Textbook Outcome in Colorectal Surgery for Cancer: An Italian Version
Abstract
1. Introduction
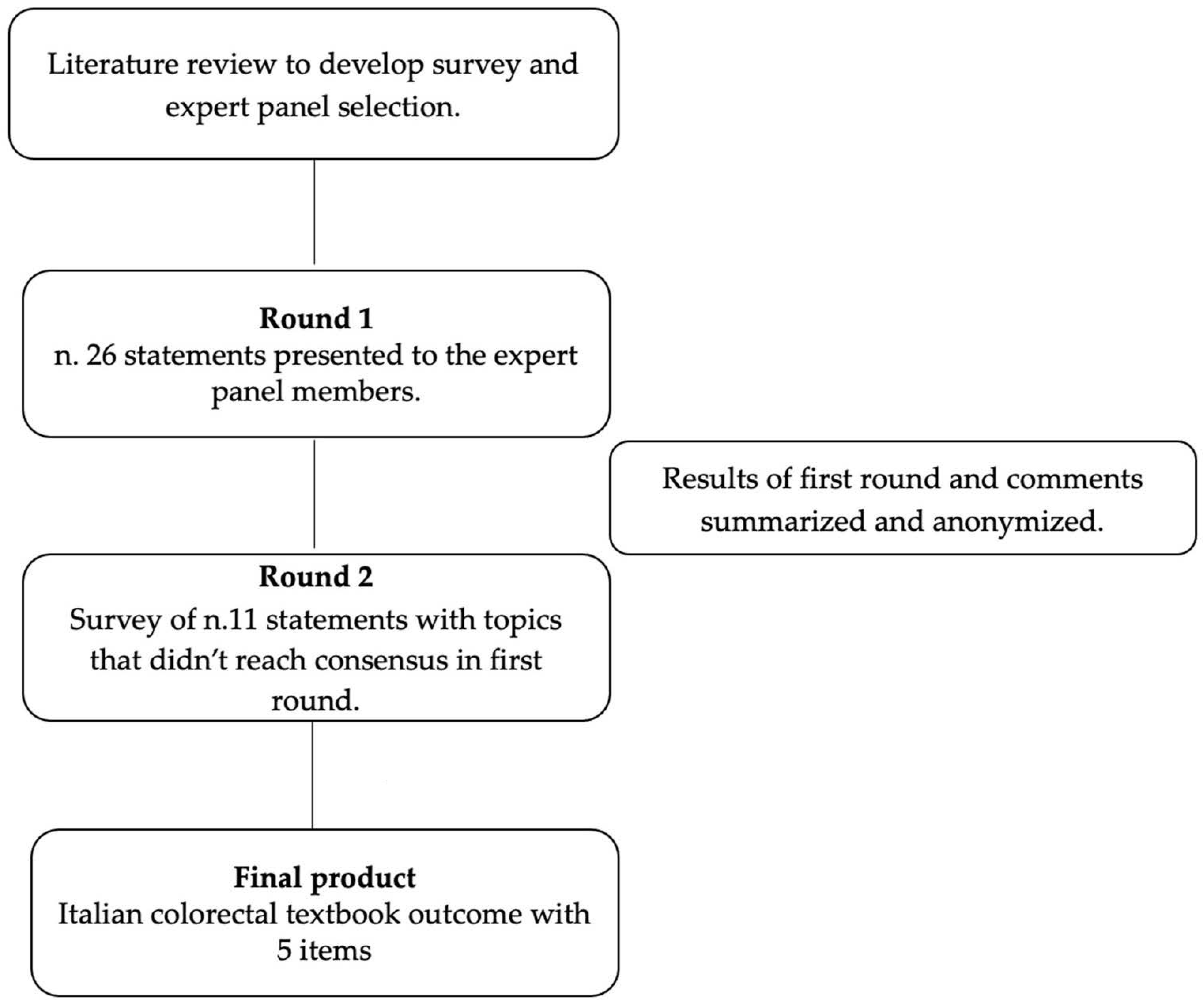
2. Materials and Methods
2.1. Selection of Expert Panel
2.2. Delphi Process
2.3. Data Management and Statistical Analysis
3. Results
- Statement 1—Postoperative Survival
- Statement 2—Oncological Radicality
- Statement 3—Surgery
- Statement 4—Postoperative course
- Statement 5—Perioperative treatments and diagnostics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Argilés, G.; Oki, E.; Martinelli, E.; Taniguchi, H.; Arnold, D.; Mishima, S.; Li, Y.; Smruti, B.K.; Ahn, J.B.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis treatment and follow-up of patients with localised colon cancer. Ann. Oncol. 2021, 32, 1496–1510. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Chang, G.J.; Chen, Y.J.; Chombor, K.; Choen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; Grem, J.L.; Harmath, C.; et al. NCCN Guidelines Version 4.2024 Colon Cancer Continue NCCN Guidelines Panel Disclosures. 2024. Available online: https://www.nccn.org/home/member (accessed on 23 July 2024).
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Elmore, U.; De Luca, R.; De Nardi, P.; Tomatis, M.; Biondi, A.; Persiani, R.; Solaini, L.; Rizzo, G.; Soriero, D.; et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Color. Dis. 2022, 24, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Reddavid, R.; Esposito, L.; Evangelista, A.; Sofia, S.; Degiuli, M. Non-anatomical colonic resections: Splenic flexure and transverse colectomy. Central vascular ligation is crucial for survival. Minerva Chir. 2018, 74, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Reddavid, R.; Ricceri, F.; Di Candido, F.; Ortenzi, M.; Elmore, U.; Belluco, C.; Rosati, R.; Guerrieri, M.; Spinelli, A. Segmental Colonic Resection Is a Safe and Effective Treatment Option for Colon Cancer of the Splenic Flexure: A Nationwide Retrospective Study of the Italian Society of Surgical Oncology-Colorectal Cancer Network Collaborative Group. Dis. Colon Rectum. 2020, 63, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Bengueddach, A.; Tidjane, A.; Tabeti, B.; Behilil, C.; Boudjenan-Serradj, N.; Bensafir, S.; Meharzi, S.E.I.; Aissat, A.; Ghouali, A.K.; Larabi, K.; et al. Evaluation of a quality improvement intervention to reduce anastomotic leak following right colectomy (EAGLE): Pragmatic, batched stepped-wedge, cluster-randomized trial in 64 countries. Br. J. Surg. 2024, 111. [Google Scholar] [CrossRef]
- Reddavid, R.; Sofia, S.; Puca, L.; Moro, J.; Ceraolo, S.; Jimenez-Rodriguez, R.; Degiuli, M. Robotic Rectal Resection for Rectal Cancer in Elderly Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5331. [Google Scholar] [CrossRef]
- Kolfschoten, N.E.; Kievit, J.; Gooiker, G.A.; Van Leersum, N.J.; Snijders, H.S.; Eddes, E.H.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M.; Marang-Van De Mheen, P.J. Focusing on desired outcomes of care after colon cancer resections; Hospital variations in “textbook outcome”. Eur. J. Surg. Oncol. 2013, 39, 156–163. [Google Scholar] [CrossRef]
- Shammas, R.L.; Sisk, G.C.; Coroneos, C.J.; Offodile, A.C.; Largo, R.D.; Momeni, A.; Berlin, N.L.; Hanson, S.E.; Momoh, A.O.; Nelson, J.A.; et al. Textbook outcomes in DIEP flap breast reconstruction: A Delphi study to establish consensus. Breast Cancer Res. Treat. 2023, 197, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Görgec, B.; Cacciaguerra, A.B.; Pawlik, T.M.; Aldrighetti, L.A.; Alseidi, A.A.; Cillo, U.; Kokudo, N.; Geller, D.A.; Wakabayashi, G.; Asbun, H.J.; et al. An International Expert Delphi Consensus on Defining Textbook Outcome in Liver Surgery (TOLS). Ann. Surg. 2023, 277, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sędłak, K.; Rawicz-Pruszyński, K.; Mlak, R.; Van Sandick, J.; Gisbertz, S.; Pera, M.; Dal Cero, M.; Baiocchi, G.L.; Celotti, A.; Morgagni, P.; et al. Textbook Oncological Outcome in European GASTRODATA. Ann. Surg. 2023, 278, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Barclay, M.; Dixon-Woods, M.; Lyratzopoulos, G. The problem with composite indicators. BMJ Qual. Saf. 2019, 28, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Manatakis, D.K.; Tzardi, M.; Souglakos, J.; Tsiaoussis, J.; Agalianos, C.; Kyriazanos, I.D.; Pechlivanides, G.; Kordelas, A.; Tasis, N.; Gouvas, N.; et al. Achieving a Textbook Outcome in Colon Cancer Surgery Is Associated with Improved Long-Term Survival. Curr. Oncol. 2023, 30, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- van Groningen, J.T.; Ceyisakar, I.E.; Gietelink, L.; Henneman, D.; van der Harst, E.; Westerterp, M.; Marang-van de Mheen, P.J.; Tollenaar, R.A.; Lingsma, H.; Wouters, M.W.; et al. Identifying best performing hospitals in colorectal cancer care; is it possible? Eur. J. Surg. Oncol. 2020, 46, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Jünger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef] [PubMed]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Aiken, T.; Abbott, D.E. Textbook oncologic outcome: A promising summary metric of high-quality care, but are we on the same page? J. Surg. Oncol. 2020, 121, 923–924. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Mackay, T.M.; van Dieren, S.; van der Schelling, G.P.; Nieuwenhuijs, V.B.; Bosscha, K.; van der Harst, E.; van Dam, R.M.; Liem, M.S.; Festen, S.; et al. Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Ann. Surg. 2020, 271, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.E.; Mamidanna, R.; Vincent, C.A.; Faiz, O. Population-based cohort study comparing 30- and 90-day institutional mortality rates after colorectal surgery. Br. J. Surg. 2013, 100, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.; Turner, M.C.; Sun, Z.; Kim, J.; Ezekian, B.; Migaly, J.; Mantyh, C.R. The appropriateness of 30-day mortality as a quality metric in colorectal cancer surgery. Am. J. Surg. 2018, 215, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Gazala, M.A.; Wexner, S.D. Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol. Rep. 2017, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Horesh, N.; Freund, M.R.; Garoufalia, Z.; Gefen, R.; Silva-Alvarenga, E.; Maron, D.J.; DaSilva, G.; Wexner, S.D. Trends in the Characteristics, Treatment, and Outcomes of Rectal Adenocarcinoma in the US from 2004 to 2019: A National Cancer Database Analysis. JAMA Oncol. 2023, 9, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Graffner, H.; Fredlund, P.; Olsson, S.Å.; Oscarson, J.; Petersson, B.G. Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument—A randomized study. Dis. Colon Rectum. 1983, 26, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Iwatsuki, M.; Mitsuura, C.; Morito, A.; Ohuchi, M.; Kosumi, K.; Eto, K.; Ogawa, K.; Baba, Y.; Iwagami, S.; et al. Textbook outcome contributes to long-term prognosis in elderly colorectal cancer patients. Langenbeck’s Arch. Surg. 2023, 408, 245. [Google Scholar] [CrossRef]
- Moris, D.; Shaw, B.I.; Gloria, J.; Kesseli, S.J.; Samoylova, M.L.; Schmitz, R.; Manook, M.; McElroy, L.M.; Patel, Y.; Berg, C.L.; et al. Textbook Outcomes in Liver Transplantation. World J. Surg. 2020, 44, 3470–3477. [Google Scholar] [CrossRef]
- Busweiler, L.A.D.; Schouwenburg, M.G.; van Berge Henegouwen, M.I.; Kolfschoten, N.E.; de Jong, P.C.; Rozema, T.; Wijnhoven, B.P.L.; van Hillegersberg, R.; Wouters, M.W.J.M.; van Sandick, J.W.; et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br. J. Surg. 2017, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Hayes, L.D.; Keegan, T.J.; Subar, D.A.; Gaffney, C.J. The Impact of Prehabilitation on Patient Outcomes in Hepatobiliary, Colorectal, and Upper Gastrointestinal Cancer Surgery: A PRISMA-Accordant Meta-analysis. Ann. Surg. 2021, 274, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kenzik, K.M.; Williams, G.R.; Bhatia, S.; Balentine, C.J. Post-Acute Care among Older Adults with Stage I to III Colorectal Cancer. J. Am. Geriatr. Soc. 2019, 67, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Tian, Y.F.; Liu, W.S.; Chou, C.L.; Cheng, L.C.; Chu, S.S.; Lee, C.C. The association between the composite quality measure “textbook outcome” and long term survival in operated colon cancer. Medicine 2020, 99, E22447. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.A.; Kim, J.; Berlth, F.; Sugita, Y.; Grimminger, P.P.; Sano, T.; Rosati, R.; Baiocchi, G.L.; Bencivenga, M.; De Manzoni, G.; et al. Defining benchmarks for total and distal gastrectomy: Global multicentre analysis. Br. J. Surg. 2024, 111, znad379. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Papapanou, M.; Routsi, E.; Mastoraki, A.; Lidoriki, I.; Zavras, N.; Avgerinos, D.V.; Lazaris, A.M.; Tsaroucha, A. Career barriers for women in surgery. Surgeon 2022, 20, 275–283. [Google Scholar] [CrossRef]
- Miller, P. Introduction: #MeToo in Surgery: Narratives by Women Surgeons. Narrat. Inq. Bioeth. 2019, 9, 179–183. [Google Scholar] [CrossRef]
| n | % | ||
|---|---|---|---|
| Gender | Male | 25 | 89.3 |
| Female | 3 | 10.7 | |
| Hospital setting | Teaching | 27 | 96.4 |
| Non-teaching | 1 | 3.6 | |
| Colorectal surgery expertise | <5 y | 1 | 3.6 |
| ≥5 y <10 y | 2 | 7.1 | |
| ≥10 y | 25 | 89.3 | |
| Number of colorectal resections | <500 | 15 | 53.5 |
| ≥500 | 13 | 46.5 | |
| Teaching activity | Yes | 27 | 96.4 |
| No | 1 | 3.6 | |
| Speaker, moderator or session president at international and national meetings | Yes | 28 | 100.0 |
| No | 0 | 0.0 | |
| Journal board activity | Yes | 23 | 82.2 |
| No | 5 | 17.8 | |
| Number of scientific publications | <50 | 8 | 28.5 |
| ≥50 <100 | 9 | 32.2 | |
| ≥100 | 11 | 39.3 | |
| H-index | <10 | 2 | 7.2 |
| ≥10 ≤20 | 5 | 17.8 | |
| >20 | 21 | 75.0 |
| Item | Median (IQR) | Round of Consensus | Agreement, % (n/N) |
|---|---|---|---|
| 1.2 30-day survival | 4 (3–5) | 1 | 75.0 (21/28) |
| 1.3 90-day survival (no mortality for all causes) | 3.5 (3–5) | 1 | 75.0 (21/28) |
| 2.2 Negative margins | 4 (3–5) | 1 | 85.7 (24/28) |
| 2.3 ≥12 LNs harvested | 4 (3–4.25) | 1 | 78.6 (22/28) |
| 2.5 Negative margins + 12 LNs | 4 (3–5) | 1 | 75.0 (21/28) |
| 2.7 Negative margins + 12 LNs + 5 cm for colon | 4 (3–5) | 1 | 85.7 (24/28) |
| 3.1 Ostomy fashioning | 4 (3–5) | 2 | 92.6 (25/27) |
| 3.2 Minimally invasive approach | 4 (3–5) | 1 | 89.3 (25/28) |
| 4.1 No reinterventions | 4 (3–5) | 1 | 82.1 (23/28) |
| 4.2 No complications | 4 (3–5) | 1 | 78.6 (22/28) |
| 4.6 No readmission (30 days) | 4 (3–5) | 1 | 85.7 (24/28) |
| 4.8 ERAS, at least 10 items | 4 (3–5) | 1 | 75.0 (21/28) |
| 4.9 ERAS, according to comorbidities of the patient | 3 (2.75–5) | 1 | 75.0 (21/28) |
| 4.9 Clavien–Dindo grade < 3b | 4 (4–5) | 2 | 88.9 (24/27) |
| 5.1 Appropriate CHT and RT regimens | 3 (2.75–4) | 1 | 75.0 (21/28) |
| 5.2 CHT and RT for rectal cancer | 4 (4–5) | 1 | 92.9 (26/28) |
| 5.3 Complete colonoscopy after or before surgery | 4.5 (2.75–5) | 1 | 75.0 (21/28) |
| 5.4 Tumor Board Evaluation | 5 (4–5) | 1 | 96.4 (21/28) |
| Item | Round 1 | Round 2 | ||
|---|---|---|---|---|
| Median (IQR) | Agreement, % (n/N) | Median (IQR) | Agreement, % (n/N) | |
| 1.1 Survival until discharge | 3 (1–4) | 53.6 (15/28) | ||
| 1.4 90 d survival (surg. causes) | 2.5 (2–4) | 50.0 (14/28) | 3 (1–5) | 59.3 (16/27) |
| 2.1 Radical resection | 1 (1–2) | 17.9 (5/28) | ||
| 2.4 No margins of 5 cm (only for colon) | 3 (2–3.25) | 60.7 (17/28) | 4 (2–5) | 66.7 (18/27) |
| 2.6 Negative margins + 5 cm for colon | 3 (2–4) | 60.7 (17/28) | ||
| 4.3 LOS < 7 days | 3 (2–4) | 57.1 (16/28) | 3 (2.5–4) | 74.1 (20/27) |
| 4.4 LOS < 75 perc. | 3 (2–4) | 64.3 (18/28) | ||
| 4.5 No readmission (90 days) | 3 (2–4) | 64.3 (18/28) | ||
| 4.7 ERAS, all items | 3 (2–4) | 57.1 (16/28) | ||
| Textbook Outcome, Italian Version | ||
|---|---|---|
| Items | Agreement, % (n/N) | |
| Postoperative survival |
| 75.0 (21/28) |
| Resection R0 |
| 75.0 (21/28) |
| 78.6 (22/28) | |
| Optimal surgery |
| 89.3 (25/28) |
| 92.6 (25/27) | |
| Regular postoperative course |
| 88.9 (24/27) |
| 77.8 (21/27) | |
| 85.7 (24/28) | |
| Adequate perioperative treatments and diagnostics |
| 75.0 (21/28) |
| 75.0 (21/28) | |
| 96.4 (21/28) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofia, S.; Degiuli, M.; Anania, G.; Baiocchi, G.L.; Baldari, L.; Baldazzi, G.; Bianco, F.; Borghi, F.; Cavaliere, D.; Coco, C.; et al. Textbook Outcome in Colorectal Surgery for Cancer: An Italian Version. J. Clin. Med. 2024, 13, 4687. https://doi.org/10.3390/jcm13164687
Sofia S, Degiuli M, Anania G, Baiocchi GL, Baldari L, Baldazzi G, Bianco F, Borghi F, Cavaliere D, Coco C, et al. Textbook Outcome in Colorectal Surgery for Cancer: An Italian Version. Journal of Clinical Medicine. 2024; 13(16):4687. https://doi.org/10.3390/jcm13164687
Chicago/Turabian StyleSofia, Silvia, Maurizio Degiuli, Gabriele Anania, Gian Luca Baiocchi, Ludovica Baldari, Gianandrea Baldazzi, Francesco Bianco, Felice Borghi, Davide Cavaliere, Claudio Coco, and et al. 2024. "Textbook Outcome in Colorectal Surgery for Cancer: An Italian Version" Journal of Clinical Medicine 13, no. 16: 4687. https://doi.org/10.3390/jcm13164687
APA StyleSofia, S., Degiuli, M., Anania, G., Baiocchi, G. L., Baldari, L., Baldazzi, G., Bianco, F., Borghi, F., Cavaliere, D., Coco, C., Coppola, R., D’Ugo, D., Delrio, P., Fumagalli Romario, U., Guerrieri, M., Milone, M., Morino, M., Muratore, A., Navarra, G., ... Reddavid, R. (2024). Textbook Outcome in Colorectal Surgery for Cancer: An Italian Version. Journal of Clinical Medicine, 13(16), 4687. https://doi.org/10.3390/jcm13164687











