Plasma Somatostatin Levels Are Lower in Patients with Coronary Stenosis and Significantly Increase after Stent Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Coronarography Procedure and Stent Implantation
2.3. Medication and Contrast Material
2.4. Blood Sampling
2.5. Determining Plasma SST-LI
2.6. Statistical Analysis
3. Results
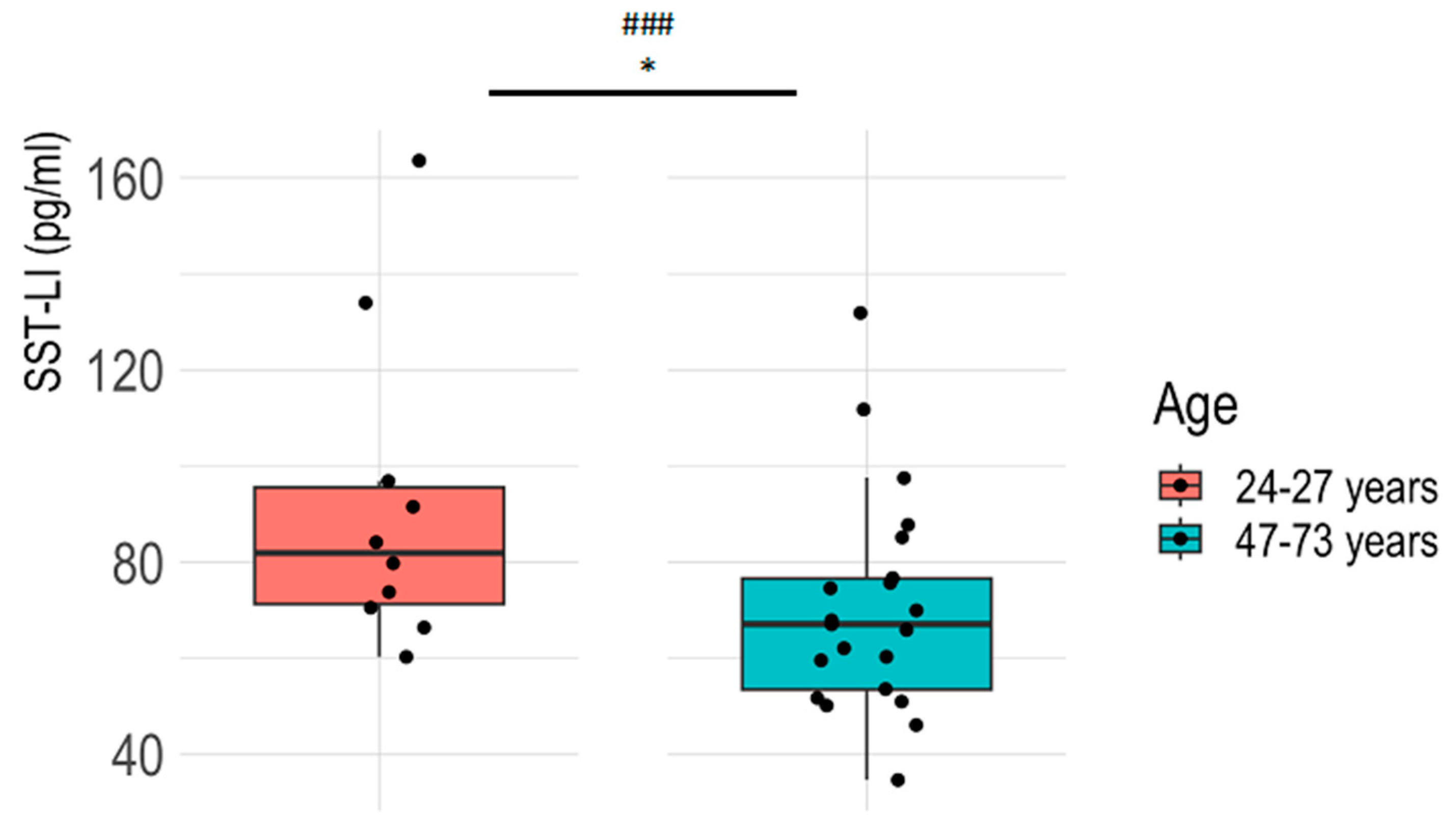
3.1. Plasma SST-LI Significantly Decreases with Aging in the Healthy Population
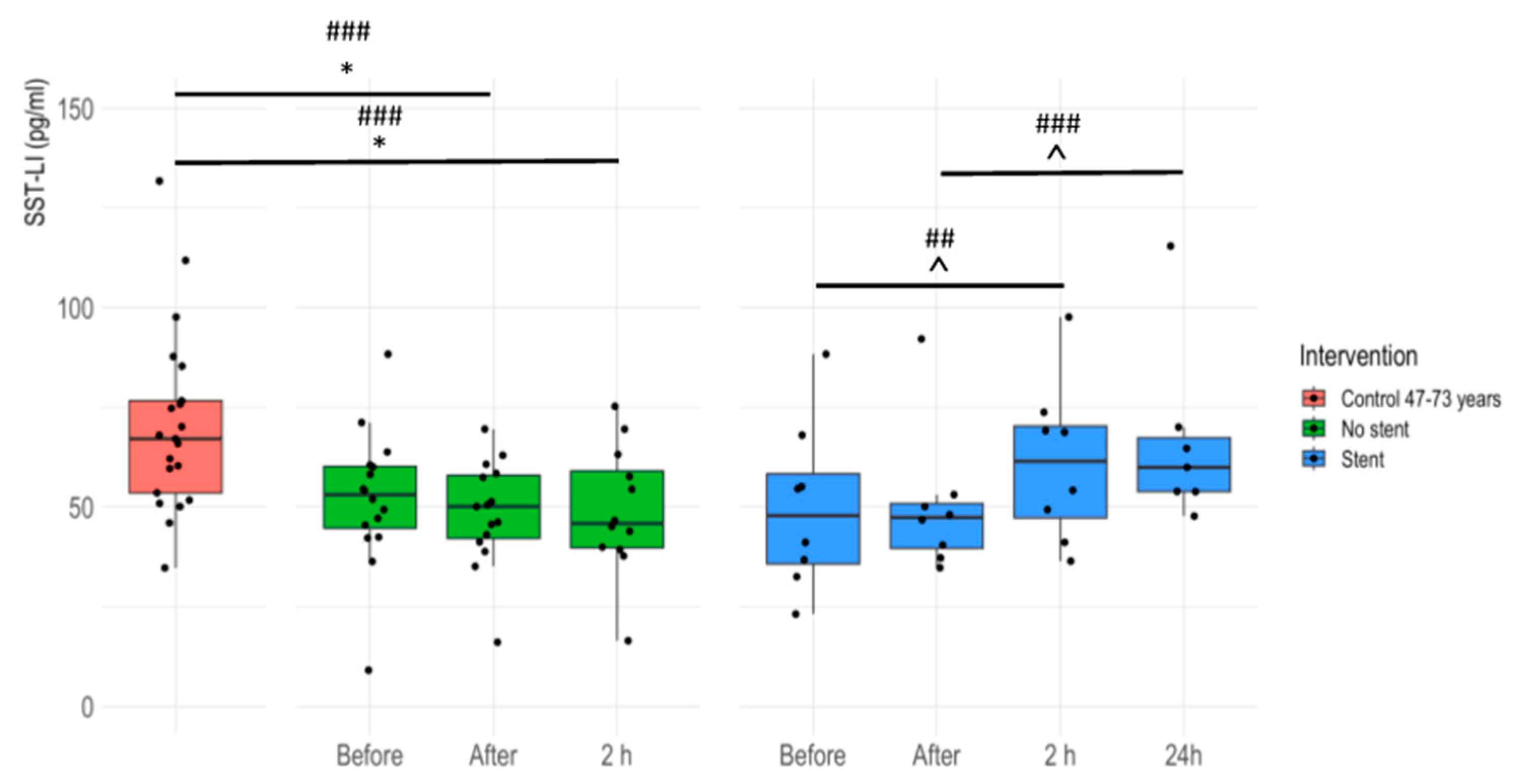
3.2. Plasma SST-LI Is Significantly Lower in IHD Patients Compared to Age-Matched Healthy Controls
3.3. Plasma SST-LI Significantly Increases in IHD Patients 24 Hours after Stent Insertion
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
References
- Onuoha, G.N.; Alpar, E.K. Calcitonin Gene-Related Peptide and Other Neuropeptides in the Plasma of Patients with Soft Tissue Injury. Life Sci. 1999, 65, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Szőke, É.; Helyes, Z. Molecular Links between Sensory Nerves, Inflammation, and Pain 2.0. Int. J. Mol. Sci. 2023, 24, 12243. [Google Scholar] [CrossRef] [PubMed]
- Börzsei, R.; Borbély, É.; Kántás, B.; Hudhud, L.; Horváth, Á.; Szőke, É.; Hetényi, C.; Helyes, Z.; Pintér, E. The Heptapeptide Somatostatin Analogue TT-232 Exerts Analgesic and Anti-Inflammatory Actions via SST4 Receptor Activation: In Silico, in Vitro and in Vivo Evidence in Mice. Biochem. Pharmacol. 2023, 209, 115419. [Google Scholar] [CrossRef]
- Tóth, L.; Juhász, M.F.; Szabó, L.; Abada, A.; Kiss, F.; Hegyi, P.; Farkas, N.; Nagy, G.; Helyes, Z. Janus Kinase Inhibitors Improve Disease Activity and Patient-Reported Outcomes in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of 24,135 Patients. Int. J. Mol. Sci. 2022, 23, 1246. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J. Forty Years in Capsaicin Research for Sensory Pharmacology and Physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Pintér, E.; Helyes, Z.; Oroszi, G.; Németh, J. Systemic Anti-Inflammatory Effect Induced by Counter-Irritation through a Local Release of Somatostatin from Nociceptors. Br. J. Pharmacol. 1998, 125, 916–922. [Google Scholar] [CrossRef]
- Karalis, K.; Mastorakos, G.; Chrousos, G.P.; Tolis, G. Somatostatin Analogues Suppress the Inflammatory Reaction in Vivo. J. Clin. Investig. 1994, 93, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Helyes, Z.; Pintér, E.; Németh, J.; Kéri, G.; Thán, M.; Oroszi, G.; Horváth, A.; Szolcsányi, J. Anti-Inflammatory Effect of Synthetic Somatostatin Analogues in the Rat. Br. J. Pharmacol. 2001, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Helyes, Z.; Szolcsányi, J. Inhibitory Effect of Somatostatin on Inflammation and Nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef]
- Ten Bokum, A.M.C.; Hofland, L.J.; Van Hagen, P.M. Somatostatin and Somatostatin Receptors in the Immune System: A Review. Eur. Cytokine Netw. 2000, 11, 161–176. [Google Scholar]
- Ferone, D.; Van Hagen, P.M.; Semino, C.; Dalm, V.A.; Barreca, A.; Colao, A.; Lamberts, S.W.J.; Minuto, F.; Hofland, L.J. Somatostatin Receptor Distribution and Function in Immune System. Dig. Liver Dis. 2004, 36, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Selmer, I.S.; Schindler, M.; Allen, J.P.; Humphrey, P.P.A.; Emson, P.C. Advances in Understanding Neuronal Somatostatin Receptors. Regul. Pept. 2000, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Somatostatin and Its Receptor Family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Reisine, T.; Bell, G.I. Molecular Properties of Somatostatin Receptors. Neuroscience 1995, 67, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Vörös, I.; Sághy, É.; Pohóczky, K.; Makkos, A.; Onódi, Z.; Brenner, G.B.; Baranyai, T.; Ágg, B.; Váradi, B.; Kemény, Á.; et al. Somatostatin and Its Receptors in Myocardial Ischemia/Reperfusion Injury and Cardioprotection. Front. Pharmacol. 2021, 12, 663655. [Google Scholar] [CrossRef] [PubMed]
- Møller, L.N.; Stidsen, C.E.; Hartmann, B.; Holst, J.J. Somatostatin Receptors. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1616, 1–84. [Google Scholar] [CrossRef]
- Helyes, Z.; Szabó, Á.; Németh, J.; Jakab, B.; Pintér, E.; Bánvölgyi, Á.; Kereskai, L.; Kéri, G.; Szolcsányi, J. Antiinflammatory and Analgesic Effects of Somatostatin Released from Capsaicin-Sensitive Sensory Nerve Terminals in a Freund’s Adjuvant-Induced Chronic Arthritis Model in the Rat. Arthritis Rheum. 2004, 50, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Nemeth, J.; Szolcsányi, J.; Pozsgai, G.; Pinter, E. Abdominal Surgery Performed under General Anesthesia Increases Somatostatin-like Immunoreactivity in Human Serum. Neuroimmunomodulation 2008, 15, 153–156. [Google Scholar] [CrossRef]
- Suto, B.; Bagoly, T.; Borzsei, R.; Lengl, O.; Szolcsanyi, J.; Nemeth, T.; Loibl, C.; Bardonicsek, Z.; Pinter, E.; Helyes, Z. Surgery and Sepsis Increase Somatostatin-like Immunoreactivity in the Human Plasma. Peptides 2010, 31, 1208–1212. [Google Scholar] [CrossRef]
- Suto, B.; Szitter, I.; Bagoly, T.; Pinter, E.; Szolcsányi, J.; Loibl, C.; Nemeth, T.; Tanczos, K.; Molnar, T.; Leiner, T.; et al. Plasma Somatostatin-like Immunoreactivity Increases in the Plasma of Septic Patients and Rats with Systemic Inflammatory Reaction: Experimental Evidence for Its Sensory Origin and Protective Role. Peptides 2014, 54, 49–57. [Google Scholar] [CrossRef]
- Sütő, B.; Kolumbán, B.; Szabó, É.; Pásztor, S.; Németh, T.; Bagoly, T.; Botz, B.; Pintér, E.; Helyes, Z. Plasma Somatostatin Levels Increase during Scoliosis Surgery, but Not Herniated Disc Operations: Results of a Pilot Study. Biomedicines 2023, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Widiapradja, A.; Chunduri, P.; Levick, S.P. The Role of Neuropeptides in Adverse Myocardial Remodeling and Heart Failure. Cell. Mol. Life Sci. 2017, 74, 2019–2038. [Google Scholar] [CrossRef]
- Zahner, M.R.; Li, D.P.; Chen, S.R.; Pan, H.L. Cardiac Vanilloid Receptor 1-Expressing Afferent Nerves and Their Role in the Cardiogenic Sympathetic Reflex in Rats. J. Physiol. 2003, 551, 515–523. [Google Scholar] [CrossRef]
- Katona, M.; Boros, K.; Sántha, P.; Ferdinandy, P.; Dux, M.; Jancsó, G. Selective Sensory Denervation by Capsaicin Aggravates Adriamycin-Induced Cardiomyopathy in Rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2004, 370, 436–443. [Google Scholar] [CrossRef]
- Jancsó, G.; Such, G. Effects of Capsaicin Applied Perineurally to the Vagus Nerve on Cardiovascular and Respiratory Functions in the Cat. J. Physiol. 1983, 341, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, P.; Gömöri, K.; Szabados, T.; Sántha, P.; Helyes, Z.; Jancsó, G.; Ferdinandy, P.; Görbe, A. Myocardial Ischaemia Reperfusion Injury and Cardioprotection in the Presence of Sensory Neuropathy: Therapeutic Options. Br. J. Pharmacol. 2020, 177, 5336–5356. [Google Scholar] [CrossRef]
- Wang, J.; Tian, W.; Wang, S.; Wei, W.; Wu, D.; Wang, H.; Wang, L.; Yang, R.; Ji, A.; Li, Y. Anti-Inflammatory and Retinal Protective Effects of Capsaicin on Ischaemia-Induced Injuries through the Release of Endogenous Somatostatin. Clin. Exp. Pharmacol. Physiol. 2017, 44, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Helyes, Z.; Thán, M.; Oroszi, G.; Pintér, E.; Németh, J.; Kéri, G.; Szolcsányi, J. Anti-Nociceptive Effect Induced by Somatostatin Released from Sensory Nerve Terminals and by Synthetic Somatostatin Analogues in the Rat. Neurosci. Lett. 2000, 278, 185–188. [Google Scholar] [CrossRef]
- Gignac, G.E.; Szodorai, E.T. Effect Size Guidelines for Individual Differences Researchers. Personal. Individ. Differ. 2016, 102, 74–78. [Google Scholar] [CrossRef]
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage Publications Limited: Washington, DC, USA, 2013. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Sample Size Justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Sladek, J.R. Age-Related Changes in Dopamine, LHRH and Somatostatin in the Rat Hypothalamus. Neurobiol. Aging 1980, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Boyd, R.L.; Booze, R.M. Somatostatin Gene Expression in Hypothalamus and Cortex of Aging Male Rats. Neurobiol. Aging 1990, 11, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Banki, E.; Sosnowska, D.; Tucsek, Z.; Gautam, T.; Toth, P.; Tarantini, S.; Tamas, A.; Helyes, Z.; Reglodi, D.; Sonntag, W.E.; et al. Age-Related Decline of Autocrine Pituitary Adenylate Cyclase-Activating Polypeptide Impairs Angiogenic Capacity of Rat Cerebromicrovascular Endothelial Cells. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2015, 70, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Hernández, C.; Arroba, A.I.; Bogdanov, P.; Ramos, H.; Simó-Servat, O.; Simó, R.; Valverde, A.M. Effect of Topical Administration of Somatostatin on Retinal Inflammation and Neurodegeneration in an Experimental Model of Diabetes. J. Clin. Med. 2020, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. TRPV1 and TRPV4 Channels: Potential Therapeutic Targets for Ischemic Conditioning-Induced Cardioprotection. Eur. J. Pharmacol. 2015, 746, 180–185. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Sándor, K.; Elekes, K.; Bánvölgyi, Á.; Keszthelyi, D.; Szoke, É.; Tóth, D.M.; Sándor, Z.; Kereskai, L.; et al. Impaired Defense Mechanism against Inflammation, Hyperalgesia, and Airway Hyperreactivity in Somatostatin 4 Receptor Gene-Deleted Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 13088–13093. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Helyes, Z.; Oroszi, G.; Németh, J.; Pintér, E. Release of Somatostatin and Its Role in the Mediation of the Anti-Inflammatory Effect Induced by Antidromic Stimulation of Sensory Fibres of Rat Sciatic Nerve. Br. J. Pharmacol. 1998, 123, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Thán, M.; Németh, J.; Szilvássy, Z.; Pintér, E.; Helyes, Z.; Szolcsányi, J. Systemic Anti-Inflammatory Effect of Somatostatin Released from Capsaicin-Sensitive Vagal and Sciatic Sensory Fibres of the Rat and Guinea-Pig. Eur. J. Pharmacol. 2000, 399, 251–258. [Google Scholar] [CrossRef]
- Day, S.M.; Gu, J.; Polak, J.M.; Bloom, S.R. Somatostatin in the Human Heart and Comparison with Guinea Pig and Rat Heart. Br. Heart J. 1985, 53, 153–157. [Google Scholar] [CrossRef]
- Tsuda, K.; Sakurai, H.; Seino, Y.; Seino, S.; Tanigawa, K.; Kuzuya, H.; Imura, H. Somatostatin-like Immunoreactivity in Human Peripheral Plasma Measured by Radioimmunoassay Following Affinity Chromatography. Diabetes 1981, 30, 471–474. [Google Scholar] [CrossRef]
- Abbasi, A.; Kieneker, L.M.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.B.; Struck, J.; Boer, R.A.D.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Plasma N-Terminal Prosomatostatin and Risk of Incident Cardiovascular Disease and All-Cause Mortality in a Prospective Observational Cohort: The PREVEND Study. Clin. Chem. 2017, 63, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Dakhel, A.; Engström, G.; Melander, O.; Acosta, S.; Fatemi, S.; Gottsäter, A.; Zarrouk, M. Vasoactive Biomarkers Associated with Long-Term Incidence of Symptomatic Peripheral Arterial Disease and Mortality. Angiology 2021, 72, 550–555. [Google Scholar] [CrossRef]
- Plášek, J.; Lazárová, M.; Dodulík, J.; Šulc, P.; Stejskal, D.; Švagera, Z.; Všianský, F.; Václavík, J. Secretoneurin as a Novel Biomarker of Cardiovascular Episodes: Are We There Yet? A Narrative Review. J. Clin. Med. 2022, 11, 7191. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Role of Inflammation in Coronary Epicardial and Microvascular Dysfunction. Eur. Cardiol. Rev. 2021, 16, e13. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells 2023, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Anzola, L.K.; Rivera, J.N.; Ramirez, J.C.; Signore, A.; Mut, F. Molecular Imaging of Vulnerable Coronary Plaque with Radiolabeled Somatostatin Receptors (Sstr). J. Clin. Med. 2021, 10, 5515. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-Immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]

| Healthy Controls | Healthy Control Group 1. | Healthy Control Group 2. (Age Matched) | Patients for Coronarography | Coronarography without Stent Implantation | Coronarography with Stent Implantation | |
|---|---|---|---|---|---|---|
| Male | 11 | 5 | 6 | 12 | 8 | 4 |
| Female | 20 | 5 | 15 | 12 | 8 | 4 |
| Total | 31 | 10 | 21 | 24 | 16 | 8 |
| Mean age ± SD | 43.74 ± 15.32 | 23.3 ± 0.82 | 53.47 ± 6.6 | 68 ± 9.29 | 66.06 ± 8.51 | 72.125 ± 9.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sütő, B.; Kun, J.; Bagoly, T.; Németh, T.; Pintér, E.; Kardos, D.; Helyes, Z. Plasma Somatostatin Levels Are Lower in Patients with Coronary Stenosis and Significantly Increase after Stent Implantation. J. Clin. Med. 2024, 13, 4727. https://doi.org/10.3390/jcm13164727
Sütő B, Kun J, Bagoly T, Németh T, Pintér E, Kardos D, Helyes Z. Plasma Somatostatin Levels Are Lower in Patients with Coronary Stenosis and Significantly Increase after Stent Implantation. Journal of Clinical Medicine. 2024; 13(16):4727. https://doi.org/10.3390/jcm13164727
Chicago/Turabian StyleSütő, Balázs, József Kun, Teréz Bagoly, Timea Németh, Erika Pintér, Dorottya Kardos, and Zsuzsanna Helyes. 2024. "Plasma Somatostatin Levels Are Lower in Patients with Coronary Stenosis and Significantly Increase after Stent Implantation" Journal of Clinical Medicine 13, no. 16: 4727. https://doi.org/10.3390/jcm13164727






