The Long-Term Impact of COVID-19 on Disability after Post-Acute Rehabilitation: A Pilot Study
Abstract
:1. Introduction
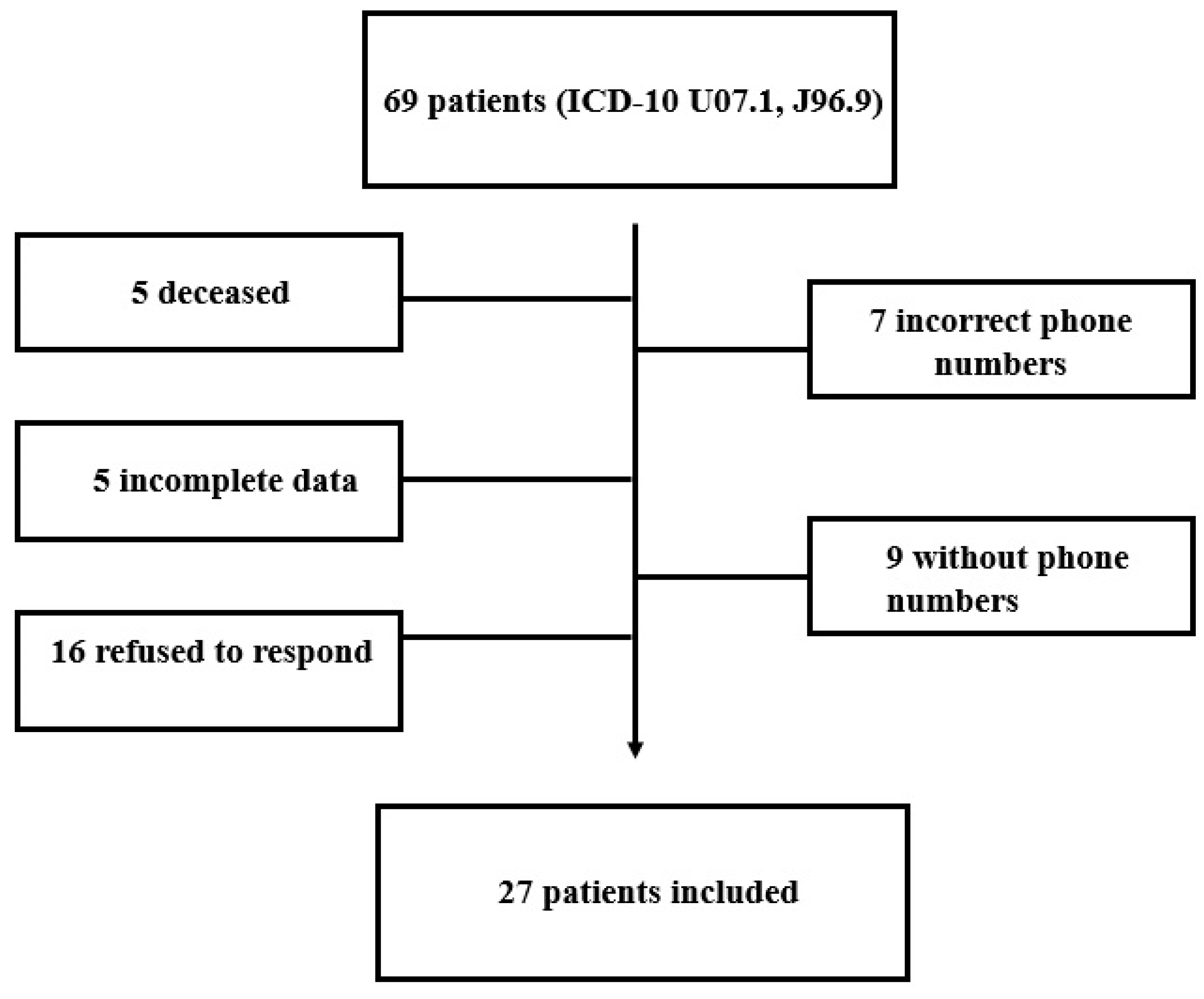
2. Materials and Methods
2.1. Study Design, Protocol, and Patients
2.2. Assessment of Disability and Health Using the WHODAS 2.0 Questionnaire
2.3. Evaluation of Patients and Grouping Them into Levels for Starting the Rehabilitation Program
- -
- Level 0 includes the patients that did not pass the BMA assessment, who were still admitted into ICU units, and who have an S5Q of 0; the rehabilitation program for them includes 2 h turning, splinting, and positioning.
- -
- Level 1 includes the patients that passed the BMA, with an S5Q score between 1 to 5, for whom transfer to a chair is not allowed because of a neurological condition; the rehabilitation program for them includes 2 h turning, splinting, Fowler′s position, a passive/active range of motion (ROM), passive/active leg and/or arm cycling in bed, neuromuscular electrical stimulation (NMES), and activities of daily living (ADL) performance.
- -
- Level 2 includes patients that passed the BMA with an S5Q score ranging between 3 to 5, for whom transfer to a chair is allowed but the patient cannot do it without the help of another person; the rehabilitation program includes 2 h turning, splinting, upright siting position in bed, passive transfer from a bed to a chair, a passive/active ROM, passive/active leg and/or arm cycling in bed, NMES, and ADL performance.
- -
- Level 3 includes patients that passed the BMA with a minimum S5Q score of 4, and an MRC sum score ≥ 36, with BBS sit to stand = 0, BBS standing = 0, and BBS sitting ≥ 1; the rehabilitation program includes 2 h turning, passive transfer from a bed to a chair, sitting out of bed, standing with at least 2 persons assisting, a passive/active ROM, resistance training for arms and legs, active leg and/or arm cycling in a bed or chair, standing (with assistance/frame), NMES, and ADL performance.
- -
- Level 4 includes patients that passed the BMA with an S5Q score of 5 and an MRC sum score ≥ 48, with BBS sit to stand ≥ 0, BBS standing ≥ 0, and BBS sitting ≥ 2; the rehabilitation program includes active transfers from a bed to a chair, sitting out of bed, standing with 1 person assisting, a passive/active ROM, resistance training for arms and legs, active leg and/or arm cycling in a bed or chair, walking (with assistance/frame), NMES, and ADL performance.
- -
- Level 5 includes patients that passed the BMA with an S5Q score of 5 and an MRC sum score ≥ 48, with BBS sit to stand ≥ 1, BBS standing ≥ 2, and BBS sitting ≥ 3; the rehabilitation program includes active transfers from a bed to a chair, sitting out of bed, standing, passive/active ROM, resistance training for arms and legs, active leg and/or arm cycling in bed or chair, walking (with assistance), NMES, and ADL performance.
2.4. Statistical Analysis
3. Results
3.1. ICU Admission History
3.2. Rehabilitation Admission Levels
3.3. Rehabilitation Hospitalization Duration
3.4. WHODAS 2.0 Domains and Their Correlations
4. Discussion
Healthcare Management Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 6 December 2022).
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Ceravolo, M.G.; Arienti, C.; de Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Negrini, S.; International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 Rapid Living Systematic Review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and Rehabilitation Needs in Survivors of COVID-19 Infection: A Cross-Sectional Evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes Mellitus Is Associated with Increased Mortality and Severity of Disease in COVID-19 Pneumonia—A Systematic Review, Meta-Analysis, and Meta-Regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Reurean-Pintilei, D.; Pantea Stoian, A.; Salmen, T.; Stoica, R.A.; Mititelu-Tartau, L.; Lazăr, S.; Timar, B. Associations between Skin Autofluorescence Levels with Cardiovascular Risk and Diabetes Complications in Patients with Type 2 Diabetes. Biomedicines 2024, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall Consensus Statement for Post-COVID-19 Rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, K.; Kim, K. Post–intensive care syndrome and health-related quality of life in long-term survivors of intensive care unit. Aust. Crit. Care 2023, 36, 477–484. [Google Scholar] [CrossRef]
- Segers, J.; Hermans, G.; Bruyninckx, F.; Meyfroidt, G.; Langer, D.; Gosselink, R. Feasibility of Neuromuscular Electrical Stimulation in Critically Ill Patients. J. Crit. Care 2014, 29, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E. Functional Disability 5 Years after Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis Acquired in the Intensive Care UnitA Prospective Multicenter Study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Jolley, S.; Moss, M.; Needham, D.; Caldwell, E.; Morris, P.; Miller, R.; Ringwood, N.; Anders, M.; Koo, K.; Gundel, S.; et al. Point Prevalence Study of Mobilization Practices for Acute Respiratory Failure Patients in the United States. Crit. Care Med. 2017, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Moggio, L.; Marotta, N.; Agostini, F.; Tasselli, A.; Drago Ferrante, V.; Curci, C.; Calafiore, D.; Ferraro, F.; Bernetti, A.; et al. Impact of Rehabilitation on Fatigue in Post-COVID-19 Patients: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 8593. [Google Scholar] [CrossRef]
- Ursescu, C.; Teodoru, G.; Bucurica, S.; Nica, R.I.; Lazăr, Ș.D.; Popescu, M.N.; Ciobanu, I.; Berteanu, M. Using the ClinFIT COVID-19 Instrument to Assess the Functional Impairments Specific to Post-COVID-19 Patients in Romania. Diagnostics 2024, 14, 1540. [Google Scholar] [CrossRef] [PubMed]
- Sen, A. Health: Perception versus Observation: Self Reported Morbidity Has Severe Limitations and Can Be Extremely Misleading. BMJ 2002, 324, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Üstün, T.B. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Üstün, T.B.; Chatterji, S.; Bickenbach, J.; Kostanjsek, N.; Schneider, M. The International Classification of Functioning, Disability and Health: A New Tool for Understanding Disability and Health. Disabil. Rehabil. 2003, 25, 565–571. [Google Scholar] [CrossRef]
- Potcovaru, C.G.; Salmen, T.; Bîgu, D.; Săndulescu, M.I.; Filip, P.V.; Diaconu, L.S.; Pop, C.; Ciobanu, I.; Cinteză, D.; Berteanu, M. Assessing the Effectiveness of Rehabilitation Interventions through the World Health Organization Disability Assessment Schedule 2.0 on Disability—A Systematic Review. J. Clin. Med. 2024, 13, 1252. [Google Scholar] [CrossRef]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and Its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Hoffman, M.; Clerckx, B.; Janssen, K.; Segers, J.; Demeyere, I.; Frickx, B.; Merckx, E.; Hermans, G.; Van der Meulen, I.; Van Lancker, T.; et al. Early Mobilization in Clinical Practice: The Reliability and Feasibility of the ‘Start To Move’ Protocol. Physiother. Theory Pract. 2022, 38, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Sommers, J.; Engelbert, R.H.; Dettling-Ihnenfeldt, D.; Gosselink, R.; Spronk, P.E.; Nollet, F.; van der Schaaf, M. Physiotherapy in the Intensive Care Unit: An Evidence-Based, Expert Driven, Practical Statement and Rehabilitation Recommendations. Clin. Rehabil. 2015, 29, 1051. [Google Scholar] [CrossRef]
- Jang, M.H.; Shin, M.-J.; Shin, Y.B. Pulmonary and Physical Rehabilitation in Critically Ill Patients. Acute Crit. Care 2019, 34, 1–13. [Google Scholar] [CrossRef]
- Hermans, G.; Clerckx, B.; Vanhullebusch, T.; Segers, J.; Vanpee, G.; Robbeets, C.; Casaer, M.P.; Wouters, P.; Gosselink, R.; Van Den Berghe, G. Interobserver Agreement of Medical Research Council Sum-Score and Handgrip Strength in the Intensive Care Unit. Muscle Nerve 2012, 45, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hohtari-Kivimäki, U.; Salminen, M.; Vahlberg, T.; Kivelä, S.-L. Short Berg Balance Scale, BBS-9, as a Predictor of Fall Risk among the Aged: A Prospective 12-Month Follow-up Study. Aging Clin. Exp. Res. 2013, 25, 645–650. [Google Scholar] [CrossRef]
- de Baptista, C.R.J.A.; Vicente, A.M.; Souza, M.A.; Cardoso, J.; Ramalho, V.M.; Mattiello-Sverzut, A.C. Methods of 10-Meter Walk Test and Repercussions for Reliability Obtained in Typically Developing Children. Rehabil. Res. Pract. 2020, 2020, 4209812. [Google Scholar] [CrossRef] [PubMed]
- Bisca, G.W.; Fava, L.R.; Morita, A.A.; Machado, F.V.C.; Pitta, F.; Hernandes, N.A. 4-Meter Gait Speed Test in Chronic Obstructive Pulmonary Disease: Interrater Reliability Using a STOPWATCH. J. Cardiopulm. Rehabil. Prev. 2018, 38, E10–E13. [Google Scholar] [CrossRef]
- Casano, H.A.M.; Anjum, F. Six Minute Walk Test; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schwartzstein, R.M. Approach to the Patient with Dyspnea; UpToDate Inc.: Waltham, MA, USA, 2006. [Google Scholar]
- Mendes, M.D.A.; Da Silva, I.; Ramires, V.; Reichert, F.; Martins, R.; Ferreira, R.; Tomasi, E. Metabolic Equivalent of Task (METs) Thresholds as an Indicator of Physical Activity Intensity. PLoS ONE 2018, 13, e0200701. [Google Scholar] [CrossRef]
- WHO. Disability Assessment Schedule (WHODAS 2.0). Available online: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health/who-disability-assessment-schedule (accessed on 30 June 2024).
- Kröönström, L.A.; Krause, J.; Larsson, S.B.; Sigström, R.; Sunnerhagen, K.S. Long-Term Self-Reported Health and Disability after COVID-19 in Public Employees. BMC Public Health 2022, 22, 2400. [Google Scholar] [CrossRef]
- Leite, V.F.; Rampim, D.B.; Jorge, V.C.; de Lima, M.D.C.C.; Cezarino, L.G.; da Rocha, C.N.; Esper, R.B.; Prevent Senior COVID-19 Rehabilitation Study. Persistent Symptoms and Disability after COVID-19 Hospitalization: Data from a Comprehensive Telerehabilitation Program. Arch. Phys. Med. Rehabil. 2021, 102, 1308–1316. [Google Scholar] [CrossRef]
- Piras, I.; Piazza, M.F.; Piccolo, C.; Azara, A.; Piana, A.; Finco, G.; Galletta, M. Experiences, Emotions, and Health Consequences among COVID-19 Survivors after Intensive Care Unit Hospitalization. Int. J. Environ. Res. Public Health 2022, 19, 6263. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, O.J.; Friedman, L.A.; Colantuoni, E.; Dinglas, V.D.; Sepulveda, K.A.; Mendez-Tellez, P.; Shanholz, C.; Pronovost, P.J.; Needham, D.M. Psychiatric Symptoms after Acute Respiratory Distress Syndrome: A 5-Year Longitudinal Study. Intensiv. Care Med. 2018, 44, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.C.D.S.F.; Costa, P.J.R.; Fernandes, J.C.J.; Vieira, T.S.; Reis, N.C.; Coimbra, I.M.M.; Paiva, J.A.O.C. The Impact of Severe COVID-19 on Health-Related Quality of Life and Disability: An Early Follow-up Perspective. Rev. Bras. Ter. Intensiv. 2022, 34, 1–6. [Google Scholar] [CrossRef]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L. One-Year Outcomes and Health Care Utilization in Survivors of Severe Acute Respiratory Syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Cisnal, A.; Alonso-Linaje, G.; Fraile, J.C.; Pérez-Turiel, J.; Álvarez, P.; Martinez, S. Tackling Post-COVID-19 Rehabilitation Challenges: A Pilot Clinical Trial Investigating the Role of Robotic-Assisted Hand Rehabilitation. J. Clin. Med. 2024, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Joyce, P.R.; O’Dempsey, R.; Kisby, G.; Anstey, C.A. Retrospective Observational Study of Sarcopenia and Outcomes in Critically Ill Patients. Anaesth. Intensiv. Care 2020, 48, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; Puthucheary, Z.A. The Impact of Extended Bed Rest on the Musculoskeletal System in the Critical Care Environment. Extrem. Physiol. Med. 2015, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Potcovaru, C.G.; Filip, P.V.; Neagu, O.M.; Diaconu, L.S.; Salmen, T.; Cinteză, D.; Pantea Stoian, A.; Bobirca, F.; Berteanu, M.; Pop, C. Diagnostic Criteria and Prognostic Relevance of Sarcopenia in Patients with Inflammatory Bowel Disease—A Systematic Review. J. Clin. Med. 2023, 12, 4713. [Google Scholar] [CrossRef]
- Oprea, E.; Berteanu, M.; Cintezã, D.; Manolescu, B.N. The Effect of the ALAnerv Nutritional Supplement on Some Oxidative Stress Markers in Postacute Stroke Patients Undergoing Rehabilitation. Appl. Physiol. Nutr. Metab. 2013, 38, 613–620. [Google Scholar] [CrossRef]
- Saghaleini, S.H.; Dehghan, K.; Shadvar, K.; Sanaie, S.; Mahmoodpoor, A.; Ostadi, Z. Pressure Ulcer and Nutrition. Indian J. Crit. Care Med. 2018, 22, 283–289. [Google Scholar] [CrossRef]
- Cinteza, D.; Munteanu, C.; Poenaru, D.; Munteanu, D.; Petrusca, I.; Dumitrascu, D. The Therapeutic Effect of Carbogaseous Natural Mineral Waters in the Metabolic Syndrome. Balneo Res. J. 2013, 4, 5–22. [Google Scholar] [CrossRef]
- Lee, C.Y.; Liang, Y.C.; Hsu, W.H.; Tsai, Y.W.; Liu, T.H.; Huang, P.Y.; Chuang, M.H.; Hung, K.C.; Lee, M.C.; Yu, T.; et al. Malnutrition and the Post-Acute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Multi-Institutional Population-Based Propensity Score-Matched Analysis. Life 2024, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the Severity of Coronavirus Disease 2019: A Model-Based Analysis. Lancet Infect. Dis. 2020, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, L.; Saggers, R.T.; Bandini, R.; Stranges, S.; Choi, Y.H.; Thornton, J.S.; Hendrie, S.; Patel, D.; Rabinowitz, S.; Patricios, J. Small Steps, Strong Shield: Directly Measured, Moderate Physical Activity in 65 361 Adults Is Associated with Significant Protective Effects from Severe COVID-19 Outcomes. Br. J. Sports Med. 2022, 56, 568–576. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. OpenSAFELY: Factors Associated with COVID-19 Death in 17 Million Patients. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Pantea Stoian, A.; Rizzo, M.; Salmen, T.; Kempler, P.; Stulnig, T.; Papanas, N.; Popovic, D.; Herder, C.; Serban, D. Post COVID-19 Syndrome Related to Diabetes—A Brief Review. Diabetes Stoffwechs. Herz 2022, 31, 126–130. [Google Scholar]
- Salmen, T.; Pietroșel, V.A.; Mihai, B.M.; Bica, I.C.; Teodorescu, C.; Păunescu, H.; Coman, O.A.; Mihai, D.A.; Pantea Stoian, A. Non-Insulin Novel Antidiabetic Drugs Mechanisms in the Pathogenesis of COVID-19. Biomedicines 2022, 10, 2624. [Google Scholar] [CrossRef]
- Sardu, C.; Gargiulo, G.; Esposito, G.; Paolisso, G.; Marfella, R. Impact of Diabetes Mellitus on Clinical Outcomes in Patients Affected by Covid-19. Cardiovasc. Diabetol. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Bassi-Dibai, D.; Santos-de-Araújo, A.D.; Dibai-Filho, A.V.; de Azevedo, L.F.S.; Goulart, C.D.L.; Luz, G.C.P.; Burke, P.R.; Garcia-Araújo, A.S.; Borghi-Silva, A. Rehabilitation of Individuals with Diabetes Mellitus: Focus on Diabetic Myopathy. Front. Endocrinol. 2022, 13, 869921. [Google Scholar] [CrossRef]
- Cârstea, A.P.; Mită, A.; Fortofoiu, M.C.; Doica, I.P.; Cârstea, D.; Beznă, C.M.; Negroiu, C.E.; Diaconu, I.D.; Georgescu, A.R.; Kamal, A.M.; et al. How Dexamethasone Used in Anti-COVID-19 Therapy Influenced Antihypertensive Treatment in Patients with SARS-CoV-2. Healthcare 2023, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Zampolini, M.; Selb, M.; Boldrini, P.; Branco, C.A.; Golyk, V.; Hu, X.; Kiekens, C.; Negrini, S.; Nulle, A.; Oral, A.; et al. The Individual Rehabilitation Project as the Core of Person-Centered Rehabilitation: The Physical and Rehabilitation Medicine Section and Board of the European Union of Medical Specialists Framework for Rehabilitation in Europe. Eur. J. Phys. Rehabil. Med. 2022, 58, 503–510. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 27 |
|---|---|
| Age (years), mean (SD) | 63.4 ± 8.6 |
| Females, %, Males, % | 51.9% 48.1% |
| Independent in the community, % | 81.5% |
| Education (years), mean (SD) | 13.14 ± 2.9 years |
| Marital status (married), % | 70.4% |
| Employment status (retirees), % | 77.8% |
| Characteristics | n = 27 |
|---|---|
| ICU unit admission (%) | 59.3% |
| Number of hospitalization days, median (IQR) | 18 (11.5,24) |
| Rehabilitation level at admission | |
| Level 2 (%) | 29.63% |
| Level 3 (%) | 29.63% |
| Level 4 (%) | 22.22% |
| Level 5 (%) | 18.52% |
| Peripheral oxygen saturation (%), median (IQR) | 90% (88.5 to 91.5) |
| Average oxygen therapy requirement (L/min), median (IQR) | 1.24 L/min (1 to 3) |
| Characteristics | n = 27 |
|---|---|
| Type 2 Diabetes Mellitus (%) | 55.5% |
| Grade 1 HBP (%) | 18.5% |
| Grade 2 HBP (%) | 62.9% |
| Urinary tract infection (%) | 74.1% |
| Clostridium difficile infection (%) | 22.2% |
| Pressure ulcers grade I (%) | 14.8% |
| Pressure ulcers grade II (%) | 18.5% |
| Pressure ulcers grade III (%) | 3.7% |
| Peripheral neurological deficit | 62.9% |
| Central neurological deficit | 14.8% |
| Disability Categories | Disability Percentages | |
|---|---|---|
| Cognition | 22.99% | |
| Mobility | 35.93% | |
| Self-care | 28.01% | |
| Getting along with people | 28.89% | |
| Life activities | Household | 51.79% |
| Work or school activities | 3.13% | |
| Participation in society | 39.81% | |
| Overall disability | 35.09% | |
| Days in Hospital | O2 Saturation | DM | Peripheral Neurologic Deficit | Central Neurologic Deficit | HBP | ICU Admission | |
|---|---|---|---|---|---|---|---|
| Cognition (d1) | r = −0.245, p = 0.217 | r = 0.308, p = 0.119 | r = 0.280, p = 0.497 | r = −0.151, p = 0.453 | r = 0.562, p = 0.002 | r = −0.082, p = 0.658 | r = −0.397, p = 0.041 |
| Mobility (d2) | r = −0.251, p = 0.207 | r = 0.261, p = 0.188 | r = 0.383, p = 0.048 | r = −0.158, p = 0.430 | r = 0.495, p = 0.009 | r = 0.028, p = 0.888 | r = −0.579, p = 0.002 |
| Self-care (d3) | r = −0.275, p = 0.165 | r = −0.293, p = 0.138 | r = −0.263, p = 0.184 | r = −0.204, p = 0.308 | r = 0.683, p = 0.000 | r = −0.093, p = 0.646 | r = −0.455, p = 0.017 |
| Getting along with people (d4) | r = −0.206, p = 0.302 | r = 0.251, p = 0.206 | r = 0.308, p = 0.119 | r = −0.190, p = 0.342 | r = 0.550, p = 0.003 | r = 0.049, p = 0.810 | r = −0.307, p = 0.119 |
| Life activities (d5) | r = −0.306, p = 0.121 | r = −0.405, p = 0.036 | r = 0.237, p = 0.234 | r = −0.114, p = 0.572 | r = 0.505, p = 0.007 | r = 0.157 p = 0.433 | r = −0.433, p = 0.024 |
| Participation in society (d6) | r = −0.214, p = 0.283 | r = 0.322, p = 0.101 | r = 0.275, p = 0.164 | r = −0199, p = 0.321 | r = 0.599, p = 0.001 | r = 0.104, p = 0.604 | r = −0.420, p = 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potcovaru, C.-G.; Salmen, T.; Potcovaru, A.M.; Săndulescu, I.-M.; Chiriac, O.; Balasa, A.-C.; Diaconu, L.S.; Poenaru, D.; Pantea Stoian, A.; Cinteza, D.; et al. The Long-Term Impact of COVID-19 on Disability after Post-Acute Rehabilitation: A Pilot Study. J. Clin. Med. 2024, 13, 4694. https://doi.org/10.3390/jcm13164694
Potcovaru C-G, Salmen T, Potcovaru AM, Săndulescu I-M, Chiriac O, Balasa A-C, Diaconu LS, Poenaru D, Pantea Stoian A, Cinteza D, et al. The Long-Term Impact of COVID-19 on Disability after Post-Acute Rehabilitation: A Pilot Study. Journal of Clinical Medicine. 2024; 13(16):4694. https://doi.org/10.3390/jcm13164694
Chicago/Turabian StylePotcovaru, Claudia-Gabriela, Teodor Salmen, Ana Mădălina Potcovaru, Ioana-Miruna Săndulescu, Ovidiu Chiriac, Ana-Cristinel Balasa, Laura Sorina Diaconu, Daniela Poenaru, Anca Pantea Stoian, Delia Cinteza, and et al. 2024. "The Long-Term Impact of COVID-19 on Disability after Post-Acute Rehabilitation: A Pilot Study" Journal of Clinical Medicine 13, no. 16: 4694. https://doi.org/10.3390/jcm13164694







