How to Improve Meniscal Repair through Biological Augmentation: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Why Repair?
4. Meniscal and Biological Augmentation
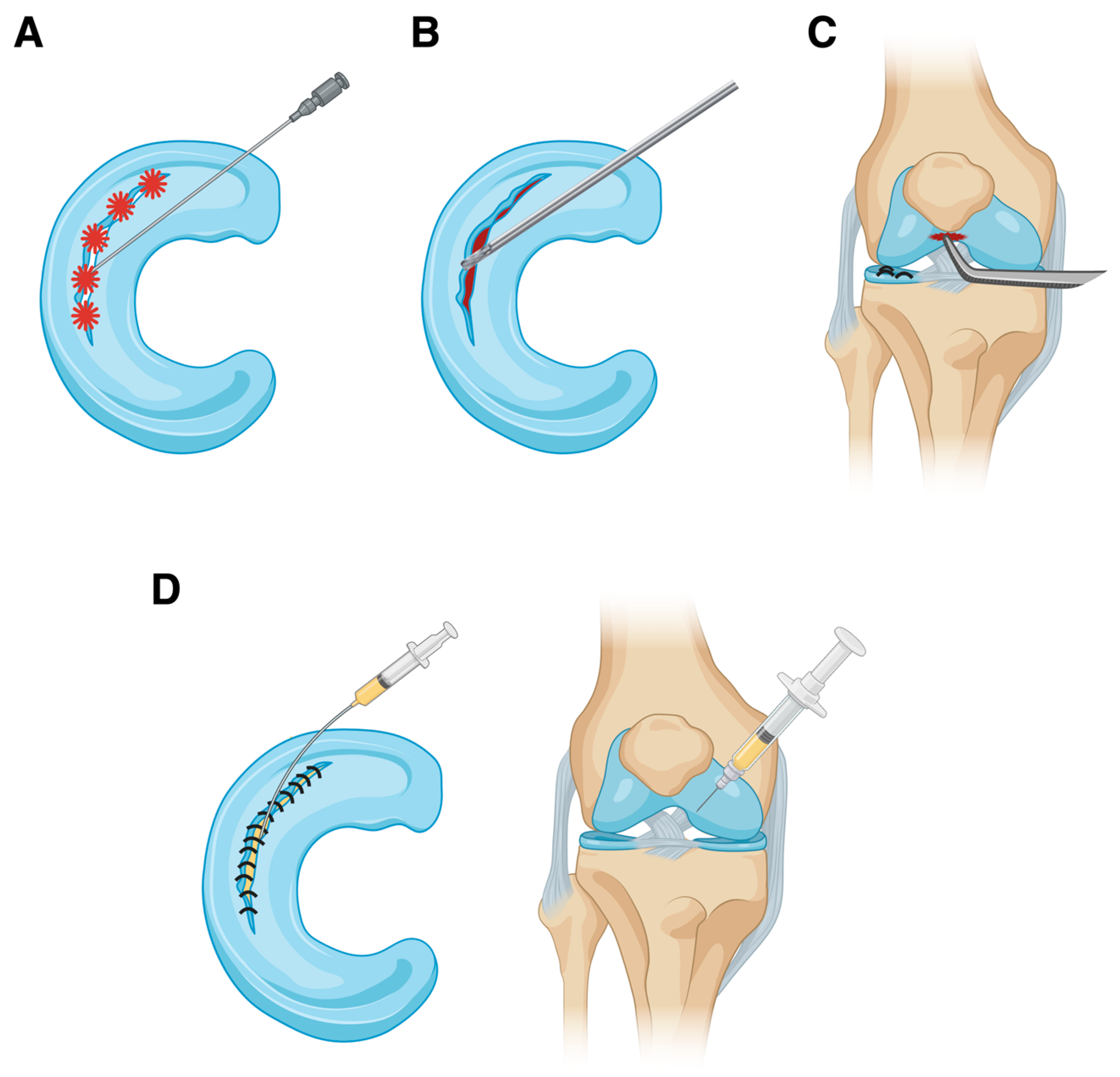
4.1. Trephination
4.2. Abrasion
4.3. Microfracture
4.4. Platelet-Rich Plasma
4.5. Fibrin Clot
4.6. Platelet-Rich Fibrin Clot
| Technique | Summary | Biology | Clinical Evidence | References |
|---|---|---|---|---|
| Trephination | A spinal needle is used arthroscopically to create multiple holes in the inner wall of the meniscus to form vascular channels connecting the vascularized area (red-red zone) with the meniscal lesion. | Redirecting the blood supply by allowing migration of cells and factors that stimulate a healing response and remodeling. | Contradictory: effective in promoting meniscal healing only if combined with other techniques. | [70,71,73,74,113] |
| Microfracture | Once meniscal repair is complete, the arthroscopic fluid flow is interrupted and a blunt tip is used arthroscopically to perform microfractures in the intercondylar notch, at the origin of the PCL, or in the medial aspect of the lateral femoral condyle. These microfractures penetrate the subchondral bone until bone marrow elements are observed entering the joint through the microfracture holes. | Exposes the joint to bone marrow mesenchymal stromal cells and growth factors such as IGF-1, TGF-β, and several BMPs. | Improved meniscal healing at arthroscopic second looks and enhanced clinical results. | [86,87,88,105] |
| Abrasions | A rasp or shaver is used arthroscopically on the edges of the meniscal lesion and parameniscal synovium to revitalize the meniscal tissue and stimulate the release of growth factors from the synovial surface. | Promotes the release of IL-1α, TGF-β1, PDGF, and PCNA. | Good results shown by arthroscopic second looks. Less effective with lesions in poorly vascularized zones. | [75,77,78,79,80,81,108] |
| Fibrin clot | Peripheral blood is taken from the patient. The blood is placed in a sterile specimen cup and then stirred using a frosted glass syringe plunger until the clot is completely formed. Afterwards, it is washed with saline and introduced into the joint. | Composed of blood factors including 5% of platelets that are progressively released in situ. | Satisfactory outcomes with high healing rates despite failures are still present in a small variable percentage. Greater efficacy compared to other techniques is uncertain. | [114,115,116,118] |
| PRF clot | The patient’s peripheral blood is drawn and placed in a sterile glass tube and centrifuged. After centrifugation, the clot is left to consolidate for 5–10 min. Then, it is removed from the centrifuge and the sterile glass tube and separated between the red clot and white clot junction. Finally, it is introduced into the joint. | Composed by 85–95% of platelets as well as a fibrin matrix polymer, leucocytes, cytokines, and stem cells. Progressive and localized release of cytokines, antimicrobial peptides, cells, and growth factors. | The theoretical advantages are numerous, although adequate clinical studies demonstrating this are lacking. Theoretical superiority over fibrin clot is debated. | [119,120,124,126,127] |
| PRP | Single or multiple intra-articular or intra-lesion injections of PRP. | Introduces growth factors (PDGF, VEGF, TGF-β1) to promote chemotaxis, angiogenesis, collagen matrix synthesis, and cell proliferation, eventually enhancing meniscal healing. | It is contradictory as it depends on the mode of use and the characteristics of the PRP. Intra-lesion injection appears more effective than intra-articular administration. | [94,95,96,97,98,99,100,101,102,105] |
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Bosque, J.; Meehan, J.P.; Jamali, A.; Marder, R. Increase in Outpatient Knee Arthroscopy in the United States: A Comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Jt. Surg. 2011, 93, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Herrlin, S.; Hållander, M.; Wange, P.; Weidenhielm, L.; Werner, S. Arthroscopic or Conservative Treatment of Degenerative Medial Meniscal Tears: A Prospective Randomised Trial. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, J.B.; Hare, K.B.; Lohmander, L.S. Large Increase in Arthroscopic Meniscus Surgery in the Middle-Aged and Older Population in Denmark from 2000 to 2011. Acta Orthop. 2014, 85, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, P.D.; Jokl, P. Surgical Outcomes After Arthroscopic Partial Meniscectomy. J. Am. Acad. Orthop. Surg. 2007, 15, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Burks, R.T.; Metcalf, M.H.; Metcalf, R.W. Fifteen-Year Follow-up of Arthroscopic Partial Meniscectomy. Arthrosc. J. Arthrosc. Relat. Surg. 1997, 13, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Brophy, R.H.; Chaisson, C.E.; De Chaves, L.; Cole, B.J.; Dahm, D.L.; Donnell-Fink, L.A.; Guermazi, A.; Haas, A.K.; Jones, M.H.; et al. Surgery versus Physical Therapy for Meniscal Tear and Osteoarthritis. N. Engl. J. Med. 2013, 369, 677–678. [Google Scholar] [CrossRef]
- Herrlin, S.V.; Wange, P.O.; Lapidus, G.; Hållander, M.; Werner, S.; Weidenhielm, L. Is Arthroscopic Surgery Beneficial in Treating Non-Traumatic, Degenerative Medial Meniscal Tears? A Five Year Follow-Up. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 358–364. [Google Scholar] [CrossRef]
- Kirkley, A.; Birmingham, T.B.; Litchfield, R.B.; Giffin, J.R.; Willits, K.R.; Wong, C.J.; Feagan, B.G.; Donner, A.; Griffin, S.H.; D’Ascanio, L.M.; et al. A Randomized Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N. Engl. J. Med. 2008, 359, 1097–1107. [Google Scholar] [CrossRef]
- Sihvonen, R.; Paavola, M.; Malmivaara, A.; Itälä, A.; Joukainen, A.; Nurmi, H.; Kalske, J.; Järvinen, T.L.N. Arthroscopic Partial Meniscectomy versus Sham Surgery for a Degenerative Meniscal Tear. N. Engl. J. Med. 2013, 369, 2515–2524. [Google Scholar] [CrossRef]
- Beaufils, P.; Hulet, C.; Dhénain, M.; Nizard, R.; Nourissat, G.; Pujol, N. Clinical Practice Guidelines for the Management of Meniscal Lesions and Isolated Lesions of the Anterior Cruciate Ligament of the Knee in Adults. Orthop. Traumatol. Surg. Res. 2009, 95, 437–442. [Google Scholar] [CrossRef]
- Sihvonen, R.; Englund, M.; Turkiewicz, A.; Järvinen, T.L.N.; Finnish Degenerative Meniscal Lesion Study Group. Mechanical Symptoms and Arthroscopic Partial Meniscectomy in Patients with Degenerative Meniscus Tear: A Secondary Analysis of a Randomized Trial. Ann. Intern. Med. 2016, 164, 449. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Bin, S.-I.; Kim, J.-M.; Lee, B.-S.; Oh, S.-M.; Cho, W.-J.; Lee, J.-H. Partial Meniscectomy Provides the Favorable Outcomes for Symptomatic Medial Meniscus Tear with an Intact Posterior Root. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.P.; Garofalo, R.; Margheritini, F. Chondrolysis after Partial Lateral Meniscectomy in Athletes. Knee Surg. Sports Traumatol. Arthr. 2008, 16, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Pioger, C.; Saithna, A.; Kandhari, V.; Thaunat, M.; Vieira, T.D.; Freychet, B.; Franck, F.; Sonnery-Cottet, B. Risk Factors for Rapid Chondrolysis After Partial Lateral Meniscectomy: A Scoping Review of the Literature. Orthop. J. Sports Med. 2021, 9, 232596712098177. [Google Scholar] [CrossRef] [PubMed]
- Duethman, N.C.; Wilbur, R.R.; Song, B.M.; Stuart, M.J.; Levy, B.A.; Camp, C.L.; Krych, A.J. Lateral Meniscal Tears in Young Patients: A Comparison of Meniscectomy and Surgical Repair. Orthop. J. Sports Med. 2021, 9, 232596712110460. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Bin, S.-I.; Kim, J.-M.; Park, M.-H.; Lee, S.-M.; Bae, K.-H. Partial Meniscectomy for Degenerative Medial Meniscal Root Tears Shows Favorable Outcomes in Well-Aligned, Nonarthritic Knees. Am. J. Sports Med. 2019, 47, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bin, S.-I.; Kim, J.-M.; Lee, B.-S.; Kim, T.-H. Progression of Radiographic Osteoarthritis after Partial Meniscectomy in Degenerative Medial Meniscal Posterior Root Tears Was Greater in Varus- than in Neutral-Aligned Knees: A Minimum 5-Year Follow-Up. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- Kluczynski, M.A.; Marzo, J.M.; Wind, W.M.; Fineberg, M.S.; Bernas, G.A.; Rauh, M.A.; Zhou, Z.; Zhao, J.; Bisson, L.J. The Effect of Body Mass Index on Clinical Outcomes in Patients Without Radiographic Evidence of Degenerative Joint Disease After Arthroscopic Partial Meniscectomy. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Feeley, B.T.; Lau, B.C. Biomechanics and Clinical Outcomes of Partial Meniscectomy. J. Am. Acad. Orthop. Surg. 2018, 26, 853–863. [Google Scholar] [CrossRef]
- Paradowski, P.T.; Lohmander, L.S.; Englund, M. Osteoarthritis of the Knee after Meniscal Resection: Long Term Radiographic Evaluation of Disease Progression. Osteoarthr. Cartil. 2016, 24, 794–800. [Google Scholar] [CrossRef]
- Thorlund, J.B.; Holsgaard-Larsen, A.; Creaby, M.W.; Jørgensen, G.M.; Nissen, N.; Englund, M.; Lohmander, L.S. Changes in Knee Joint Load Indices from before to 12 Months after Arthroscopic Partial Meniscectomy: A Prospective Cohort Study. Osteoarthr. Cartil. 2016, 24, 1153–1159. [Google Scholar] [CrossRef]
- Brophy, R.H.; Gray, B.L.; Nunley, R.M.; Barrack, R.L.; Clohisy, J.C. Total Knee Arthroplasty After Previous Knee Surgery: Expected Interval and the Effect on Patient Age. J. Bone Jt. Surg. 2014, 96, 801–805. [Google Scholar] [CrossRef]
- Aprato, A.; Sordo, L.; Costantino, A.; Sabatini, L.; Barberis, L.; Testa, D.; Massè, A. Outcomes at 20 Years after Meniscectomy in Young Patients. Knee 2021, 29, 49–54. [Google Scholar] [CrossRef]
- Aprato, A.; Sordo, L.; Costantino, A.; Sabatini, L.; Barberis, L.; Testa, D.; Massè, A. Outcomes at 20 Years After Meniscectomy in Patients Aged 50 to 70 Years. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 1547–1553. [Google Scholar] [CrossRef]
- Available online: https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-31-no-3-2011/report-summary-life-with-arthritis-canada-personal-public-health-challenge.html (accessed on 17 June 2024).
- Musahl, V.; Citak, M.; O’Loughlin, P.F.; Choi, D.; Bedi, A.; Pearle, A.D. The Effect of Medial Versus Lateral Meniscectomy on the Stability of the Anterior Cruciate Ligament-Deficient Knee. Am. J. Sports Med. 2010, 38, 1591–1597. [Google Scholar] [CrossRef]
- Hasan, J.; Fisher, J.; Ingham, E. Current Strategies in Meniscal Regeneration. J. Biomed. Mater. Res. 2014, 102, 619–634. [Google Scholar] [CrossRef]
- Brown, M.J.; Farrell, J.P.; Kluczynski, M.A.; Marzo, J.M. Biomechanical Effects of a Horizontal Medial Meniscal Tear and Subsequent Leaflet Resection. Am. J. Sports Med. 2016, 44, 850–854. [Google Scholar] [CrossRef]
- Koh, J.L.; Yi, S.J.; Ren, Y.; Zimmerman, T.A.; Zhang, L.-Q. Tibiofemoral Contact Mechanics with Horizontal Cleavage Tear and Resection of the Medial Meniscus in the Human Knee. J. Bone Jt. Surg. 2016, 98, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.S.; Pan, T.J.; Tran, D.; Dumpe, S.C.; Zhang, X.; Harner, C.D. Vertical Tears of the Lateral Meniscus: Effects on In Vitro Tibiofemoral Joint Mechanics. Orthop. J. Sports Med. 2014, 2, 232596711454123. [Google Scholar] [CrossRef]
- Ode, G.E.; Van Thiel, G.S.; McArthur, S.A.; Dishkin-Paset, J.; Leurgans, S.E.; Shewman, E.F.; Wang, V.M.; Cole, B.J. Effects of Serial Sectioning and Repair of Radial Tears in the Lateral Meniscus. Am. J. Sports Med. 2012, 40, 1863–1870. [Google Scholar] [CrossRef]
- Bedi, A.; Kelly, N.; Baad, M.; Fox, A.J.S.; Ma, Y.; Warren, R.F.; Maher, S.A. Dynamic Contact Mechanics of Radial Tears of the Lateral Meniscus: Implications for Treatment. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sturnieks, D.L.; Besier, T.F.; Mills, P.M.; Ackland, T.R.; Maguire, K.F.; Stachowiak, G.W.; Podsiadlo, P.; Lloyd, D.G. Knee Joint Biomechanics Following Arthroscopic Partial Meniscectomy. J. Orthop. Res. 2008, 26, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Wrigley, T.V.; Metcalf, B.R.; Hinman, R.S.; Cicuttini, F.M.; Dempsey, A.R.; Mills, P.M.; Lloyd, D.G.; Bennell, K.L. Mechanisms Underpinning the Peak Knee Flexion Moment Increase over 2-Years Following Arthroscopic Partial Meniscectomy. Clin. Biomech. 2015, 30, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Beamer, B.S.; Walley, K.C.; Okajima, S.; Manoukian, O.S.; Perez-Viloria, M.; DeAngelis, J.P.; Ramappa, A.J.; Nazarian, A. Changes in Contact Area in Meniscus Horizontal Cleavage Tears Subjected to Repair and Resection. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Krause, W.R.; Pope, M.H.; Johnson, R.J.; Wilder, D.G. Mechanical Changes in the Knee after Meniscectomy. J Bone Jt. Surg Am 1976, 58, 599–604. [Google Scholar] [CrossRef]
- Barber, F.A.; McGarry, J.E. Meniscal Repair Techniques. Sports Med. Arthrosc. Rev. 2007, 15, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Beaufils, P.; Pujol, N. Meniscal Repair: Technique. Orthop. Traumatol. Surg. Res. 2018, 104, S137–S145. [Google Scholar] [CrossRef]
- Rothermel, S.D.; Smuin, D.; Dhawan, A. Are Outcomes After Meniscal Repair Age Dependent? A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; McIntosh, A.L.; Voll, A.E.; Stuart, M.J.; Dahm, D.L. Arthroscopic Repair of Isolated Meniscal Tears in Patients 18 Years and Younger. Am. J. Sports Med. 2008, 36, 1283–1289. [Google Scholar] [CrossRef]
- Yang, C.-P.; Hung, K.-T.; Weng, C.-J.; Chen, A.C.-Y.; Hsu, K.-Y.; Chan, Y.-S. Clinical Outcomes of Meniscus Repair with or without Multiple Intra-Articular Injections of Platelet Rich Plasma after Surgery. J. Clin. Med. 2021, 10, 2546. [Google Scholar] [CrossRef]
- Shieh, A.K.; Edmonds, E.W.; Pennock, A.T. Revision Meniscal Surgery in Children and Adolescents: Risk Factors and Mechanisms for Failure and Subsequent Management. Am. J. Sports Med. 2016, 44, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Paxton, E.S.; Stock, M.V.; Brophy, R.H. Meniscal Repair Versus Partial Meniscectomy: A Systematic Review Comparing Reoperation Rates and Clinical Outcomes. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1275–1288. [Google Scholar] [CrossRef]
- Chahla, J.; Kennedy, N.I.; Geeslin, A.G.; Moatshe, G.; Cinque, M.E.; DePhillipo, N.N.; LaPrade, R.F. Meniscal Repair with Fibrin Clot Augmentation. Arthrosc. Tech. 2017, 6, e2065–e2069. [Google Scholar] [CrossRef]
- Krych, A.J.; Reardon, P.; Sousa, P.; Levy, B.A.; Dahm, D.L.; Stuart, M.J. Clinical Outcomes After Revision Meniscus Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Seil, R.; Krych, A.J.; Koga, H. Surgical Treatment of Complex Meniscus Tear and Disease: State of the Art. J. ISAKOS 2021, 6, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhao, J. A Meta-Analysis Comparing Meniscal Repair with Meniscectomy in the Treatment of Meniscal Tears: The More Meniscus, the Better Outcome? Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 164–170. [Google Scholar] [CrossRef]
- Bottomley, J.; Al-Dadah, O. Arthroscopic Meniscectomy vs Meniscal Repair: Comparison of Clinical Outcomes. Cureus 2023, 15, e44122. [Google Scholar] [CrossRef]
- Ro, K.-H.; Kim, J.-H.; Heo, J.-W.; Lee, D.-H. Clinical and Radiological Outcomes of Meniscal Repair Versus Partial Meniscectomy for Medial Meniscus Root Tears: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2020, 8, 232596712096207. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosi, R.; Meena, A.; Raj, A.; Ursino, N.; Mangiavini, L.; Herbort, M.; Fink, C. In Elite Athletes with Meniscal Injuries, Always Repair the Lateral, Think about the Medial! A Systematic Review. Knee Surg Sports Traumatol. Arthrosc. 2023, 31, 2500–2510. [Google Scholar] [CrossRef]
- Migliorini, F.; Schäfer, L.; Bell, A.; Weber, C.D.; Vecchio, G.; Maffulli, N. Meniscectomy Is Associated with a Higher Rate of Osteoarthritis Compared to Meniscal Repair Following Acute Tears: A Meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5485–5495. [Google Scholar] [CrossRef]
- Stein, T.; Mehling, A.P.; Welsch, F.; Von Eisenhart-Rothe, R.; Jäger, A. Long-Term Outcome After Arthroscopic Meniscal Repair Versus Arthroscopic Partial Meniscectomy for Traumatic Meniscal Tears. Am. J. Sports Med. 2010, 38, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Hagmeijer, M.H.; Kennedy, N.I.; Tagliero, A.J.; Levy, B.A.; Stuart, M.J.; Saris, D.B.F.; Dahm, D.L.; Krych, A.J. Long-Term Results After Repair of Isolated Meniscal Tears Among Patients Aged 18 Years and Younger: An 18-Year Follow-up Study. Am. J. Sports Med. 2019, 47, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Ronnblad, E.; Barenius, B.; Engstrom, B.; Eriksson, K. Predictive Factors for Failure of Meniscal Repair: A Retrospective Dual-Center Analysis of 918 Consecutive Cases. Orthop. J. Sports Med. 2020, 8, 232596712090552. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, J.J.; Arciero, R.A. Arthroscopic Evaluation of Meniscal Repairs: Factors That Effect Healing. Am. J. Sports Med. 1994, 22, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.D.; Vittori, J.M. The Incidence of Healing in Arthroscopic Meniscal Repairs in Anterior Cruciate Ligament-Reconstructed Knees versus Stable Knees. Am. J. Sports Med. 1992, 20, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.D.; Frank, R.M.; Gupta, A.K.; Harris, J.D.; McCormick, F.M.; Cole, B.J. Trends in Meniscus Repair and Meniscectomy in the United States, 2005–2011. Am. J. Sports Med. 2013, 41, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Jin Hwan, A.; Lee, Y.S.; Yoo, J.C.; Chang, M.J.; Koh, K.H.; Kim, M.H. Clinical and Second-Look Arthroscopic Evaluation of Repaired Medial Meniscus in Anterior Cruciate Ligament—Reconstructed Knees. Am. J. Sports Med. 2010, 38, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, C.; Hanreich, C.; Tscholl, P.M.; Blatter, S.; Windhager, R.; Waldstein, W. Meniscal Repair Outcome in 3829 Patients with a Minimum Follow-up from 2 Years up to 5 Years: A Meta-Analysis on the Overall Failure Rate and Factors Influencing Failure. Am. J. Sports Med. 2024, 52, 822–831. [Google Scholar] [CrossRef]
- Barrett, G. Clinical Results of Meniscus Repair in Patients 40 Years and Older. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 824–829. [Google Scholar] [CrossRef]
- Kise, N.J.; Drogset, J.O.; Ekeland, A.; Sivertsen, E.A.; Heir, S. All-inside Suture Device Is Superior to Meniscal Arrows in Meniscal Repair: A Prospective Randomized Multicenter Clinical Trial with 2-Year Follow-Up. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 211–218. [Google Scholar] [CrossRef]
- Steadman, J.R.; Matheny, L.M.; Singleton, S.B.; Johnson, N.S.; Rodkey, W.G.; Crespo, B.; Briggs, K.K. Meniscus Suture Repair: Minimum 10-Year Outcomes in Patients Younger Than 40 Years Compared with Patients 40 and Older. Am. J. Sports Med. 2015, 43, 2222–2227. [Google Scholar] [CrossRef]
- Wouters, D.B. Repair of a Meniscus Tear within 3 Weeks after Trauma Significantly Reduces the Likelihood of a Recurrent Tear Compared with Later Repairs. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2246–2250. [Google Scholar] [CrossRef]
- O’Donnell, K.; Freedman, K.B.; Tjoumakaris, F.P. Rehabilitation Protocols After Isolated Meniscal Repair: A Systematic Review. Am. J. Sports Med. 2017, 45, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, C.; Hanreich, C.; Tscholl, P.M.; Ristl, R.; Apprich, S.; Windhager, R.; Waldstein, W. Nineteen Percent of Meniscus Repairs Are Being Revised and Failures Frequently Occur after the Second Postoperative Year: A Systematic Review and Meta-Analysis with a Minimum Follow-up of 5 Years. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, E.R.; Hadley, C.J.; Wicks, E.D.; Emper, W.; Cohen, S.B. Return to Play After Isolated Meniscal Repairs in Athletes: A Systematic Review. Orthop. J. Sports Med. 2020, 8, 232596712096209. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Leng, X.; Wang, J.; Hu, X.; Ao, Y. Second-Look Arthroscopic Evaluation of Healing Rates After Arthroscopic Repair of Meniscal Tears: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 232596712110382. [Google Scholar] [CrossRef] [PubMed]
- Abdelkafy, A.; Aigner, N.; Zada, M.; Elghoul, Y.; Abdelsadek, H.; Landsiedl, F. Two to Nineteen Years Follow-up of Arthroscopic Meniscal Repair Using the Outside-in Technique: A Retrospective Study. Arch. Orthop. Trauma Surg. 2007, 127, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Campi, S.; Romeo, G.; Spiezia, F.; Maffulli, N.; Denaro, V. Biological Strategies to Enhance Healing of the Avascular Area of the Meniscus. Stem Cells Int. 2012, 2012, 528359. [Google Scholar] [CrossRef]
- Cook, J.L.; Fox, D.B. A Novel Bioabsorbable Conduit Augments Healing of Avascular Meniscal Tears in a Dog Model. Am. J. Sports Med. 2007, 35, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tu, K.; Xu, Y.; Zhang, W.; Liu, Z.; Ou, S. Treatment of Longitudinal Injuries in Avascular Area of Meniscus in Dogs by Trephination. Arthrosc. J. Arthrosc. Relat. Surg. 1988, 4, 151–159. [Google Scholar] [CrossRef]
- Forriol, F.; Longo, U.; Duart, J.; Ripalda, P.; Vaquero, J.; Loppini, M.; Romeo, G.; Campi, S.; Khan, W.; Muda, A.; et al. VEGF, BMP-7, MatrigelTM, Hyaluronic Acid, In Vitro Cultured Chondrocytes and Trephination for Healing of the Avascular Portion of the Meniscus. An Experimental Study in Sheep. Curr. Stem Cell Res. Ther. 2014, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Arnold, J.A. Trephination and Suturing of Avascular Meniscal Tears: A Clinical Study of the Trephination Procedure. Arthrosc. J. Arthrosc. Relat. Surg. 1996, 12, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Rintz, K.G.; Ferkel, R.D. Trephination of Incomplete Meniscal Tears. Arthrosc. J. Arthrosc. Relat. Surg. 1993, 9, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Uchio, Y.; Okuda, K.; Shu, N.; Yamaguchi, H.; Sakai, Y. Expression of Cytokines after Meniscal Rasping to Promote Meniscal Healing. Arthrosc. J. Arthrosc. Relat. Surg. 2001, 17, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Warren, R.F. The Microvasculature of the Meniscus and Its Response to Injury: An Experimental Study in the Dog. Am. J. Sports Med. 1983, 11, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.R.; Miller, M.D.; Bents, R.T.; Smith, D.K. Meniscal Repair in the Goat Model. Am. J. Sports Med. 1998, 26, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Ochi, M.; Shu, N.; Uchio, Y. Meniscal Rasping for Repair of Meniscal Tear in the Avascular Zone. Arthrosc. J. Arthrosc. Relat. Surg. 1999, 15, 281–286. [Google Scholar] [CrossRef]
- Uchio, Y.; Ochi, M.; Adachi, N.; Kawasaki, K.; Iwasa, J. Results of Rasping of Meniscal Tears with and without Anterior Cruciate Ligament Injury as Evaluated by Second-Look Arthroscopy. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 463–469. [Google Scholar] [CrossRef]
- Ghazi Zadeh, L.; Chevrier, A.; Farr, J.; Rodeo, S.; Buschmann, M. Augmentation Techniques for Meniscus Repair. J. Knee Surg. 2018, 31, 099–116. [Google Scholar] [CrossRef]
- Tetik, O.; Kocabey, Y.; Johnson, D.L. Synovial Abrasion for Isolated, Partial Thickness, Undersurface, Medial Meniscus Tears. Orthopedics 2002, 25, 675–678. [Google Scholar] [CrossRef]
- Krych, A.J.; Pitts, R.T.; Dajani, K.A.; Stuart, M.J.; Levy, B.A.; Dahm, D.L. Surgical Repair of Meniscal Tears with Concomitant Anterior Cruciate Ligament Reconstruction in Patients 18 Years and Younger. Am. J. Sports Med. 2010, 38, 976–982. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, C.; James, E.; LaPrade, R.; Engebretsen, L. How Should We Evaluate Outcomes for Use of Biologics in the Knee? J. Knee Surg. 2014, 28, 035–044. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, I.D.; Moran, C.J.; Potter, H.G.; Warren, R.F.; Rodeo, S.A. Restoration of the Meniscus: Form and Function. Am. J. Sports Med. 2014, 42, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The Influence of Platelet-Derived Products on Angiogenesis and Tissue Repair: A Concise Update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Howarth, W.R.; Brochard, K.; Campbell, S.E.; Grogan, B.F. Effect of Microfracture on Meniscal Tear Healing in a Goat (Capra Hircus) Model. Orthopedics 2016, 39, 105–110. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kwon, O.-J.; Nam, T.-S. Arthroscopic Repair of Horizontal Meniscal Cleavage Tears with Marrow-Stimulating Technique. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 92–98. [Google Scholar] [CrossRef]
- Freedman, K.B.; Nho, S.J.; Cole, B.J. Marrow Stimulating Technique to Augment Meniscus Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.S.; Chahla, J.; Matheny, L.M.; Mitchell, J.J.; LaPrade, R.F. Outcomes After Biologically Augmented Isolated Meniscal Repair with Marrow Venting Are Comparable with Those After Meniscal Repair With Concomitant Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2017, 45, 1341–1348. [Google Scholar] [CrossRef]
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wasko, M.K.; Langner, M.; Pomianowski, S. Repair Augmentation of Unstable, Complete Vertical Meniscal Tears with Bone Marrow Venting Procedure: A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 1500–1508.e1. [Google Scholar] [CrossRef]
- Blough, C.L.; Bobba, C.M.; DiBartola, A.C.; Everhart, J.S.; Magnussen, R.A.; Kaeding, C.; Flanigan, D.C. Biologic Augmentation during Meniscal Repair. J. Knee Surg. 2023, 36, 498–506. [Google Scholar] [CrossRef]
- Keller, R.E.; O’Donnell, E.A.; Medina, G.I.S.; Linderman, S.E.; Cheng, T.T.W.; Sabbag, O.D.; Oh, L.S. Biological Augmentation of Meniscal Repair: A Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Aggarwal, S.; Baker, H.; Athiviraham, A. Biologic Augmentation of Isolated Meniscal Repair. Curr. Rev. Musculoskelet. Med. 2024, 17, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Kuroda, R.; Miwa, M.; Tabata, Y.; Hokugo, A.; Kawamoto, T.; Sasaki, K.; Doita, M.; Kurosaka, M. The Regenerative Effects of Platelet-Rich Plasma on Meniscal Cells In Vitro and Its In Vivo Application with Biodegradable Gelatin Hydrogel. Tissue Eng. 2007, 13, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zellner, J.; Mueller, M.; Berner, A.; Dienstknecht, T.; Kujat, R.; Nerlich, M.; Hennemann, B.; Koller, M.; Prantl, L.; Angele, M.; et al. Role of Mesenchymal Stem Cells in Tissue Engineering of Meniscus. J. Biomed. Mater. Res. 2010, 94A, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.P.; Mayes, C.E.; Miller, R.R.; Imro, A.K.; Davidson, J.M. Regional Mitogenic Response of the Meniscus to Platelet-derived Growth Factor (PDGF-AB). J. Orthop. Res. 1995, 13, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zou, X.; Xia, P.; Aboudi, M.A.K.; Chen, R.; Huang, H. Differential Effects of Platelets Selectively Activated by Protease-Activated Receptors on Meniscal Cells. Am. J. Sports Med. 2020, 48, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-C.; Gao, S.-G.; Xu, M.; Jiang, W.; Tian, J.; Lei, G.-H. A Novel Hypothesis: The Application of Platelet-Rich Plasma Can Promote the Clinical Healing of White-White Meniscal Tears. Med. Sci. Monit. 2012, 18, HY47–HY50. [Google Scholar] [CrossRef]
- Pujol, N.; Salle De Chou, E.; Boisrenoult, P.; Beaufils, P. Platelet-Rich Plasma for Open Meniscal Repair in Young Patients: Any Benefit? Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sochacki, K.R.; Safran, M.R.; Abrams, G.D.; Donahue, J.; Chu, C.; Sherman, S.L. Platelet-Rich Plasma Augmentation for Isolated Arthroscopic Meniscal Repairs Leads to Significantly Lower Failure Rates: A Systematic Review of Comparative Studies. Orthop. J. Sports Med. 2020, 8, 232596712096453. [Google Scholar] [CrossRef]
- Kemmochi, M.; Sasaki, S.; Takahashi, M.; Nishimura, T.; Aizawa, C.; Kikuchi, J. The Use of Platelet-Rich Fibrin with Platelet-Rich Plasma Support Meniscal Repair Surgery. J. Orthop. 2018, 15, 711–720. [Google Scholar] [CrossRef]
- Dai, W.-L.; Zhang, H.; Lin, Z.-M.; Shi, Z.-J.; Wang, J. Efficacy of Platelet-Rich Plasma in Arthroscopic Repair for Discoid Lateral Meniscus Tears. BMC Musculoskelet. Disord. 2019, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.W.; Hadeed, M.M.; Werner, B.C.; Diduch, D.R.; Carson, E.W.; Miller, M.D. Platelet-Rich Plasma in Meniscal Repair: Does Augmentation Improve Surgical Outcomes? Clin. Orthop. Relat. Res. 2015, 473, 1665–1672. [Google Scholar] [CrossRef]
- Everhart, J.S.; Cavendish, P.A.; Eikenberry, A.; Magnussen, R.A.; Kaeding, C.C.; Flanigan, D.C. Platelet-Rich Plasma Reduces Failure Risk for Isolated Meniscal Repairs but Provides No Benefit for Meniscal Repairs with Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019, 47, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Kulinski, K.; Kozar-Kaminska, K.; Wielgus, M.; Langner, M.; Wasko, M.K.; Kowalczewski, J.; Pomianowski, S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes, and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. BioMed Res. Int. 2018, 2018, 9315815. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Poggi, A.; Reale, D.; Andriolo, L.; Flanigan, D.C.; Filardo, G. Biologic Augmentation Reduces the Failure Rate of Meniscal Repair: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 232596712098162. [Google Scholar] [CrossRef] [PubMed]
- Haunschild, E.D.; Huddleston, H.P.; Chahla, J.; Gilat, R.; Cole, B.J.; Yanke, A.B. Platelet-Rich Plasma Augmentation in Meniscal Repair Surgery: A Systematic Review of Comparative Studies. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1765–1774. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F.; Spivak, J.M. Meniscal Repair Using an Exogenous Fibrin Clot. An Experimental Study in Dogs. J. Bone Jt. Surg. Am. 1988, 70, 1209–1217. [Google Scholar] [CrossRef]
- Henning, C.E.; Lynch, M.A.; Yearout, K.M.; Vequist, S.W.; Stallbaumer, R.J.; Decker, K.A. Arthroscopic Meniscal Repair Using an Exogenous Fibrin Clot. Clin. Orthop. Relat. Res. 1990, 252, 64–72. [Google Scholar] [CrossRef]
- Ishimura, M.; Tamai, S.; Fujisawa, Y. Arthroscopic Meniscal Repair with Fibrin Glue. Arthrosc. J. Arthrosc. Relat. Surg. 1991, 7, 177–181. [Google Scholar] [CrossRef]
- Van Trommel, M.; Simonian, P.; Potter, H.; Wickiewicz, T. Arthroscopic Meniscal Repair with Fibrin Clot of Complete Radial Tears of the Lateral Meniscus in the Avascular Zone. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 360–365. [Google Scholar] [CrossRef]
- Forriol, F.; Denaro, V.; Denaro, L.; Longo, U.G.; Taira, H. Bone Lengthening Osteogenesis, a Combination of Intramembranous and Endochondral Ossification: An Experimental Study in Sheep. Strateg. Trauma Limb Reconstr. 2010, 5, 71–78. [Google Scholar] [CrossRef]
- Forriol, F.; Longo, U.G.; Concejo, C.; Ripalda, P.; Maffulli, N.; Denaro, V. Platelet-Rich Plasma, rhOP-1® (rhBMP-7) and Frozen Rib Allograft for the Reconstruction of Bony Mandibular Defects in Sheep. A Pilot Experimental Study. Injury 2009, 40, S44–S49. [Google Scholar] [CrossRef]
- Kamimura, T.; Kimura, M. Meniscal Repair of Degenerative Horizontal Cleavage Tears Using Fibrin Clots: Clinical and Arthroscopic Outcomes in 10 Cases. Orthop. J. Sports Med. 2014, 2, 232596711455567. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Kanto, R.; Kambara, S.; Iseki, T.; Onishi, S.; Yoshiya, S. Successful Treatment of Degenerative Medial Meniscal Tears in Well-Aligned Knees with Fibrin Clot Implantation. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3466–3473. [Google Scholar] [CrossRef]
- Ra, H.J.; Ha, J.K.; Jang, S.H.; Lee, D.W.; Kim, J.G. Arthroscopic Inside-out Repair of Complete Radial Tears of the Meniscus with a Fibrin Clot. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2126–2130. [Google Scholar] [CrossRef]
- Nakayama, H.; Kanto, R.; Kambara, S.; Kurosaka, K.; Onishi, S.; Yoshiya, S.; Yamaguchi, M. Clinical Outcome of Meniscus Repair for Isolated Meniscus Tear in Athletes. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 10, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Matsushita, T.; Nagai, K.; Araki, D.; Hoshino, Y.; Kuroda, R. Fibrin Clot and Leukocyte-Rich Platelet-Rich Fibrin Show Similar Release Kinetics and Amount of Growth Factors: A Pilot Study. J. Orthop Surg. Res. 2023, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part V: Histologic Evaluations of PRF Effects on Bone Allograft Maturation in Sinus Lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Sha, I. Arthroscopic Meniscal Repair with Second-Generation Platelet-Rich Fibrin Clot Augmentation. Arthrosc. Tech. 2022, 11, e1569–e1575. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Autologous Liquid Platelet Rich Fibrin: A Novel Drug Delivery System. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef]
- Elbehwashy, M.T.; Hosny, M.M.; Elfana, A.; Nawar, A.; Fawzy El-Sayed, K. Clinical and Radiographic Effects of Ascorbic Acid-Augmented Platelet-Rich Fibrin versus Platelet-Rich Fibrin Alone in Intra-Osseous Defects of Stage-III Periodontitis Patients: A Randomized Controlled Clinical Trial. Clin. Oral Invest. 2021, 25, 6309–6319. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lee, S.; Tsai, C.; Lu, K.; Zhao, J.; Chang, Y. Platelet-rich Fibrin Increases Cell Attachment, Proliferation and Collagen-related Protein Expression of Human Osteoblasts. Aust. Dent. J. 2012, 57, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, P.; Sharma, S.D.; Chhabra, N.; Gupta, A.; Shukla, D. Platelet-Rich Fibrin as an Aid to Soft- and Hard-Tissue Healing. J. Maxillofac. Oral Surg. 2021, 20, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Chai, J.; Zheng, S.; Feng, M.; Sculean, A.; Zhang, Y. A Novel Method for Evaluating and Quantifying Cell Types in Platelet Rich Fibrin and an Introduction to Horizontal Centrifugation. J Biomed. Mater. Res 2019, 107, 2257–2271. [Google Scholar] [CrossRef]
- Desai, T.; Babu, S.S.; Lal, J.V.; Kaushik, Y.S.; Lukose, A.M.; Sandesh, G.M.; Amaravathi, R.S. Fibrin Clot Augmented Repair of Longitudinal Tear of Medial Meniscus. Arthrosc. Tech. 2021, 10, e2449–e2455. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltrán, V.; Fuentes, R. Platelet-Rich Fibrin Application in Dentistry: A Literature Review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cannata, F.; Cicione, C.; Papalia, R.; Denaro, V. Irisin recovers osteoarthritic chondrocytes in vitro. Cells 2020, 9, 1478. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, G.; Vadalà, G.; Ambrosio, L.; Cicione, C.; Tilotta, V.; Cannata, F.; Russo, F.; Papalia, R.; Denaro, V. Irisin inhibits tenocytes response to inflammation in vitro: New insights into tendon-muscle cross-talk. J. Orthop. Res. 2023, 41, 2195–2204. [Google Scholar] [CrossRef]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cicione, C.; Tilotta, V.; Russo, F.; Papalia, R.; Denaro, V. The Effect of Irisin on Human Nucleus Pulposus Cells: New Insights into the Biological Crosstalk between the Muscle and Intervertebral Disc. Spine 2023, 48, 468–475. [Google Scholar] [CrossRef]
| Study (Year) | Study Design (LOE) | Mean Age or Range (Years) | Mean Follow-Up (Months) | Tear Location | PROMs | Radiological Outcomes | Failure Rate | Reoperation Rate | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IKDC | Lysholm Score | TAS | KOOS | ||||||||
| Xu et al. [47] (2015) | Meta-analysis (III) | 22–33 | 84 | NS | MR: ↑ | MR: ↑ | MR: ↑ | NS | NS | MR: ↓ | NS |
| Bottomley et al. [48] (2023) | Retrospective study (III) | MR: 47 ME: 61 | MR: 57 | Mixed | MR: ↑ | MR: ↑ | MR: ↑ | MR: ↑ | NS | NS | NS |
| ME: 47 | |||||||||||
| Ro et al. [49] (2020) | Meta-analysis (IV) | MR: 54 ME: 56 | MR: 33.5 | MMPRT | NS | MR: ↑ | MR = ME | NS | MR showed lower rates of OA progression | NS | MR 4.2% |
| ME: 47.2 | ME: 32% | ||||||||||
| Duethman et al. [15] (2021) | Retrospective study (III) | 17.4 | 100 | LM | MR: ↑ | NS | NS | NS | ME showed higher rates of symptomatic OA | MR: 27% ME: 27% | MR: 23% ME: 18% |
| Paxton et al. [43] (2011) | Systematic review (IV) | MR: 26.0–46.8 ME: 15.3–44.2 | >120 | Mixed | NS | MR = ME | NS | NS | MR showed lower rates of OA progression | NS | MR: 16.5% (0–48 months), 20.7% (>120 months) |
| ME: 1.4% (0–48 months), 3.9% (>120 months) | |||||||||||
| D’Ambrosi et al. [50] (2022) | Systematic review (IV) | MR: 22.1 ME: 23.3 | NS | NS | NS | NS | NS | NS | NS | NS | MR: 17% (LM 25%, MM 25%, 50% NS) |
| ME: 3.7% (LM 83%, MM 17%) | |||||||||||
| Migliorini et al. [51] (2023) | Meta-analysis (III) | 37.6 | 63 | NS | MR = ME | ME: ↑ | NS | NS | MR showed lower rates of OA progression | No differences | MR: 14% |
| Stein et al. [52] (2010) | Cohort study (III) | MR: 31.3 ME: 32.5 | 40 (mid-term | MM | NS | NS | NS | NS | ME showed higher OA progression in the long-term | NS | ME: 10% |
| 104 (long-term) | NS | MR = ME | NS | NS | |||||||
| Shieh et al. [42] (2016) | Case–control study (III) | 15.3 | 40 | NS | NS | NS | NS | NS | NS | NS | MR: 18% |
| ME: 7% | |||||||||||
| Study (Year) | Study Design (LOE) | Mean Follow-Up (Months) | Tear Localization | Overall Failure Rate | Reoperation Rate |
|---|---|---|---|---|---|
| Schweizer et al. [65] (2021) | Meta-analysis (III) | 86 | MM and LM | 19.1% | MM 24.4%, LM 19.5% |
| Schweizer et al. [59] (2023) | Meta-analysis (IV) | 24–60 | MM and LM | 14.8% (MM 10.8%, LM 6.1%) | NS |
| Ro et al. [49] (2020) | Meta-analysis (IV) | 33.5 | MMPRT | NS | 4.2% |
| Migliorini et al. [51] (2023) | Meta-analysis (IV) | 63 | NS | NS | 14% |
| Migliorini et al. [51] (2023) | Systematic review (III) | 67 | MM and LM | 5.9% | 1.1% |
| Blanchard et al. [66] (2020) | Systematic review (IV) | 40.5 | MM and LM | 12.4% | |
| Dai et al. [67] (2021) | Systematic review (IV) | 19–58 (range) | MM and LM | MM: 15%; LM 14% | NS |
| D’Ambrosi et al. [50] (2022) | Systematic review (IV) | NS | NS | NS | 17% (LM 25%, MM 25%, 50% NS) |
| Paxton et al. [43] (2011) | Systematic review (IV) | >120 | MM and LM | NS | 16.5% (0–48 months), 20.7% (>120 months) |
| Study (Year) | Study Design (LOE) | Mean Follow-Up (Months) | Tear Location | Overall Failure Rate | Reoperation Rate |
|---|---|---|---|---|---|
| Hagmeijer et al. [53] (2019) | Case series (IV) | 210 | MM and LM | 42% (complex tears 80%; bucket-handle tears 47%; simple tears 18.2%) | 36% |
| Ronnblad et al. [54] (2020) | Case–control study (III) | 96 | MM and LM | 22.5% | MM 28.3%, LM 11.7% |
| Abdelkafy et al. [68] (2005) | Retrospective study (III) | 139 | 12% | ||
| Duethman et al. [15] (2021) | Retrospective study (III) | 100 | LM | 27% | 23% |
| Shieh et al. [42] (2016) | Case–control study (III) | 40 | 18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Za, P.; Ambrosio, L.; Vasta, S.; Russo, F.; Papalia, G.F.; Vadalà, G.; Papalia, R. How to Improve Meniscal Repair through Biological Augmentation: A Narrative Review. J. Clin. Med. 2024, 13, 4688. https://doi.org/10.3390/jcm13164688
Za P, Ambrosio L, Vasta S, Russo F, Papalia GF, Vadalà G, Papalia R. How to Improve Meniscal Repair through Biological Augmentation: A Narrative Review. Journal of Clinical Medicine. 2024; 13(16):4688. https://doi.org/10.3390/jcm13164688
Chicago/Turabian StyleZa, Pierangelo, Luca Ambrosio, Sebastiano Vasta, Fabrizio Russo, Giuseppe Francesco Papalia, Gianluca Vadalà, and Rocco Papalia. 2024. "How to Improve Meniscal Repair through Biological Augmentation: A Narrative Review" Journal of Clinical Medicine 13, no. 16: 4688. https://doi.org/10.3390/jcm13164688
APA StyleZa, P., Ambrosio, L., Vasta, S., Russo, F., Papalia, G. F., Vadalà, G., & Papalia, R. (2024). How to Improve Meniscal Repair through Biological Augmentation: A Narrative Review. Journal of Clinical Medicine, 13(16), 4688. https://doi.org/10.3390/jcm13164688








