Role of Cardiac Magnetic Resonance in Inflammatory and Infiltrative Cardiomyopathies: A Narrative Review
Abstract
:1. Introduction
2. Inflammatory Phenotypes and “Hot Phase” Presentation in Cardiomyopathies
3. Cardiac Sarcoidosis
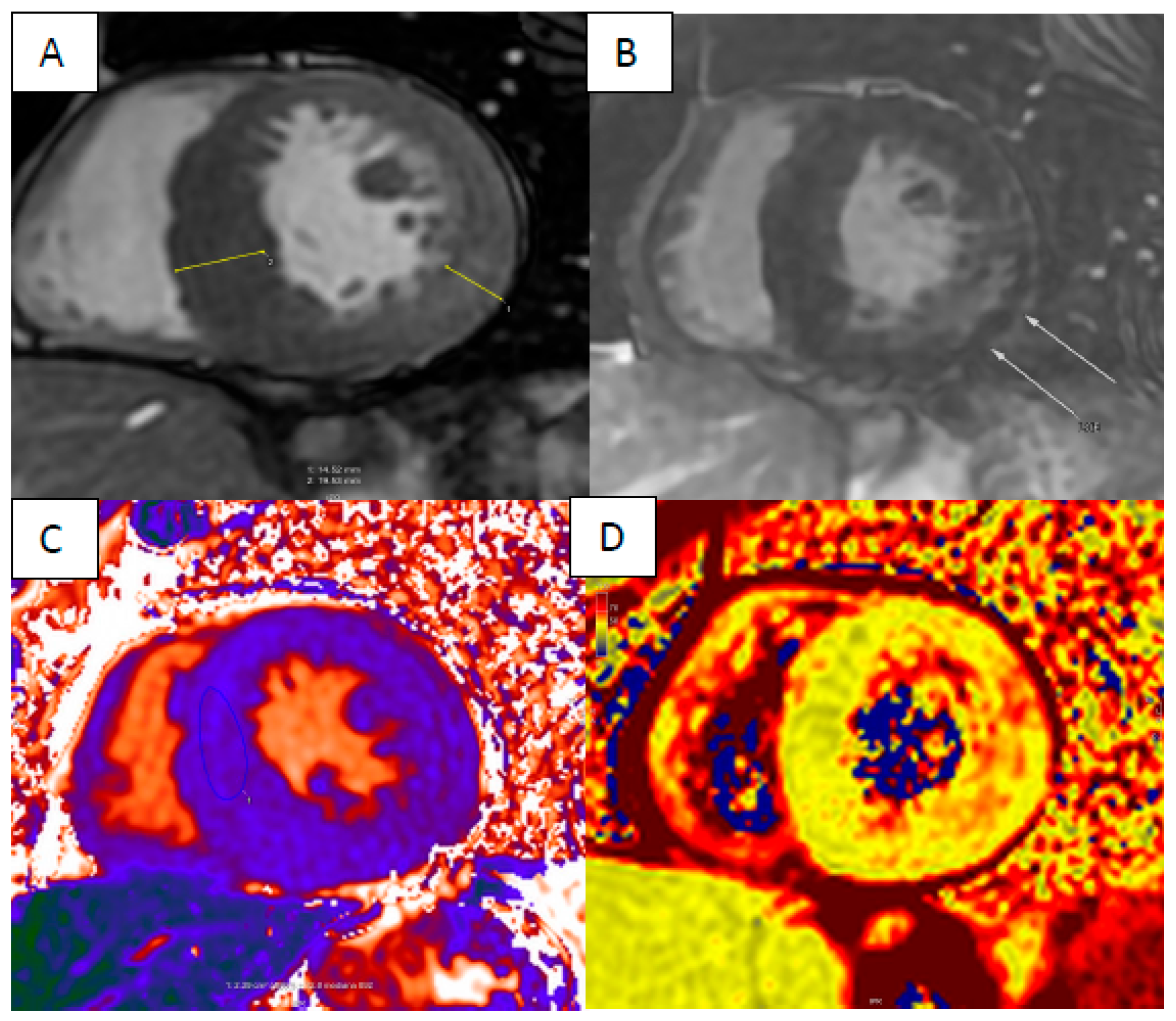
4. Cardiac Amyloidosis
5. Anderson–Fabry Disease
6. Myocardial Iron Overload
7. Limitation of Cardiac MRI
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology: A comprehensive summary and update. J. Cardiovasc. Magn. Reson. 2023, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Todiere, G.; Barison, A.; Baritussio, A.; Cipriani, A.; Guaricci, A.I.; Pica, S.; Indolfi, C.; Pontone, G.; Dellegrottaglie, S.; Working Group on Cardiac Magnetic Resonance of the Italian Society of Cardiology. Acute clinical presentation of nonischemic cardiomyopathies: Early detection by cardiovascular magnetic resonance. J. Cardiovasc. Med. 2023, 24 (Suppl. S1), e36–e46. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.D.; Lakdawala, N.K.; Papoutsidakis, N.; Aubert, G.; Mazzanti, A.; McCanta, A.C.; Agarwal, P.P.; Arscott, P.; Dellefave-Castillo, L.M.; Vorovich, E.E.; et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1872–1884. [Google Scholar] [CrossRef]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Carbone, I.; Agati, L.; Bucciarelli Ducci, C.; Mangia, M.; Iacucci, I.; Catalano, C.; Passariello, R. Utility of T2-weighted short-tau inversion recovery (STIR) sequences in cardiac MRI: An overview of clinical applications in ischaemic and non-ischaemic heart disease. Radiol. Med. 2011, 116, 32–46. [Google Scholar] [CrossRef]
- Verhaert, D.; Thavendiranathan, P.; Giri, S.; Mihai, G.; Rajagopalan, S.; Simonetti, O.P.; Raman, S.V. Direct T2 Quantification of Myocardial Edema in Acute Ischemic Injury. JACC Cardiovasc. Imaging 2011, 4, 269–278. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. T1 Mapping for the Diagnosis of Acute Myocarditis Using CMR. JACC Cardiovasc. Imaging 2013, 6, 1048–1058. [Google Scholar] [CrossRef]
- O’Brien, A.T.; Gil, K.E.; Varghese, J.; Simonetti, O.P.; Zareba, K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. 2022, 24, 33. [Google Scholar] [CrossRef] [PubMed]
- Bariani, R.; Rigato, I.; Cipriani, A.; Bueno Marinas, M.; Celeghin, R.; Basso, C.; Corrado, D.; Pilichou, K.; Bauce, B. Myocarditis-like Episodes in Patients with Arrhythmogenic Cardiomyopathy: A Systematic Review on the So-Called Hot-Phase of the Disease. Biomolecules 2022, 12, 1324. [Google Scholar] [CrossRef]
- Lopez-Ayala, J.M.; Pastor-Quirante, F.; Gonzalez-Carrillo, J.; Lopez-Cuenca, D.; Sanchez-Munoz, J.J.; Oliva-Sandoval, M.J.; Gimeno, J.R. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2015, 12, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, O.; Fernández-Falgueras, A.; Sarquella-Brugada, G.; Sanchez, O.; Cesar, S.; Mademont, I.; Allegue, C.; Mates, J.; Pérez-Serra, A.; Coll, M.; et al. A Genetically Vulnerable Myocardium May Predispose to Myocarditis. J. Am. Coll. Cardiol. 2015, 66, 2913–2914. [Google Scholar] [CrossRef] [PubMed]
- Doeblin, P.; Hashemi, D.; Tanacli, R.; Lapinskas, T.; Gebker, R.; Stehning, C.; Motzkus, L.A.; Blum, M.; Tahirovic, E.; Dordevic, A.; et al. CMR Tissue Characterization in Patients with HFmrEF. J. Clin. Med. 2019, 8, 1877. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Bertrand, P.B.; Willems, E.; Gielen, E.; Mullens, W.; Giri, S.; Tang, W.H.W.; Raman, S.V.; Verhaert, D. Global myocardial oedema in advanced decompensated heart failure. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Lota, A.S.; Hazebroek, M.R.; Theotokis, P.; Wassall, R.; Salmi, S.; Halliday, B.P.; Tayal, U.; Verdonschot, J.; Meena, D.; Owen, R.; et al. Genetic Architecture of Acute Myocarditis and the Overlap with Inherited Cardiomyopathy. Circulation 2022, 146, 1123–1134. [Google Scholar] [CrossRef]
- Sikking, M.A.; Stroeks, S.L.V.M.; Henkens, M.T.H.M.; Venner, M.F.G.H.M.; Li, X.; Heymans, S.R.B.; Hazebroek, M.R.; Verdonschot, J.A.J. Cardiac Inflammation in Adult-Onset Genetic Dilated Cardiomyopathy. J. Clin. Med. 2023, 12, 3937. [Google Scholar] [CrossRef]
- Kuusisto, J.; Kärjä, V.; Sipola, P.; Kholová, I.; Peuhkurinen, K.; Jääskeläinen, P.; Naukkarinen, A.; Ylä-Herttuala, S.; Punnonen, K.; Laakso, M. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 2012, 98, 1007–1013. [Google Scholar] [CrossRef]
- Lillo, R.; Graziani, F.; Franceschi, F.; Iannaccone, G.; Massetti, M.; Olivotto, I.; Crea, F.; Liuzzo, G. Inflammation across the spectrum of hypertrophic cardiac phenotypes. Heart Fail. Rev. 2023, 28, 1065–1075. [Google Scholar] [CrossRef]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef]
- Imran, M.; Wang, L.; McCrohon, J.; Yu, C.; Holloway, C.; Otton, J.; Huang, J.; Stehning, C.; Moffat, K.J.; Ross, J.; et al. Native T1 Mapping in the Diagnosis of Cardiac Allograft Rejection. JACC Cardiovasc. Imaging 2019, 12, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Kouranos, V.; Sharma, R. Cardiac sarcoidosis: State-of-the-art review. Heart 2021, 107, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Kouranos, V.; Tzelepis, G.E.; Rapti, A.; Mavrogeni, S.; Aggeli, K.; Douskou, M.; Prasad, S.; Koulouris, N.; Sfikakis, P.; Wells, A.; et al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1437–1447. [Google Scholar] [CrossRef]
- Smedema, J.P.; Snoep, G.; Van Kroonenburgh, M.P.G.; van Geuns, R.J.; Dassen, W.R.; Gorgels, A.P.; Crijns, H.J. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J. Am. Coll. Cardiol. 2005, 45, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Fong, H.K.; Birati, E.Y.; Han, Y. Cardiac Sarcoidosis. Am. J. Cardiol. 2019, 123, 513–522. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; Judd, R.M.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.C.; Shaw, P.W.; Balfour, P.C., Jr.; Gonzalez, J.A.; Kramer, C.M.; Patel, A.R.; Salerno, M. Prognostic Value of Myocardial Scarring on CMR in Patients with Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 411–420. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Ise, T.; Hasegawa, T.; Morita, Y.; Yamada, N.; Funada, A.; Takahama, H.; Amaki, M.; Kanzaki, H.; Okamura, H.; Kamakura, S.; et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 2014, 100, 1165–1172. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Hosadurg, N.; Iwanaga, Y.; Chen, Y.; Liu, W.; Wan, K.; Patel, A.R.; Wicks, E.C.; Gkoutos, G.V.; et al. Prognostic Value of RV Abnormalities on CMR in Patients With Known or Suspected Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2023, 16, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; Kitterer, D.; Latus, J.; Aguor, E.; Steubing, H.; Kaesemann, P.; Patrascu, A.; Greiser, A.; Groeninger, S.; Mayr, A.; et al. Comprehensive Cardiovascular Magnetic Resonance Assessment in Patients with Sarcoidosis and Preserved Left Ventricular Ejection Fraction. Circ. Cardiovasc. Imaging 2016, 9, e005022. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; Gatidis, S.; Gräni, C.; Blankstein, R.; Glatthaar, A.; Mezger, K.; Müller, K.A.L.; Castor, T.; Mahrholdt, H.; Häntschel, M.; et al. Hybrid Cardiac Magnetic Resonance/Fluorodeoxyglucose Positron Emission Tomography to Differentiate Active from Chronic Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2022, 15, 445–456. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Isted, A.; Hinojar, R.; Foote, L.; Carr-White, G.; Nagel, E. T1 and T2 Mapping in Recognition of Early Cardiac Involvement in Systemic Sarcoidosis. Radiology 2017, 285, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Pozo, E.; Kanwar, A.; Deochand, R.; Castellano, J.M.; Naib, T.; Pazos-López, P.; Osman, K.; Cham, M.; Narula, J.; Fuster, V.; et al. Cardiac magnetic resonance evaluation of left ventricular remodelling distribution in cardiac amyloidosis. Heart 2014, 100, 1688–1695. [Google Scholar] [CrossRef]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Knight, D.S.; Zumbo, G.; Barcella, W.; Steeden, J.A.; Muthurangu, V.; Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Kotecha, T.; et al. Cardiac Structural and Functional Consequences of Amyloid Deposition by Cardiac Magnetic Resonance and Echocardiography and Their Prognostic Roles. JACC Cardiovasc. Imaging 2019, 12, 823–833. [Google Scholar] [CrossRef]
- Cohen, O.C.; Ismael, A.; Pawarova, B.; Manwani, R.; Ravichandran, S.; Law, S.; Foard, D.; Petrie, A.; Ward, S.; Douglas, B.; et al. Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis. Eur. Heart J. 2022, 43, 333–341. [Google Scholar] [CrossRef]
- Rapezzi, C.; Aimo, A.; Pavasini, R. Longitudinal strain in the management of cardiac AL amyloidosis: Do we need it? Eur. Heart J. 2022, 43, 342–344. [Google Scholar] [CrossRef]
- Fine, N.M.; White, J.A.; Jimenez-Zepeda, V.; Howlett, J.G. Determinants and Prognostic Significance of Serial Right Heart Function Changes in Patients With Cardiac Amyloidosis. Can. J. Cardiol. 2020, 36, 432–440. [Google Scholar] [CrossRef]
- Bodez, D.; Ternacle, J.; Guellich, A.; Galat, A.; Lim, P.; Radu, C.; Guendouz, S.; Bergoend, E.; Couetil, J.P.; Hittinger, L.; et al. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid 2016, 23, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Chung, R.; Hawkins, P.N.; Moon, J.C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail. Rev. 2015, 20, 133–144. [Google Scholar] [CrossRef]
- Gerbaud, É.; Lederlin, M.; Laurent, F. Value of phase-sensitive inversion recovery sequence to perform and analyse late gadolinium enhancement in cardiac amyloidosis. Arch. Cardiovasc. Dis. 2009, 102, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Maceira, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.; White, S.K.; Piechnik, S.K.; Flett, A.S.; Ferreira, V.M.; Loudon, M.; Francis, J.M.; Karamitsos, T.D.; Prendergast, B.D.; Robson, M.D.; et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013, 99, 932–937. [Google Scholar] [CrossRef]
- Lavall, D.; Vosshage, N.H.; Geßner, R.; Stöbe, S.; Ebel, S.; Denecke, T.; Hagendorff, A.; Laufs, U. Native T1 mapping for the diagnosis of cardiac amyloidosis in patients with left ventricular hypertrophy. Clin. Res. Cardiol. 2023, 112, 334–342. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef]
- Ioannou, A.; Patel, R.K.; Martinez-Naharro, A.; Razvi, Y.; Porcari, A.; Rauf, M.U.; Bolhuis, R.E.; Fernando-Sayers, J.; Virsinskaite, R.; Bandera, F.; et al. Tracking Treatment Response in Cardiac Light-Chain Amyloidosis with Native T1 Mapping. JAMA Cardiol. 2023, 8, 848. [Google Scholar] [CrossRef]
- Boretto, P.; Patel, N.H.; Patel, K.; Rana, M.; Saglietto, A.; Soni, M.; Ahmad, M.; Sin Ying Ho, J.; De Filippo, O.; Providencia, R.A.; et al. Prognosis prediction in cardiac amyloidosis by cardiac magnetic resonance imaging: A systematic review with meta-analysis. Eur. Heart J. Open 2023, 3, oead092. [Google Scholar] [CrossRef]
- Chacko, L.; Kotecha, T.; Ioannou, A.; Patel, N.; Martinez-Naharro, A.; Razvi, Y.; Patel, R.; Massa, P.; Venneri, L.; Brown, J.; et al. Myocardial perfusion in cardiac amyloidosis. Eur. J. Heart Fail. 2024, 26, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Ridouani, F.; Damy, T.; Tacher, V.; Derbel, H.; Legou, F.; Sifaoui, I.; Audureau, E.; Bodez, D.; Rahmouni, A.; Deux, J.-F. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J. Cardiovasc. Magn. Reson. 2018, 20, 58. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Kotecha, T.; Norrington, K.; Boldrini, M.; Rezk, T.; Quarta, C.; Treibel, T.A.; Whelan, C.J.; Knight, D.S.; Kellman, P.; et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2019, 12, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Lama, N.; Rempakos, A.; Theodorakakou, F.; Stamatelopoulos, K.; Dimopoulos, M.A.; Kelekis, N.; Kastritis, E. Diagnostic and Prognostic Value of Non-late Gadolinium Enhancement Cardiac Magnetic Resonance Parameters in Cardiac Amyloidosis. Curr. Probl. Cardiol. 2023, 48, 101573. [Google Scholar] [CrossRef] [PubMed]
- Banypersad, S.M.; Fontana, M.; Maestrini, V.; Sado, D.M.; Captur, G.; Petrie, A.; Piechnik, S.K.; Whelan, C.J.; Herrey, A.S.; Gillmore, J.D.; et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur. Heart J. 2015, 36, 244–251. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Patel, R.; Kotecha, T.; Karia, N.; Ioannou, A.; Petrie, A.; Chacko, L.A.; Razvi, Y.; Ravichandran, S.; Brown, J.; et al. Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment. Eur. Heart J. 2022, 43, 4722–4735. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Myocardial Edema and Prognosis in Amyloidosis. J. Am. Coll. Cardiol. 2018, 71, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Zarate, Y.A.; Hopkin, R.J. Fabry’s disease. Lancet 2008, 372, 1427–1435. [Google Scholar] [CrossRef]
- Yousef, Z.; Elliott, P.M.; Cecchi, F.; Escoubet, B.; Linhart, A.; Monserrat, L.; Namdar, M.; Weidemann, F. Left ventricular hypertrophy in Fabry disease: A practical approach to diagnosis. Eur. Heart J. 2013, 34, 802–808. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Medina-Menacho, K.; Abdel-Gadir, A.; Baig, S.; Sado, D.M.; Lobascio, I.; Murphy, E.; Lachmann, R.H.; Mehta, A.; et al. Proposed Stages of Myocardial Phenotype Development in Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef]
- Kozor, R.; Callaghan, F.; Tchan, M.; Hamilton-Craig, C.; Figtree, G.A.; Grieve, S.M. A disproportionate contribution of papillary muscles and trabeculations to total left ventricular mass makes choice of cardiovascular magnetic resonance analysis technique critical in Fabry disease. J. Cardiovasc. Magn. Reson. 2015, 17, 22. [Google Scholar] [CrossRef]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; Packman, S.; Sims, K.; Solomon, S.D. Cardiovascular manifestations of fabry disease: Relationships between left ventricular hypertrophy, disease severity, and-galactosidase a activity. Eur. Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.; Gambardella, M.; Green, R.; Cantoni, V.; Nappi, C.; Ascione, R.; De Giorgi, M.; Cuocolo, R.; Pisani, A.; Petretta, M.; et al. Cardiovascular magnetic resonance native T1 mapping in Anderson-Fabry disease: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2022, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, A.; Pieroni, M.; Pieruzzi, F.; Lusardi, P.; Pica, S.; Spada, M.; Mignani, R.; Burlina, A.; Bandera, F.; Guazzi, M.; et al. Predictors of Clinical Evolution in Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008424. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; De Gori, C.; Faggioni, L.; Parisella, M.L.; Aringhieri, G.; Cioni, D.; Lencioni, R.; Neri, E. Cardiac Magnetic Resonance in Fabry Disease: Morphological, Functional, and Tissue Features. Diagnostics 2022, 12, 2652. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Bulluck, H.; Castelletti, S.; Rosmini, S.; Abdel-Gadir, A.; Baig, S.; Mehta, A.; Hughes, D.; Moon, J.C. Cardiac Fabry Disease with Late Gadolinium Enhancement Is a Chronic Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Wald, R.M.; Iwanochko, R.M.; Morel, C.F. Left ventricular hypertrophy and late gadolinium enhancement at cardiac MRI are associated with adverse cardiac events in Fabry disease. Radiology 2020, 294, 42–49. [Google Scholar] [CrossRef]
- Augusto, J.B.; Nordin, S.; Vijapurapu, R.; Baig, S.; Bulluck, H.; Castelletti, S.; Alfarih, M.; Knott, K.; Captur, G.; Kotecha, T.; et al. Myocardial Edema, Myocyte Injury, and Disease Severity in Fabry Disease. Circ. Cardiovasc. Imaging 2020, 13, E010171. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Augusto, J.B.; Nordin, S.; Kozor, R.; Camaioni, C.; Xue, H.; Hughes, R.K.; Manisty, C.; Brown, L.A.E.; Kellman, P.; et al. Quantitative myocardial perfusion in Fabry disease. Circ. Cardiovasc. Imaging 2019, 12, e008872. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Dreisbach, J.G.; Karur, G.R.; Iwanochko, R.M.; Morel, C.F.; Wasim, S.; Nguyen, E.T.; Wintersperger, B.J.; Hanneman, K. Loss of base-to-apex circumferential strain gradient assessed by cardiovascular magnetic resonance in Fabry disease: Relationship to T1 mapping, late gadolinium enhancement and hypertrophy. J. Cardiovasc. Magn. Reson. 2019, 21, 45. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K. CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, T.; Carpenter, J.P.; Jabbour, A.; Alam, M.H.; Gatehouse, P.D.; Greiser, A.; Messroghli, D.; Firmin, D.N.; Pennell, D.J. In vivo comparison of myocardial T1 with T2 and T2* in thalassaemia major. J. Magn. Reson. Imaging 2013, 38, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Hardy, P.A.; Henkelman, R.M. Transverse relaxation rate enhancement caused by magnetic particulates. Magn. Reson. Imaging 1989, 7, 265–275. [Google Scholar] [CrossRef]
- Brendel, J.M.; Kratzenstein, A.; Berger, J.; Hagen, F.; Nikolaou, K.; Gawaz, M.; Greulich, S.; Krumm, P. T2* map at cardiac MRI reveals incidental hepatic and cardiac iron overload. Diagn. Interv. Imaging 2023, 104, 552–559. [Google Scholar] [CrossRef]
- Ojha, V.; Ganga, K.P.; Seth, T.; Roy, A.; Naik, N.; Jagia, P.; Gulati, G.S.; Kumar, S.; Sharma, S. Role of CMR feature-tracking derived left ventricular strain in predicting myocardial iron overload and assessing myocardial contractile dysfunction in patients with thalassemia major. Eur. Radiol. 2021, 31, 6184–6192. [Google Scholar] [CrossRef]
- Sado, D.M.; Maestrini, V.; Piechnik, S.K.; Banypersad, S.M.; White, S.K.; Flett, A.S.; Robson, M.D.; Neubauer, S.; Ariti, C.; Arai, A.; et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J. Magn. Reson. Imaging 2015, 41, 1505–1511. [Google Scholar] [CrossRef]
- Saadatifar, H.; Niayeshfar, A.; Mard-Soltani, M.; Bahrampour, E.; Khalili, S.; Alinezhad Dezfuli, D.; Pouriamehr, S. The correlation of cardiac biomarkers and myocardial iron overload based on T2* MRI in major beta-thalassemia. Int. J. Cardiovasc. Imaging 2021, 38, 833–840. [Google Scholar] [CrossRef] [PubMed]

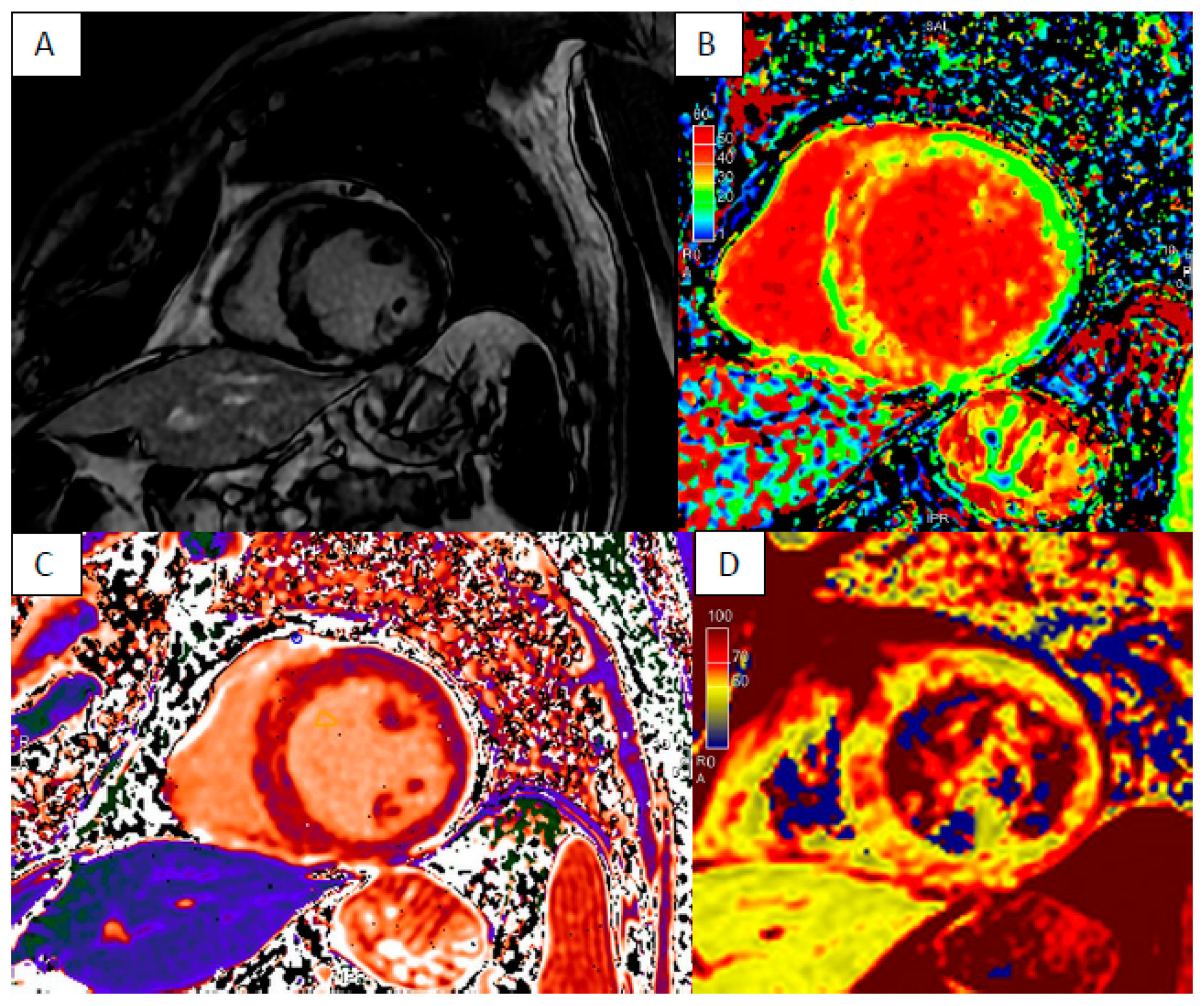
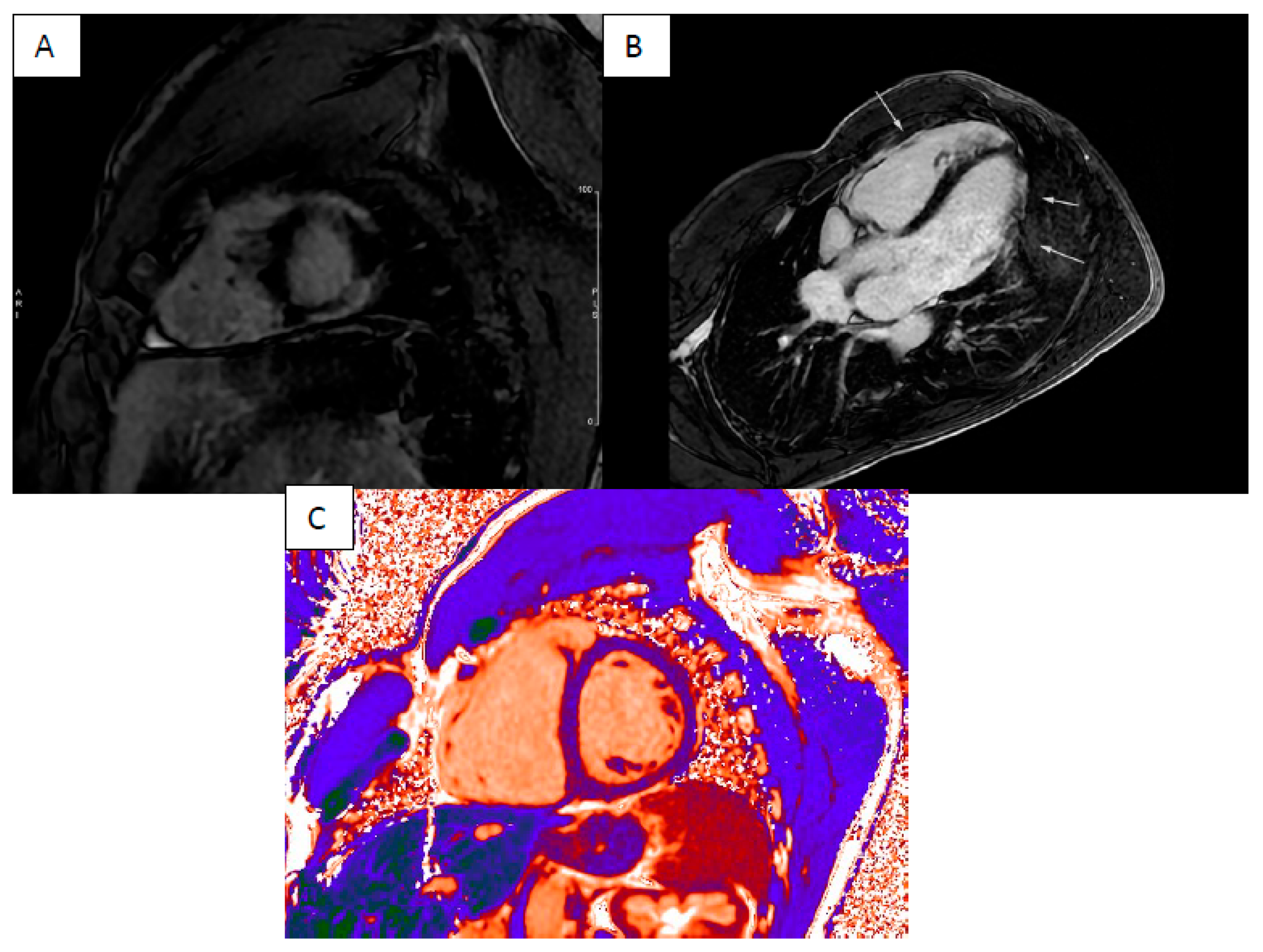
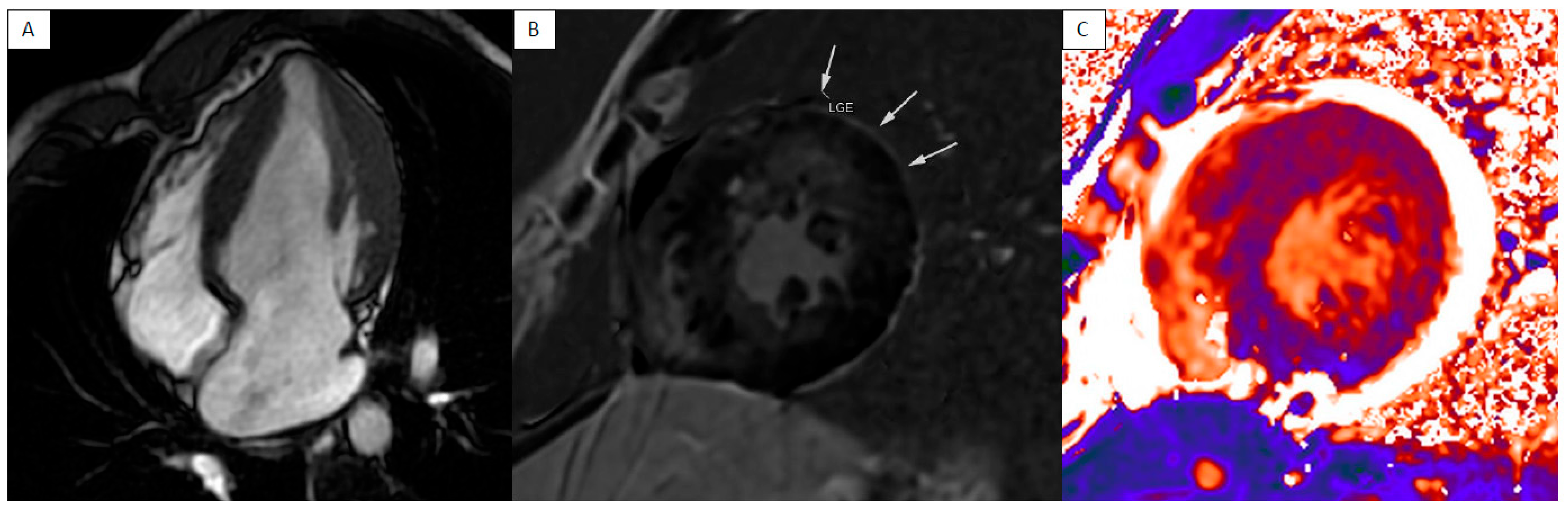
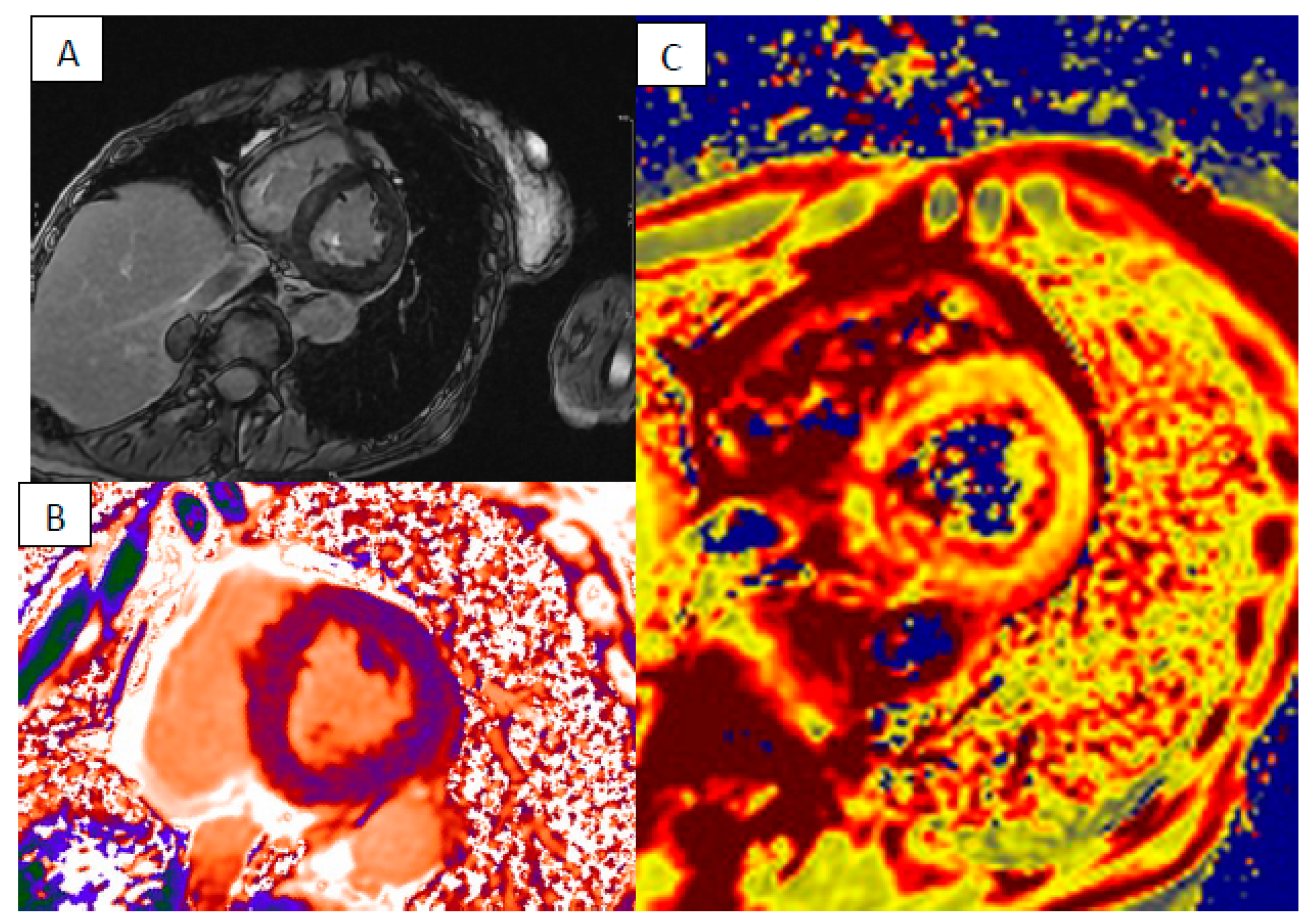

| LV Systolic Function | LV Diastolic Volume | LV Hypertrophy | LGE Pattern | T1 Mapping | T2 Mapping | ECV | T2* | |
|---|---|---|---|---|---|---|---|---|
| Cardiomiopathy Hot Phase | normal/reduced | normal/dilated | variable | specific pattern | elevated | elevated | elevated | normal |
| Amyloidosis | normal | normal | Concentric Hypertophy | diffuse | elevated | normal/elevated | elevated | normal |
| Sarcoidosis | normal/reduced | normal/dilated | not present | patchy | elevated | elevated | elevated | normal |
| Anderson–Fabry | normal | normal | Concentric Hypertrophy | inferolateral | reduced | elevated | normal | normal |
| Iron overload | normal/reduced | normal/dilated | not present | mild/absent | reduced | normal/elevated | normal/reduced | reduced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, D.; Buzzi, F.; Di Febo, R.; Modugno, S.; Schillaci, M.; Paolisso, P.; Doldi, M.; Melotti, E.; Ratti, A.; Provera, A.; et al. Role of Cardiac Magnetic Resonance in Inflammatory and Infiltrative Cardiomyopathies: A Narrative Review. J. Clin. Med. 2024, 13, 4733. https://doi.org/10.3390/jcm13164733
Marchetti D, Buzzi F, Di Febo R, Modugno S, Schillaci M, Paolisso P, Doldi M, Melotti E, Ratti A, Provera A, et al. Role of Cardiac Magnetic Resonance in Inflammatory and Infiltrative Cardiomyopathies: A Narrative Review. Journal of Clinical Medicine. 2024; 13(16):4733. https://doi.org/10.3390/jcm13164733
Chicago/Turabian StyleMarchetti, Davide, Federica Buzzi, Riccardo Di Febo, Sara Modugno, Matteo Schillaci, Pasquale Paolisso, Marco Doldi, Eleonora Melotti, Angelo Ratti, Andrea Provera, and et al. 2024. "Role of Cardiac Magnetic Resonance in Inflammatory and Infiltrative Cardiomyopathies: A Narrative Review" Journal of Clinical Medicine 13, no. 16: 4733. https://doi.org/10.3390/jcm13164733





