Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes
Abstract
1. Introduction
2. How Were Normal Amniotic Fluid Volumes Established?
3. Clinical Implications of Abnormal Fluid Volumes
3.1. Adverse Outcomes Associated with Oligohydramnios
3.2. Adverse Outcomes Associated with Polyhydramnios
4. Guideline Review—Evidence Acquisition
5. Discussion
5.1. How Do Professional Organizations and Societies Define Normal and Abnormal Amniotic Fluid Volumes?
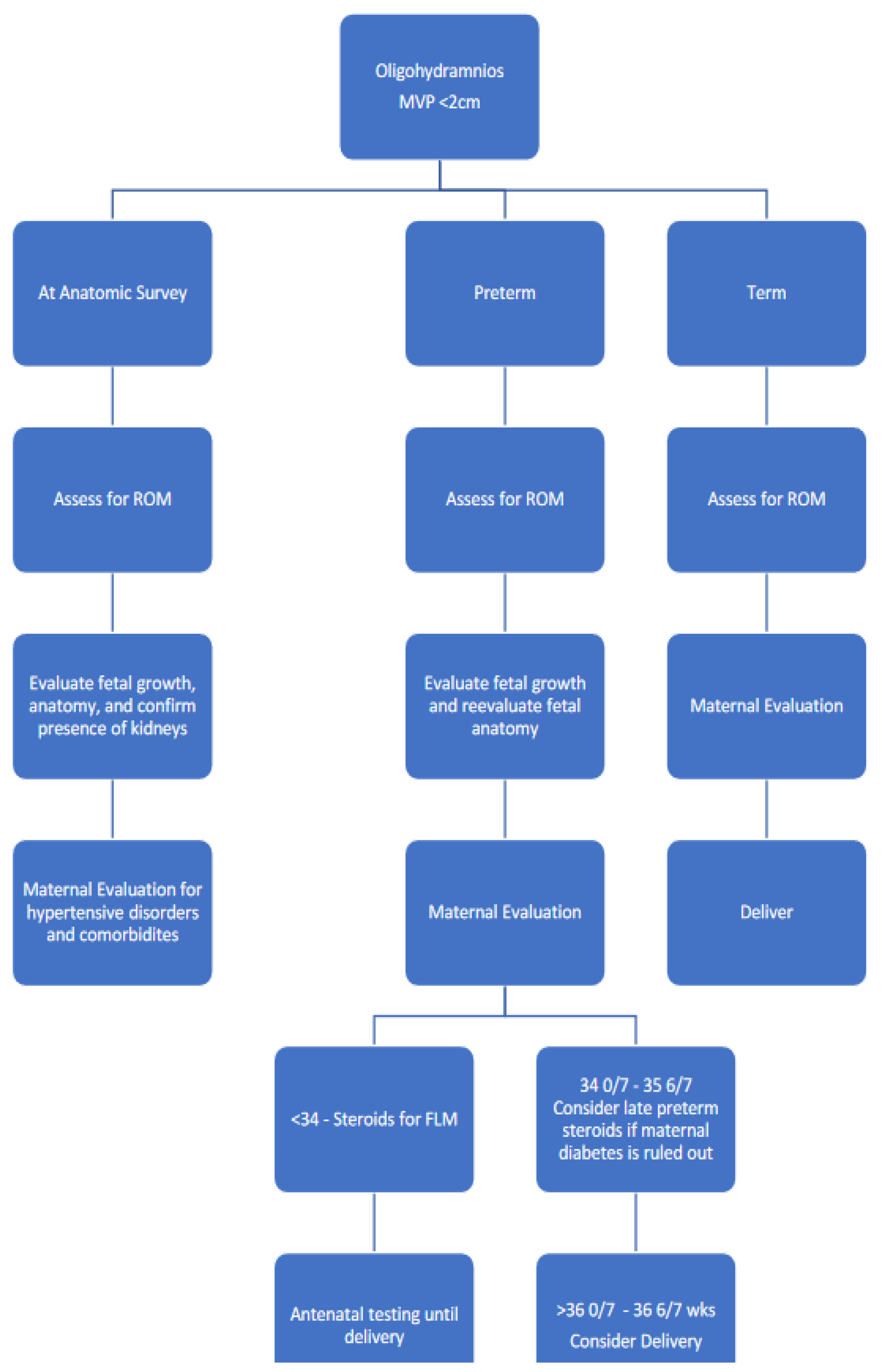
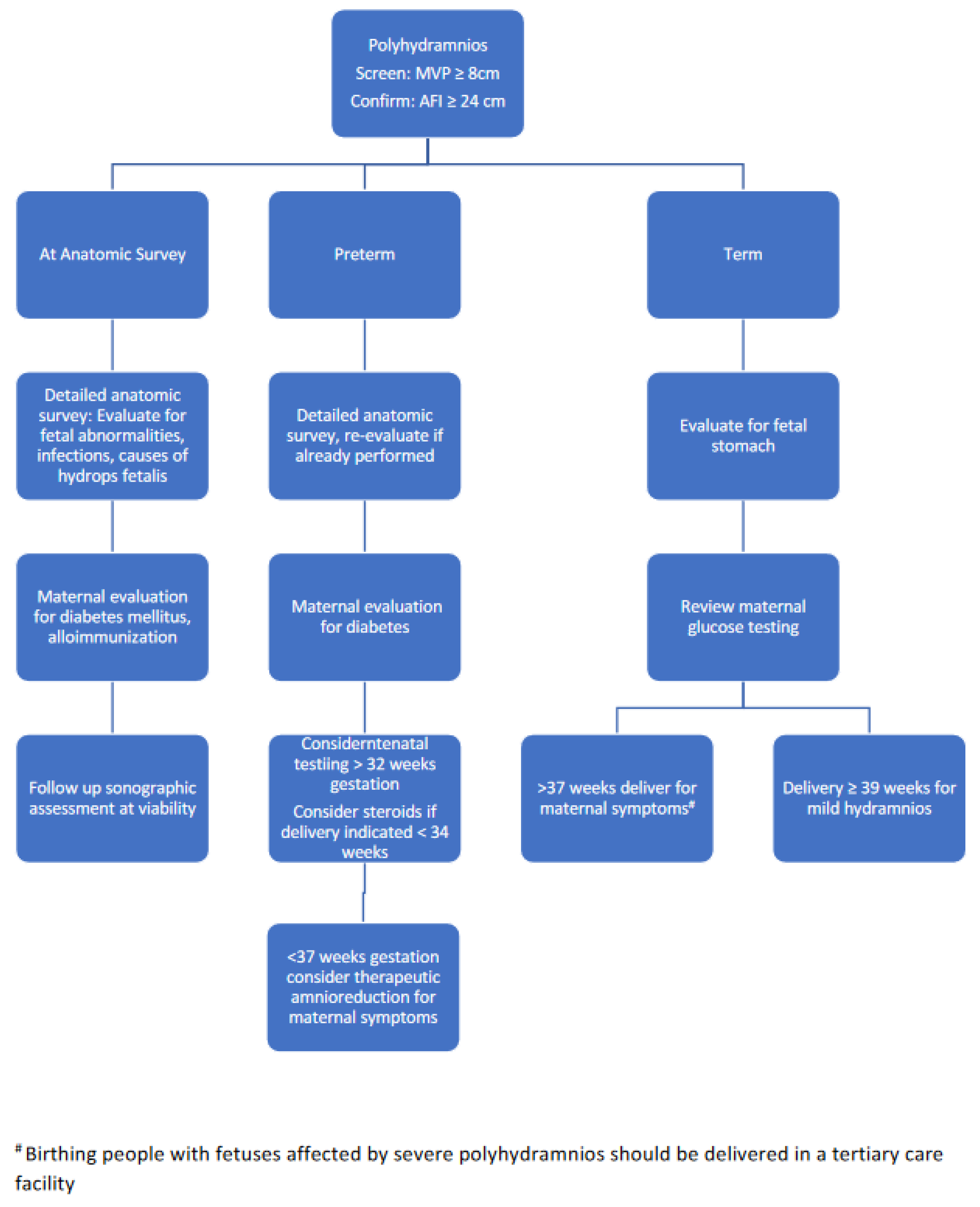
5.2. Management of Abnormal Amniotic Fluid Volumes
5.3. Knowledge Gaps
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creatsas, G.K.; Lolis, D.E.; Pavlatos, M.P.; Kaskarelis, D.B. Bacteriology of amniotic fluid. Gynecol. Obstet. Invest. 1980, 11, 174–176. [Google Scholar] [CrossRef]
- Lim, E.S.; Rodriguez, C.; Holtz, L.R. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E. Recognizing Congenital Pressure Injuries: A Case Series. J. Wound Ostomy Cont. Nurs. 2019, 46, 65–68. [Google Scholar] [CrossRef]
- Moore, T.R. The role of amniotic fluid assessment in evaluating fetal well-being. Clin. Perinatol. 2011, 38, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.P.; Sanderson, M.; Hendrix, N.W.; Magann, E.F.; Devoe, L.D. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: A meta-analysis. Am. J. Obstet. Gynecol. 1999, 181, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R. Amniotic fluid dynamics reflect fetal and maternal health and disease. Obstet. Gynecol. 2010, 116, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; Cayle, J.E. The amniotic fluid index in normal human pregnancy. Am. J. Obstet. Gynecol. 1990, 162, 1168–1173. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Doherty, D.D.; Magann, E.F.; Cahanding, F.; Moreno, F.; Klausen, J.H. Amniotic fluid index vs. single deepest pocket technique during modified biophysical profile: A randomized clinical trial. Am. J. Obstet. Gynecol. 2004, 191, 661–667; discussion 667–668. [Google Scholar] [CrossRef] [PubMed]
- Magann, E.F.; Chauhan, S.P.; Bofill, J.A.; Martin, J.N., Jr. Comparability of the amniotic fluid index and single deepest pocket measurements in clinical practice. Aust. N Z J. Obstet. Gynaecol. 2003, 43, 75–77. [Google Scholar] [CrossRef]
- Magann, E.F.; Doherty, D.A.; Lutgendorf, M.A.; Magann, M.I.; Chauhan, S.P.; Morrison, J.C. Peripartum outcomes of high-risk pregnancies complicated by oligo- and polyhydramnios: A prospective longitudinal study. J. Obstet. Gynaecol. Res. 2010, 36, 268–277. [Google Scholar] [CrossRef]
- Magann, E.F.; Doherty, D.A.; Chauhan, S.P.; Busch, F.W.; Mecacci, F.; Morrison, J.C. How well do the amniotic fluid index and single deepest pocket indices (below the 3rd and 5th and above the 95th and 97th percentiles) predict oligohydramnios and hydramnios? Am. J. Obstet. Gynecol. 2004, 190, 164–169. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Hendrix, N.W.; Morrison, J.C.; Magann, E.F.; Devoe, L.D. Intrapartum oligohydramnios does not predict adverse peripartum outcome among high-risk parturients. Am. J. Obstet. Gynecol. 1997, 176, 1130–1136; discussion 1136–1138. [Google Scholar] [CrossRef]
- Phelan, J.P.; Smith, C.V.; Broussard, P.; Small, M. Amniotic fluid volume assessment with the four-quadrant technique at 36–42 weeks’ gestation. J. Reprod. Med. 1987, 32, 540–542. [Google Scholar] [PubMed]
- Rutherford, S.E.; Phelan, J.P.; Smith, C.V.; Jacobs, N. The four-quadrant assessment of amniotic fluid volume: An adjunct to antepartum fetal heart rate testing. Obstet. Gynecol. 1987, 70 Pt 1, 353–356. [Google Scholar]
- Peixoto, A.B.; Caldas, T.M.; Martins, W.P.; Da Silva Costa, F.; Araujo Junior, E. Unconditional reference values for the amniotic fluid index measurement between 26w0d and 41w6d of gestation in low-risk pregnancies. J. Matern. Fetal Neonatal Med. 2016, 29, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P.F.; Manning, F.A.; Morrison, I.; Harman, C.R.; Lange, I.R. Ultrasound evaluation of amniotic fluid volume. II. The relationship of increased amniotic fluid volume to perinatal outcome. Am. J. Obstet. Gynecol. 1984, 150, 250–254. [Google Scholar] [CrossRef]
- Chamberlain, P.F.; Manning, F.A.; Morrison, I.; Harman, C.R.; Lange, I.R. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am. J. Obstet. Gynecol. 1984, 150, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.M.; Thompson, K.; Smithey, J.; Gaffney, G.; Cooke, I.; Chamberlain, P.; MacKenzie, I.Z. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: A prospective blinded observational study. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 989–994. [Google Scholar] [CrossRef]
- Rutherford, S.E.; Smith, C.V.; Phelan, J.P.; Kawakami, K.; Ahn, M.O. Four-quadrant assessment of amniotic fluid volume. Interobserver and intraobserver variation. J. Reprod. Med. 1987, 32, 587–589. [Google Scholar]
- Pagan, M.; Magann, E.F.; Rabie, N.; Steelman, S.C.; Hu, Z.; Ounpraseuth, S. Idiopathic polyhydramnios and pregnancy outcomes: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 61, 302–309. [Google Scholar] [CrossRef]
- Magann, E.F.; Sandlin, A.T.; Ounpraseuth, S.T. Amniotic fluid and the clinical relevance of the sonographically estimated amniotic fluid volume: Oligohydramnios. J. Ultrasound Med. 2011, 30, 1573–1585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gramellini, D.; Fieni, S.; Verrotti, C.; Piantelli, G.; Cavallotti, D.; Vadora, E. Ultrasound evaluation of amniotic fluid volume: Methods and clinical accuracy. Acta Bio-Medica Atenei Parm. 2004, 75 (Suppl. S1), 40–44. [Google Scholar]
- Chishom, T.; Stephens, A.; Raley, S.; Ange, B.; Looney, S.; Street, L.; Browne, P. Amniotic fluid index curves in the obese gravida. J. Neonatal-Perinat. Med. 2021, 14, 131–137. [Google Scholar] [CrossRef]
- Bicocca, M.J.; Qureshey, E.J.; Chauhan, S.P.; Hernandez-Andrade, E.; Sibai, B.M.; Nowlen, C.; Stafford, I. Semiquantitative Assessment of Amniotic Fluid Among Individuals with and Without Diabetes Mellitus. J. Ultrasound Med. 2022, 41, 447–455. [Google Scholar] [CrossRef]
- Blitz, M.J.; Rochelson, B.; Stork, L.B.; Augustine, S.; Greenberg, M.; Sison, C.P.; Vohra, N. Maternal Body Mass Index and Amniotic Fluid Index in Late Gestation. J. Ultrasound Med. 2018, 37, 561–568. [Google Scholar] [CrossRef]
- Magann, E.F.; Nolan, T.E.; Hess, L.W.; Martin, R.W.; Whitworth, N.S.; Morrison, J.C. Measurement of amniotic fluid volume: Accuracy of ultrasonography techniques. Am. J. Obstet. Gynecol. 1992, 167, 1533–1537. [Google Scholar] [CrossRef]
- Moses, J.; Doherty, D.A.; Magann, E.F.; Chauhan, S.P.; Morrison, J.C. A randomized clinical trial of the intrapartum assessment of amniotic fluid volume: Amniotic fluid index versus the single deepest pocket technique. Am. J. Obstet. Gynecol. 2004, 190, 1564–1569; discussion 1569–1570. [Google Scholar] [CrossRef]
- Odibo, I.N.; Whittemore, B.S.; Hughes, D.S.; Simmons, P.M.; Ounpraseuth, S.T.; Magann, E.F. Addition of Color Doppler Sonography for Detection of Amniotic Fluid Disturbances and Its Implications on Perinatal Outcomes. J. Ultrasound Med. 2017, 36, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J., Jr. Toward consistent terminology: Assessment and reporting of amniotic fluid volume. Semin. Perinatol. 2013, 37, 370–374. [Google Scholar] [CrossRef]
- Hughes, D.S.; Whittington, J.R.; Kim, H.; Gunderman, B.; Ounpraseuth, S.; Magann, E.F. Is There a Difference in Sonographic Estimation of Amniotic Fluid Volume When Measuring with the Probe Perpendicular to the Floor Compared with Perpendicular to the Uterine Contour? J. Obstet. Gynaecol. Can. 2019, 41, 1295–1301. [Google Scholar] [CrossRef]
- Magann, E.F.; Sanderson, M.; Martin, J.N.; Chauhan, S. The amniotic fluid index, single deepest pocket, and two-diameter pocket in normal human pregnancy. Am. J. Obstet. Gynecol. 2000, 182, 1581–1588. [Google Scholar] [CrossRef]
- Donald, I.; Macvicar, J.; Brown, T.G. Investigation of abdominal masses by pulsed ultrasound. Lancet 1958, 1, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S. A short history of sonography in obstetrics and gynaecology. Facts Views Vis. ObGyn 2013, 5, 213–229. [Google Scholar]
- Hobbins, J.C.; Grannum, P.A.; Berkowitz, R.L.; Silverman, R.; Mahoney, M.J. Ultrasound in the diagnosis of congenital anomalies. Am. J. Obstet. Gynecol. 1979, 134, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.A.; Platt, L.D.; Sipos, L. Antepartum fetal evaluation: Development of a fetal biophysical profile. Am. J. Obstet. Gynecol. 1980, 136, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.A.; Hill, L.M.; Platt, L.D. Qualitative amniotic fluid volume determination by ultrasound: Antepartum detection of intrauterine growth retardation. Am. J. Obstet. Gynecol. 1981, 139, 254–258. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Obstetrics and the American Institute of Ultrasound in Medicine. Practice Bulletin No. 175: Ultrasound in Pregnancy. Obstet. Gynecol. 2016, 128, e241–e256. [Google Scholar] [CrossRef] [PubMed]
- Brace, R.A.; Wolf, E.J. Normal amniotic fluid volume changes throughout pregnancy. Am. J. Obstet. Gynecol. 1989, 161, 382–388. [Google Scholar] [CrossRef]
- Melamed, N.; Pardo, J.; Milstein, R.; Chen, R.; Hod, M.; Yogev, Y. Perinatal outcome in pregnancies complicated by isolated oligohydramnios diagnosed before 37 weeks of gestation. Am. J. Obstet. Gynecol. 2011, 205, 241.e1–241.e6. [Google Scholar] [CrossRef]
- Rabie, N.; Magann, E.; Steelman, S.; Ounpraseuth, S. Oligohydramnios in complicated and uncomplicated pregnancy: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017, 49, 442–449. [Google Scholar] [CrossRef]
- Zhang, J.; Troendle, J.; Meikle, S.; Klebanoff, M.A.; Rayburn, W.F. Isolated oligohydramnios is not associated with adverse perinatal outcomes. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 220–225. [Google Scholar] [CrossRef]
- Dashe, J.S.; Pressman, E.K.; Hibbard, J.U. SMFM Consult Series #46: Evaluation and management of polyhydramnios. Am. J. Obstet. Gynecol. 2018, 219, B2–B8. [Google Scholar] [CrossRef] [PubMed]
- Abele, H.; Starz, S.; Hoopmann, M.; Yazdi, B.; Rall, K.; Kagan, K.O. Idiopathic polyhydramnios and postnatal abnormalities. Fetal Diagn. Ther. 2012, 32, 251–255. [Google Scholar] [CrossRef]
- Odibo, I.N.; Newville, T.M.; Ounpraseuth, S.T.; Dixon, M.; Lutgendorf, M.A.; Foglia, L.M.; Magann, E.F. Idiopathic polyhydramnios: Persistence across gestation and impact on pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Donnelly, J. Outcome of pregnancy in women diagnosed with idiopathic polyhydramnios. Aust. N Z J. Obstet. Gynaecol. 2017, 57, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pri-Paz, S.; Khalek, N.; Fuchs, K.M.; Simpson, L.L. Maximal amniotic fluid index as a prognostic factor in pregnancies complicated by polyhydramnios. Ultrasound Obstet. Gynecol. 2012, 39, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.L.; Beamon, C.J.; Chescheir, N.C.; Stamilio, D. Idiopathic Polyhydramnios: Severity and Perinatal Morbidity. Am. J. Perinatol. 2016, 33, 658–664. [Google Scholar] [CrossRef]
- Panting-Kemp, A.; Nguyen, T.; Chang, E.; Quillen, E.; Castro, L. Idiopathic polyhydramnios and perinatal outcome. Am. J. Obstet. Gynecol. 1999, 181 Pt 1, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Biggio, J.R., Jr.; Wenstrom, K.D.; Dubard, M.B.; Cliver, S.P. Hydramnios prediction of adverse perinatal outcome. Obstet. Gynecol. 1999, 94 Pt 1, 773–777. [Google Scholar] [CrossRef]
- Luo, Q.Q.; Zou, L.; Gao, H.; Zheng, Y.F.; Zhao, Y.; Zhang, W.Y. Idiopathic polyhydramnios at term and pregnancy outcomes: A multicenter observational study. J. Matern. Fetal Neonatal Med. 2017, 30, 1755–1759. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. 2022. Available online: www.acog.org (accessed on 5 December 2022).
- American Institute of Ultrasound in Medicine. 2022. Available online: www.aium.org (accessed on 1 December 2022).
- College National des Gynecologues et Obstetriciens Francais. 2022. Available online: www.cngof.org (accessed on 1 December 2022).
- Fetal Medicine Foundation. 2022. Available online: www.fetalmedicine.org (accessed on 5 December 2022).
- International Federation of Gynecololgy and Obstetrics. Available online: www.figo.org (accessed on 1 December 2022).
- International Society of Ultrasound in Obstetrics and Gynecology. 2022. Available online: https://www.isuog.org (accessed on 1 December 2022).
- Japan Association of Obstetricians and Gynecologists. Japan Association of Obstetricians and Gynecologists. 2022. Available online: https://www.jaog.or.jp/ (accessed on 1 December 2022).
- National Institute for Health and Care Excellence. National Institute for Health and Care Excellence. 2022. Available online: https://www.nice.org.uk (accessed on 1 December 2022).
- National Health Service of Glasgow and Clyde. 2022. Available online: https://www.nhsggc.scot (accessed on 1 December 2022).
- Royal College of Australian New Zealand College of Obstetricians and Gynecologists. 2022. Available online: https://ranzcog.edu.au (accessed on 1 December 2022).
- Society for Maternal-Fetal Medicine. 2022. Available online: https://www.smfm.org (accessed on 5 December 2022).
- Society of Obstetricians and Gynaecologists of Canada. 2022. Available online: https://sogc.org (accessed on 1 December 2022).
- Royal College of Obstetricians and Gynaecologists. 2022. Available online: https://www.rcog.org.uk (accessed on 2 December 2022).
- Lim, K.I.; Butt, K.; Naud, K.; Smithies, M. Amniotic Fluid: Technical Update on Physiology and Measurement. J. Obstet. Gynaecol. Can. 2017, 39, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.A.; Morrison, I.; Lange, I.R.; Harman, C.R.; Chamberlain, P.F. Fetal assessment based on fetal biophysical profile scoring: Experience in 12,620 referred high-risk pregnancies. I. Perinatal mortality by frequency and etiology. Am. J. Obstet. Gynecol. 1985, 151, 343–350. [Google Scholar] [CrossRef] [PubMed]
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Intrapartum Fetal Surveillance, 4th ed.; Clincal Guideline; The Royal Australian and New Zealand College of Obstetricians and Gynaecologists: Melbourne, Australia, 2019. [Google Scholar]
- Magann, E.F.; Chauhan, S.P.; Doherty, D.A.; Lutgendorf, M.A.; Magann, M.I.; Morrison, J.C. A review of idiopathic hydramnios and pregnancy outcomes. Obstet. Gynecol. Surv. 2007, 62, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.M.; Chalouhi, G.E.; Costa FD, S.; Lee, W. ISUOG Practice Guidelines (updated): Performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2022, 59, 840–856. [Google Scholar] [CrossRef]
- Itakura, A.; Satoh, S.; Aoki, S.; Fukushima, K.; Hasegawa, J.; Hyodo, H.; Shozu, M. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J. Obstet. Gynaecol. Res. 2023, 49, 5–53. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, J.; Bromley, B.; Allison, S.; Chauhan, A.; Destounis, S.; Dickman, E.; Wilkins, I. AIUM-ACR-ACOG-SMFM-SRU Practice Parameter for the Performance of Standard Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2018, 37, E13–E24. [Google Scholar] [CrossRef]
- AIUM Practice Parameter for the Performance of Detailed Second- and Third-Trimester Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2019, 38, 3093–3100. [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Antepartum Fetal Surveillance: ACOG Practice Bulletin, Number 229. Obstet. Gynecol. 2021, 137, e116–e127. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists, Committee on Obstetric Practice; Society for Maternal-Fetal Medicine. Medically Indicated Late-Preterm and Early-Term Deliveries: ACOG Committee Opinion, Number 831. Obstet. Gynecol. 2021, 138, e35–e39. [Google Scholar] [CrossRef]
- Zizzo, A.R.; Hvidman, L.; Salvig, J.D.; Holst, L.; Kyng, M.; Petersen, O.B. Home management by remote self-monitoring in intermediate- and high-risk pregnancies: A retrospective study of 400 consecutive women. Acta Obstet. Et Gynecol. Scand. 2022, 101, 135–144. [Google Scholar] [CrossRef]
- Owen, J.; Albert, P.S.; Buck Louis, G.M.; Fuchs, K.M.; Grobman, W.A.; Kim, S.; Grantz, K.L. A contemporary amniotic fluid volume chart for the United States: The NICHD Fetal Growth Studies-Singletons. Am. J. Obstet. Gynecol. 2019, 221, 67.e1–67.e12. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.L.; Leung, A.; Henry, O.A.; Victor, M.R.; Paul, R.H.; Medearis, A.L. Antepartum surveillance in diabetic pregnancies: Predictors of fetal distress in labor. Am. J. Obstet. Gynecol. 1995, 173, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, H.; Barwick, M.; Carpenter, C.R.; Eldridge, S.; Grandes, G.; Griffiths, C.J.; Taylor, S.J. Standards for Reporting Implementation Studies (StaRI) Statement. Bmj 2017, 356, i6795. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; STARD Group. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Bmj 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.W.; Moher, D.; Mayo-Wilson, E.; Offringa, M. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. Jama 2022, 328, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J. Births in the United States, 2021. Natl. Cent. Health Stat. 2022, 1–8. [Google Scholar]
- Buck Louis, G.M.; Grewal, J.; Albert, P.S.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Grantz, K.L. Racial/ethnic standards for fetal growth: The NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2015, 213, 449.e1–449.e41. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whittington, J.R.; Chauhan, S.P.; Wendel, M.P.; Ghahremani, T.L.; Pagan, M.E.; Carter, M.M.; Magann, E.F. Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes. J. Clin. Med. 2024, 13, 4702. https://doi.org/10.3390/jcm13164702
Whittington JR, Chauhan SP, Wendel MP, Ghahremani TL, Pagan ME, Carter MM, Magann EF. Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes. Journal of Clinical Medicine. 2024; 13(16):4702. https://doi.org/10.3390/jcm13164702
Chicago/Turabian StyleWhittington, Julie R., Suneet P. Chauhan, Michael P. Wendel, Taylor L. Ghahremani, Megan E. Pagan, Meagen M. Carter, and Everett F. Magann. 2024. "Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes" Journal of Clinical Medicine 13, no. 16: 4702. https://doi.org/10.3390/jcm13164702
APA StyleWhittington, J. R., Chauhan, S. P., Wendel, M. P., Ghahremani, T. L., Pagan, M. E., Carter, M. M., & Magann, E. F. (2024). Historical Assessment, Practical Management, and Future Recommendations for Abnormal Amniotic Fluid Volumes. Journal of Clinical Medicine, 13(16), 4702. https://doi.org/10.3390/jcm13164702





