Carré et al., Warkentin et al., Ong et al. [29,34,35], which included (i) only; Kunk et al. defined platelet recovery as count of ≥50 × 109/L; not defined in Wang et al. [28]; we applied both (i) and (ii) to report these statistics. See full text for more details. # Platelet count 56 (range 25–107) × 109/L. ** One patient with known gastric varices who was taking clopidogrel developed gastrointestinal bleeding; another patient with locally advanced squamous cell lung cancer developed severe hemoptysis. † One patient never had thrombocytopenia. § Includes 3 cases published by Ng et al. in 2015 [34,38]. ‡ Ten patients had thrombocytopenia at study entry when they commenced or transitioned to rivaroxaban. Nine patients recovered platelet count CI: confidence interval, CRNMB: clinically relevant non-major bleeding, DOAC: direct-acting oral anticoagulants, DVT: deep venous thrombosis, HIT: heparin-induced thrombocytopenia, HITT: heparin-induced thrombocytopenia with thrombosis, IQR: interquartile range.
4.2.2. Danaparoid
Danaparoid inhibits factor Xa and, to a much lesser extent, factor IIa by its effect on antithrombin [39]. It is mainly metabolized by the kidneys, with a half-life elimination of approximately 25 and 2 h for its anti-Xa and anti-IIa activity, respectively. As protamine only partially neutralizes danaparoid, there is no reliable reversal agent. Thus, danaparoid may not be suitable for patients requiring emergency surgery, cardiac surgery, or at high risk of bleeding [9,40]. It is usually administered as a continuous intravenous (IV) infusion after a loading dose bolus. In approximately 10% of cases, danaparoid cross-reactivity is observed with the HIT antibody. In vivo cross-reactivity is rare; however, it is important to consider the possibility in cases with suboptimal platelet response to treatment and/or new thrombotic events [41,42,43]. Danaparoid does not cross the placenta and is suitable for pregnant patients with HIT [10,40,44]. It is also suitable for patients who are breastfeeding [10].
4.2.3. Bivalirudin
Bivalirudin is a direct thrombin inhibitor (DTI). It is metabolized by proteolytic cleavage (80%) and renal excretion (20%) with a short half-life of 25 min [40]. It is administered as a continuous IV infusion. Hence, the agent may be preferred in patients at high risk of bleeding, those who are critically ill, or those who may require urgent surgery. ASH suggests bivalirudin in patients with acute HIT or subacute HIT A who require percutaneous coronary intervention (PCI) over other non-heparin anticoagulants. It is also suggested over UFH in patients with subacute HIT B or remote HIT undergoing PCI [9].
4.2.4. Argatroban
Argatroban is a DTI. It is predominantly metabolized in the liver with a short half-life of 40–50 min [12,19]. Hence, it is not suitable or requires a dose reduction in patients with moderate to severe liver dysfunction (Child–Pugh Class B and C). Due to the short half-life, the agent may be favored in patients who may require emergency surgery or are at high risk of bleeding [9]. It is administered as a continuous IV infusion. For patients with acute HIT or subacute HIT A who require PCI and bivalirudin is not available, the agent may be a suitable alternative [9]. It is an option for pregnant patients with HIT [10].
4.2.5. Fondaparinux
Fondaparinux is a synthetic pentasaccharide that selectively inhibits factor Xa by its effects on antithrombin. It is predominantly excreted by the kidneys, with a long half-life of 17–24 h [19]. It is a subcutaneous injection administered once daily without the need for monitoring. Fondaparinux is an attractive option for clinically stable patients without a high risk of bleeding. Due to the long half-life, the agent is not suitable for cardiac surgery [9]. It is an option for pregnant patients with HIT [10]. However, there is a small amount of in vivo placental transfer; hence, ACCP recommends its use as an alternative if danaparoid is not available [8]. Note that although the risk is low, fondaparinux is associated with autoimmune HIT.
4.2.6. Comparison of Non-Heparin Anticoagulants
The selection of an agent would depend on the availability, cost, clinician experience, and various patient factors, as summarized in Table 3 below, incorporating recent guidelines, reviews, and databases [9,10,12,19,22,40,45,46,47].

Table 3.
Comparison of non-heparin anticoagulants during acute HIT.
4.3. Screening for Asymptomatic DVT
Asymptomatic lower-limb DVTs are common in patients with HIT, with an incidence rate as high as 50% reported [48]. In addition, 9.7% of patients who received a central venous catheter (CVC) up to 2 weeks before a diagnosis of HIT were reported to have an upper-limb DVT ipsilateral to the CVC site [49]. Hence, ASH and Thrombosis Canada suggest screening for asymptomatic proximal DVTs in patients with acute isolated HIT using bilateral lower-limb compression ultrasound. In addition, in patients with an upper-limb CVC, ASH also suggests ultrasound of the ipsilateral upper limb [9,11]. Identification of a DVT has significant implications on the duration of anticoagulation, as discussed in Section 4.5.
4.4. Transition to Oral Anticoagulant
Platelet count recovery marks the transition to subacute HIT, an important time point to guide the transition to oral and/or outpatient anticoagulation [50]. Other considerations include the need for urgent procedures requiring immediate anticoagulation cessation and safety for oral administration [51].
4.4.1. Direct-Acting Oral Anticoagulants
DOACs offer several advantages over warfarin, particularly without the need for monitoring or titration. ASH suggests DOACs over warfarin in patients with subacute HIT A, with the choice of agent determined by drug/patient factors and clinician experience [9]. BSH supports DOACs in patients with HIT who are clinically stable [10]. Thrombosis Canada recommends either DOACs or warfarin for patients who have recovered their platelet count [11]. THANZ recommends DOACs as an alternative to warfarin for patients who have responded to parenteral anticoagulation [12].
DOACs should be commenced within 2 h of stopping argatroban or bivalirudin, 8–12 h after stopping danaparoid, and 24 h after the last dose of fondaparinux, based on the half-life of each of the drugs, respectively [9,10,52].
The doses of DOACs for subacute HIT are also listed in Table 2.
4.4.2. Warfarin
Patients commencing warfarin in subacute HIT should continue parenteral non-heparin anticoagulation for at least five days and until the target international normalized ratio (INR) is reached [8]. When transitioning from bivalirudin and argatroban, it is important to be aware that these agents also prolong the INR [9].
4.5. Duration of Therapeutic Anticoagulation
For HIT patients with thrombosis, three months of anticoagulation is recommended (BSH: Grade 1A) [10]. For HIT patients without thrombosis, ASH suggests anticoagulation until platelet recovery at a minimum [9]. ACCP suggests four weeks of anticoagulation, whereas the BSH recommends anticoagulation until platelet count recovery or at least four weeks, whichever is later [8,10]. Thrombosis Canada recommends at least four weeks of anticoagulation and until platelet recovery [11].
4.6. Avoidance of Heparin
Following a diagnosis of HIT, all heparin products should be avoided except those absolutely required, primarily during cardiovascular surgery, coronary angiography, or percutaneous coronary intervention [9,10,11,20]. The diagnosis should be noted in the patient’s medical record, and an alert card or emergency bracelet may be provided to the patient [9,10]. The decision for heparin re-exposure may be informed by evidence of persistent HIT antibodies, positive functional assays, and a careful evaluation of available expertise with alternative anticoagulants (e.g., cardiopulmonary bypass with bivalirudin).
4.7. Management during Cardiovascular Intervention
Patients with HIT undergoing cardiovascular surgery or surgeries requiring cardiopulmonary bypass are a challenge due to the need for an intraoperative anticoagulant where heparin is normally the preferred agent in this setting. In general, surgeries should be delayed in patients with HIT until they are negative for the HIT antibody (usually >100 days after diagnosis) [10]. Where this is not possible, recommendations from various societal guidelines pertaining to cardiovascular surgery are discussed below.
ACCP suggests intraoperative bivalirudin over non-heparin anticoagulants or heparin combined with antiplatelets for patients with acute or subacute HIT [8]. ASH suggests one of these options for patients with acute or subacute HIT A depending on the cost, availability, and experience of the treating team: intraoperative bivalirudin, heparin plus a potent antiplatelet (e.g., tirofiban or prostacyclin analogs), or plasma exchange and intraoperative heparin [9]. BSH recommends intraoperative bivalirudin in patients with active or recent HIT with positive antibodies. If bivalirudin is not available and intraoperative heparin is required, plasma exchange with or without intravenous immunoglobulin may be offered [10].
In patients with subacute HIT B or remote HIT, intraoperative anticoagulation with heparin is recommended over non-heparin anticoagulant, plasma exchange and heparin, or heparin combined with an antiplatelet agent. Patients who receive heparin intraoperatively may require platelet count monitoring post-surgery due to the risk of delayed-onset HIT [9]. Otherwise, heparin should always be avoided pre- and post-surgery.
For patients with acute HIT or subacute HIT A who require percutaneous coronary intervention (PCI), bivalirudin is recommended over other non-heparin anticoagulants. Argatroban is an alternative if bivalirudin is not available [9].
4.8. Management for Patients Undergoing Renal Replacement Therapy
In acute HIT patients undergoing renal replacement therapy (RRT), a parenteral non-heparin anticoagulant is suggested over heparin and citrate. The options are argatroban, danaparoid, or bivalirudin. After the acute phase, patients requiring ongoing RRT without other indications for anticoagulation can receive citrate or other non-heparin agents to prevent thrombosis of the dialysis circuit [8,9,10].
While DOACs have increasingly become the preferred agents to treat HIT, the optimal dosing regimens and safety in patients with severe renal impairment remain poorly defined.
4.9. Summary of Guidelines and Grades of Evidence
Table 4 summarizes the guidelines on management of HIT with grades of evidence included where available.

Table 4.
Summary of guidelines on management of HIT.
5. Future Directions
We discussed the emerging benefits of delineating HIT into different phases according to the platelet count, immunoassay, and functional assay results. In addition, their use in the research setting may help to reduce heterogeneity in study design, patent recruitment, and streamlining reporting outcomes.
We also reviewed data on the use of DOACs in acute HIT published within the last 10 years. Accumulated evidence is mainly retrospective, with only two prospective studies published to date. Additional prospective data would be helpful, particularly a comparison between DOAC and parenteral-heparin anticoagulants [9]. However, it is important to note challenges reported by previous prospective studies. While heparin is widely used in various clinical settings, HIT is not common. These factors pose difficulties in enrollment and prospective selection of patients [53]. In addition, patients with HIT require timely treatment and are often critically unwell. Hence, thorough consideration of the study methodology, enrollment, and a consent process are required. In addition, the eligibility criteria, such as the threshold for concomitant renal impairment given the frequency in HIT patients, need to be carefully selected [36,53]. Given the increasing acceptance of DOACs for suitable patients with acute HIT and the challenges in designing prospective studies in HIT, establishing an international registry may help facilitate clinician reporting and capturing the real-world outcomes of acute HIT treated with DOACs [9,22].
Better defining of non-canonical HIT presentations remains another important frontier [22]. As we approach consensus definitions of terms such as persistent HIT, refractory HIT, and delayed-onset HIT, we may be better placed to understand the mechanisms of these phenomena, plot their natural history, and design treatment strategies to better target these scenarios.
We have not discussed other PF4-immune diseases in this paper, but we recognize the emerging recognition these disorders have in the field and the future challenges these conditions will bring as we attempt to better identify and treat them [54].
6. Conclusions
In this contemporary review on the management of HIT, we discussed the management of HIT with an emphasis on recent developments, namely incorporating the phases of HIT into its management and DOACs for acute HIT.
The delineation of the phases of HIT, incorporating platelet count, immunoassay, and functional assays, may offer several advantages in clinical and research settings. This includes streamlining patient management and care requirements according to the different phases of HIT and facilitating communication between healthcare providers. Additionally, incorporating the phases of HIT in the research setting may help optimize study design and outcome reporting.
There is increasing acceptance of DOACs worldwide in suitable patients with acute HIT, especially clinically stable patients without high risk of bleeding. They offer several advantages over parenteral anticoagulants, such as convenience of use, ease of administration, broad availability, and lower costs. They do not require monitoring or inpatient management and can be continued to the subacute phase of HIT.
Future research on the efficacy of DOACs in HIT would be beneficial, in particular, comparison with parenteral non-heparin anticoagulants. However, the challenges of designing prospective studies in HIT need to be considered. An international registry may capture the real-world outcomes of patients with HIT treated with DOACs.
Author Contributions
Conceptualization, P.C.; writing—original draft preparation, J.Y.N., M.D., and F.H.; writing—review and editing, P.C. and J.Y.N.; visualization, J.Y.N.; supervision, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tardy-Poncet, B.; Tardy, B.; Grelac, F.; Reynaud, J.; Mismetti, P.; Bertrand, J.C.; Guyotat, D. Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am. J. Hematol. 1994, 45, 252–257. [Google Scholar] [CrossRef]
- Martel, N.; Lee, J.; Wells, P.S. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: A meta-analysis. Blood 2005, 106, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.I.; Coats, R.; Liem, T.K.; Silver, D. Reduced morbidity and mortality rates of the heparin-induced thrombocytopenia syndrome. J. Vasc. Surg. 1998, 27, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Demasi, R.; Bode, A.P.; Knupp, C.; Bogey, W.; Powell, S. Heparin-induced thrombocytopenia. Am. Surg. 1994, 60, 26–29. [Google Scholar] [PubMed]
- Gruel, Y.; Vayne, C.; Rollin, J.; Weber, P.; Faille, D.; Bauters, A.; Macchi, L.; Alhenc-Gelas, M.; Lebreton, A.; De Maistre, E.; et al. Comparative Analysis of a French Prospective Series of 144 Patients with Heparin-Induced Thrombocytopenia (FRIGTIH) and the Literature. Thromb. Haemost. 2020, 120, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, S.; Adla Jala, S.R.; Verma, R.; Chennapragada, S.S.; Pramudita, A.H.; Dandwani, M.; Aggarwal, S.; Savani, S.; Singh, V.; Thevuthasan, S.; et al. Trends in Heparin-Induced Thrombocytopenia in the Pre-Pandemic and Peak-Pandemic Era, and Impact of COVID-19 on Mortality; An Analysis Via the National Inpatient Sample. Blood 2023, 142, 5519. [Google Scholar] [CrossRef]
- Pishko, A.M.; Lefler, D.S.; Gimotty, P.; Paydary, K.; Fardin, S.; Arepally, G.M.; Crowther, M.; Rice, L.; Vega, R.; Cines, D.B.; et al. The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J. Thromb. Haemost. 2019, 17, 1956–1965. [Google Scholar] [CrossRef]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e495S–e530S. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.M.; Chong, B.H.; Cines, D.B.; Greinacher, A.; Gruel, Y.; Linkins, L.A.; Rodner, S.B.; Selleng, S.; Warkentin, T.E.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018, 2, 3360–3392. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Thachil, J.; Anderson, J.A.M.; Baker, P.; Poles, A.; Kitchen, S.; Laffan, M.; Committee, T.B. Diagnosis and management of heparin-induced thrombocytopenia: Third edition. Br. J. Haematol. 2024, 204, 459–475. [Google Scholar] [CrossRef]
- Thrombosis Canada. Heparin-Induced Thrombocytopenia (HIT). Available online: https://thrombosiscanada.ca/hcp/practice/clinical_guides?language=en-ca&guideID=HEPARININDUCEDTHROMBOCYTOPENIA (accessed on 2 April 2024).
- Joseph, J.; Rabbolini, D.; Enjeti, A.K.; Favaloro, E.; Kopp, M.C.; McRae, S.; Pasalic, L.; Tan, C.W.; Ward, C.M.; Chong, B.H. Diagnosis and management of heparin-induced thrombocytopenia: A consensus statement from the Thrombosis and Haemostasis Society of Australia and New Zealand HIT Writing Group. Med. J. Aust. 2019, 210, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Brieger, D.B.; Mak, K.-H.; Kottke-Marchant, K.; Topol, E.J. Heparin-Induced Thrombocytopenia. J. Am. Coll. Cardiol. 1998, 31, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H. Heparin-induced thrombocytopenia. J. Thromb. Haemost. 2003, 1, 1471–1478. [Google Scholar] [CrossRef]
- Chong, B.H. Evolving concepts of pathogenesis of heparin-induced thrombocytopenia: Diagnostic and therapeutic implications. Int. J. Lab. Hematol. 2020, 42 (Suppl. S1), 25–32. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.M.; Chong, B.H. Further characterization of antibody and antigen in heparin-induced thrombocytopenia. Br. J. Haematol. 1999, 107, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A. Management of the multiple phases of heparin-induced thrombocytopenia. Thromb. Haemost. 2016, 116, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.; Pishko, A. Management of heparin-induced thrombocytopenia. In UpToDate; Leung, L.L., Tirnauer, J.S., Post, T., Eds.; Wolters Kluwer: Albany, NY, USA, 2024. [Google Scholar]
- Weiss, P.; Soff, G.A.; Halkin, H.; Seligsohn, U. Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb. Res. 1987, 45, 783–790. [Google Scholar] [CrossRef]
- Choi, P.Y.; Uzun, G.; Bakchoul, T. Results of an international survey of opinions on the definitions and treatments for heparin-induced thrombocytopenia: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J. Thromb. Haemost. 2024, 22, 1772–1778. [Google Scholar] [CrossRef]
- Tuleja, A.; Salvador, D.; Muka, T.; Bernhard, S.; Lenz, A.; Baumgartner, I.; Schindewolf, M. Cost-effectiveness analysis of alternative anticoagulation in suspected heparin-induced thrombocytopenia. Blood Adv. 2022, 6, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Sebaaly, J.; Wooten, L.; Khouli, C.; Mihm, A.; Nisly, S.A. A Multicenter Retrospective Evaluation of Direct Oral Anticoagulants for the Treatment of Heparin-Induced Thrombocytopenia. Am. J. Cardiovasc. Drugs 2022, 22, 417–424. [Google Scholar] [CrossRef]
- Cirbus, K.; Simone, P.; Austin Szwak, J. Rivaroxaban and apixaban for the treatment of suspected or confirmed heparin-induced thrombocytopenia. J. Clin. Pharm. Ther. 2022, 47, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Albuloushi, A.; Rhoten, M.; Kelly, J.; Sylvester, K.W.; Grandoni, J.; Connors, J.M. Evaluation of the use of direct oral anticoagulants for the management of heparin-induced thrombocytopenia. J. Thromb. Thrombolysis 2022, 54, 597–604. [Google Scholar] [CrossRef]
- Farasatinasab, M.; Balouchzehi, S.; Moghaddam, O.M.; Ansarinejad, N.; Mohammadi, M.; Nasiripour, S. An Open-Label, Single-Arm, Pilot Intervention Study to Assess the Efficacy and Safety of Apixaban in Heparin-Induced Thrombocytopenia. J. Clin. Pharmacol. 2022, 62, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Yin, L.; Fu, G.; Liu, Z. Dabigatran Use after Argatroban for Heparin-induced Thrombocytopenia with Thrombosis: A Case Series and Literature Review. Ann. Vasc. Surg. 2022, 80, 392.e1–392.e7. [Google Scholar] [CrossRef]
- Carré, J.; Guérineau, H.; Le Beller, C.; Mauge, L.; Huynh, B.; Nili, R.; Planquette, B.; Clauser, S.; Smadja, D.M.; Helley, D.; et al. Direct Oral Anticoagulants as Successful Treatment of Heparin-Induced Thrombocytopenia: A Parisian Retrospective Case Series. Front. Med. 2021, 8, 713649. [Google Scholar] [CrossRef]
- Farasatinasab, M.; Zarei, B.; Moghtadaei, M.; Nasiripour, S.; Ansarinejad, N.; Zarei, M. Rivaroxaban as an Alternative Agent for Heparin-Induced Thrombocytopenia. J. Clin. Pharmacol. 2020, 60, 1362–1366. [Google Scholar] [CrossRef]
- Nasiripour, S.; Saif, M.; Farasatinasab, M.; Emami, S.; Amouzegar, A.; Basi, A.; Mokhtari, M. Dabigatran as a Treatment Option for Heparin-Induced Thrombocytopenia. J. Clin. Pharmacol. 2019, 59, 107–111. [Google Scholar] [CrossRef]
- Davis, K.A.; Davis, D.O. Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur. J. Haematol. 2017, 99, 332–335. [Google Scholar] [CrossRef]
- Kunk, P.R.; Brown, J.; McShane, M.; Palkimas, S.; Gail Macik, B. Direct oral anticoagulants in hypercoagulable states. J. Thromb. Thrombolysis 2017, 43, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Pai, M.; Linkins, L.A. Direct oral anticoagulants for treatment of HIT: Update of Hamilton experience and literature review. Blood 2017, 130, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Chin, Y.A.; Than, H.; Tan, C.W.; Yap, E.S.; Wong, W.H.; Ng, H.J. Rivaroxaban for heparin-induced thrombocytopenia: Adding to the evidence. Ann. Hematol. 2017, 96, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Linkins, L.A.; Warkentin, T.E.; Pai, M.; Shivakumar, S.; Manji, R.A.; Wells, P.S.; Wu, C.; Nazi, I.; Crowther, M.A. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J. Thromb. Haemost. 2016, 14, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Bay, C.; Vajo, Z.; Freeman, W.; Sharifi, M.; Schwartz, F. New Oral Anticoagulants in the Treatment of Heparin-Induced Thrombocytopenia. Thromb. Res. 2015, 135, 607–609. [Google Scholar] [CrossRef]
- Ng, H.J.; Than, H.; Teo, E.C.Y. First experiences with the use of rivaroxaban in the treatment of heparin-induced thrombocytopenia. Thromb. Res. 2015, 135, 205–207. [Google Scholar] [CrossRef]
- Wilde, M.I.; Markham, A. Danaparoid. A review of its pharmacology and clinical use in the management of heparin-induced thrombocytopenia. Drugs 1997, 54, 903–924. [Google Scholar] [CrossRef]
- Danaparoid (United States: Not available): Drug Information. Available online: https://www.uptodate.com/contents/danaparoid-united-states-not-available-drug-information?search=danaparoid&source=panel_search_result&selectedTitle=1%7E18&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 31 July 2024).
- Keng, T.B.; Chong, B.H. Heparin-induced thrombocytopenia and thrombosis syndrome: In vivo cross-reactivity with danaparoid and successful treatment with r-Hirudin. Br. J. Haematol. 2001, 114, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H.; Magnani, H.N. Orgaran in heparin-induced thrombocytopenia. Haemostasis 1992, 22, 85–91. [Google Scholar] [CrossRef]
- Ronchard, T.; Salaun, E.; Theron, A.; Grisoli, D.; Jaussaud, N.; Collart, F.; Habib, G.; Camoin-Jau, L. Cross-Reactivity Between Heparin and Danaparoid Antibodies in Cardiac Surgery. Ann. Thorac. Surg. 2017, 103, e9–e10. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Akl, E.A.; Crowther, M.; Gutterman, D.D.; Schuünemann, H.J. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, 7s–47s. [Google Scholar] [CrossRef] [PubMed]
- Bivalirudin: Drug information. Available online: https://www.uptodate.com/contents/bivalirudin-drug-information?search=bivalirudin&source=panel_search_result&selectedTitle=1%7E39&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 31 July 2024).
- Apixaban: Drug information. Available online: https://www.uptodate.com/contents/apixaban-drug-information?search=apixaban&source=panel_search_result&selectedTitle=1%7E150&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 31 July 2024).
- Rivaroxaban: Drug information. Available online: https://www.uptodate.com/contents/rivaroxaban-drug-information?search=rivaroxaban&source=panel_search_result&selectedTitle=1%7E150&usage_type=panel&kp_tab=drug_general&display_rank=1 (accessed on 31 July 2024).
- Tardy, B.; Tardy-Poncet, B.; Fournel, P.; Venet, C.; Jospe, R.; Dacosta, A. Lower limb veins should be systematically explored in patients with isolated heparin-induced thrombocytopenia. Thromb. Haemost. 1999, 82, 1199–1200. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.P.; Cook, D.J.; Sigouin, C.S.; Warkentin, T.E. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood 2003, 101, 3049–3051. [Google Scholar] [CrossRef]
- Hvas, A.M.; Favaloro, E.J.; Hellfritzsch, M. Heparin-induced thrombocytopenia: Pathophysiology, diagnosis and treatment. Expert. Rev. Hematol. 2021, 14, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; Berger, J.S. Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc. Med. 2020, 25, 160–173. [Google Scholar] [CrossRef]
- Pishko, A.M.; Linkins, L.A.; Warkentin, T.E.; Cuker, A. Diagnosis and Management of Heparin-Induced Thrombocytopenia (HIT). In A Pocket Guide for the Clinician; American Society of Haematology: Washington, DC, USA, 2018. [Google Scholar]
- Boyce, S.W. Challenges to the design and execution of controlled clinical studies of anticoagulants in patients with heparin-induced thrombocytopenia: Lessons learned. J. Thromb. Thrombolysis 2012, 33, 124–128. [Google Scholar] [CrossRef]
- Schönborn, L.; Esteban, O.; Wesche, J.; Dobosz, P.; Broto, M.; Puig, S.R.; Fuhrmann, J.; Torres, R.; Serra, J.; Llevadot, R.; et al. Anti-PF4 immunothrombosis without proximate heparin or adenovirus vector vaccine exposure. Blood 2023, 142, 2305–2314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).