Omentoplasty for Cervical Lymphocele after Aortic Arch Replacement
Abstract
:1. Introduction
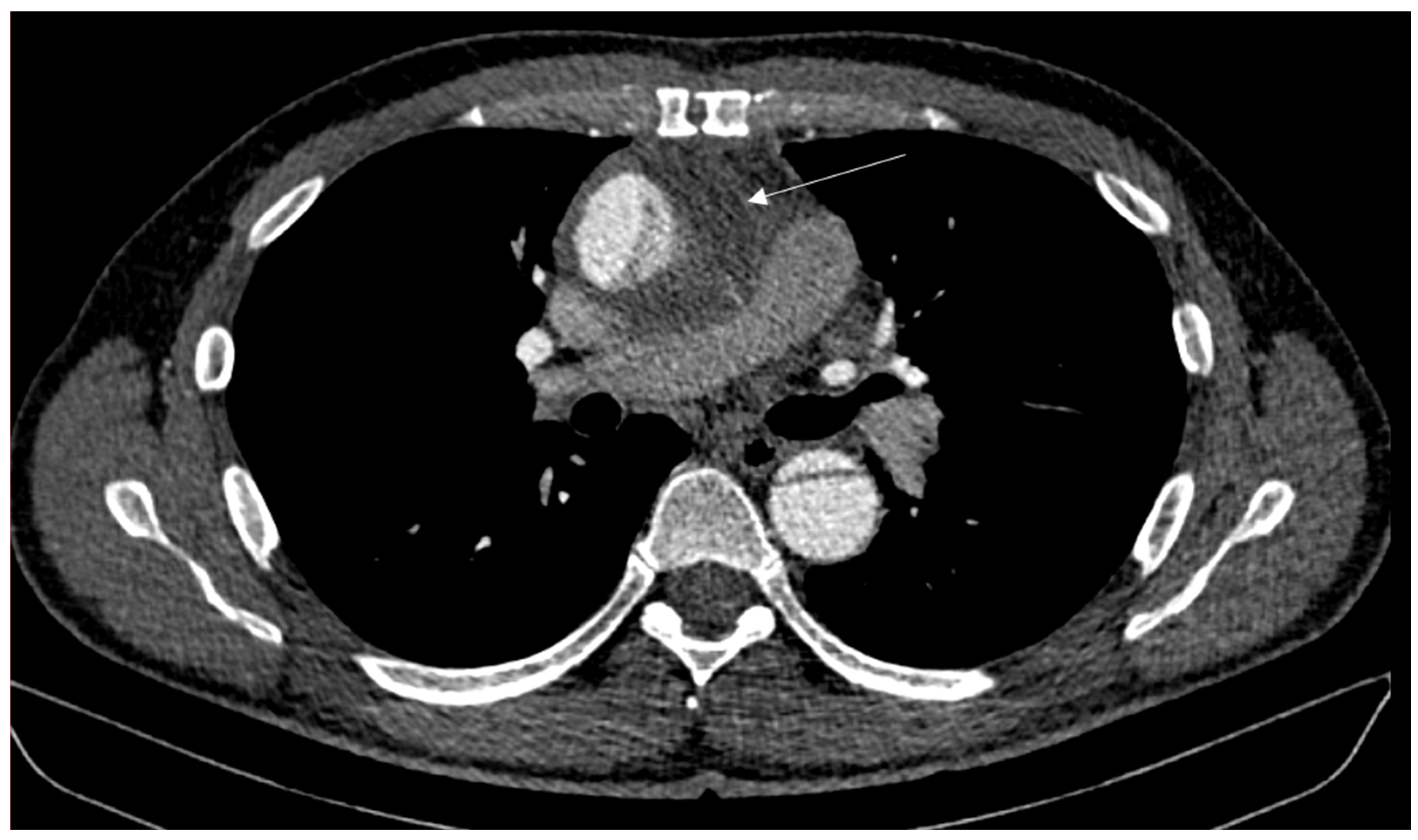
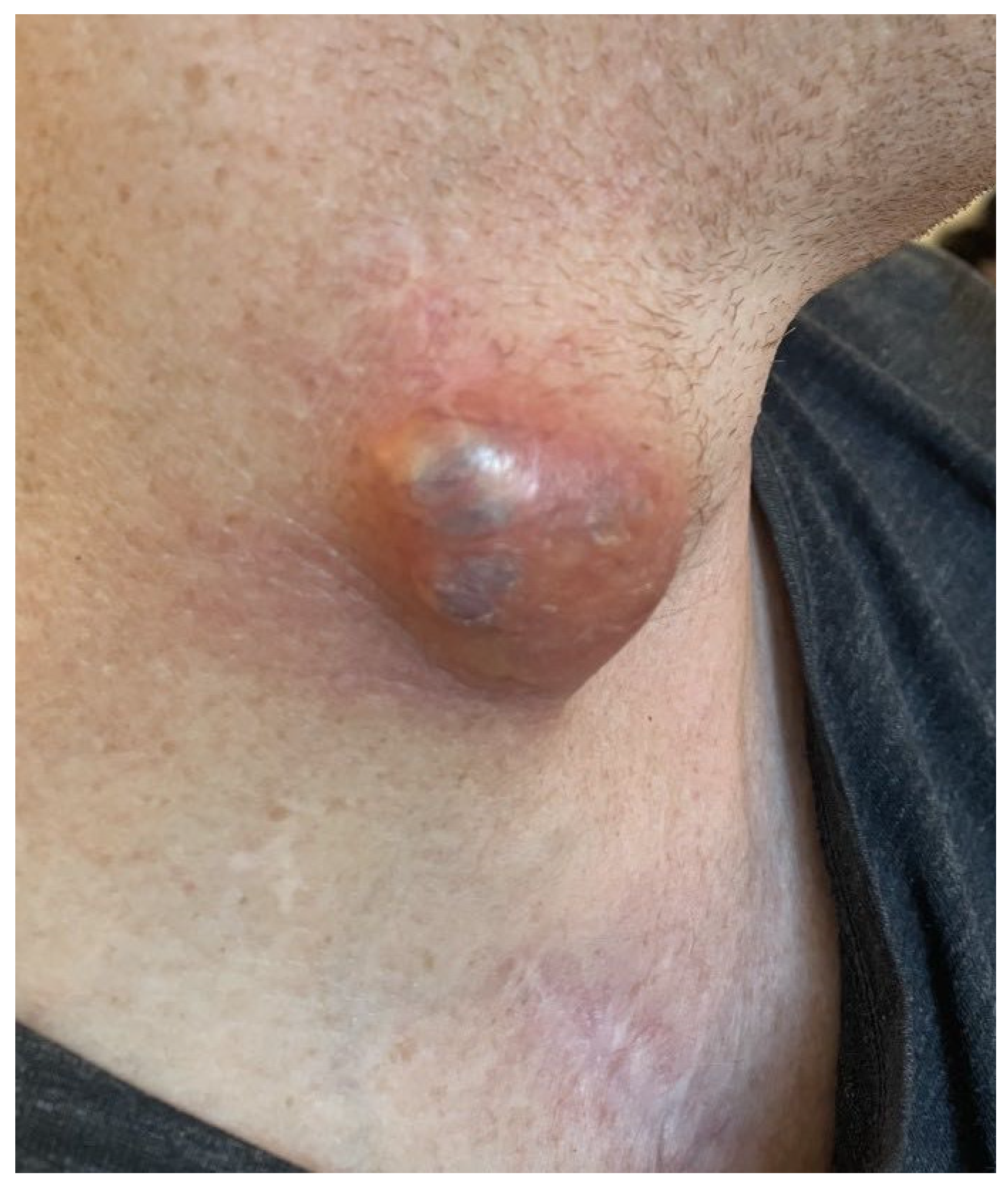
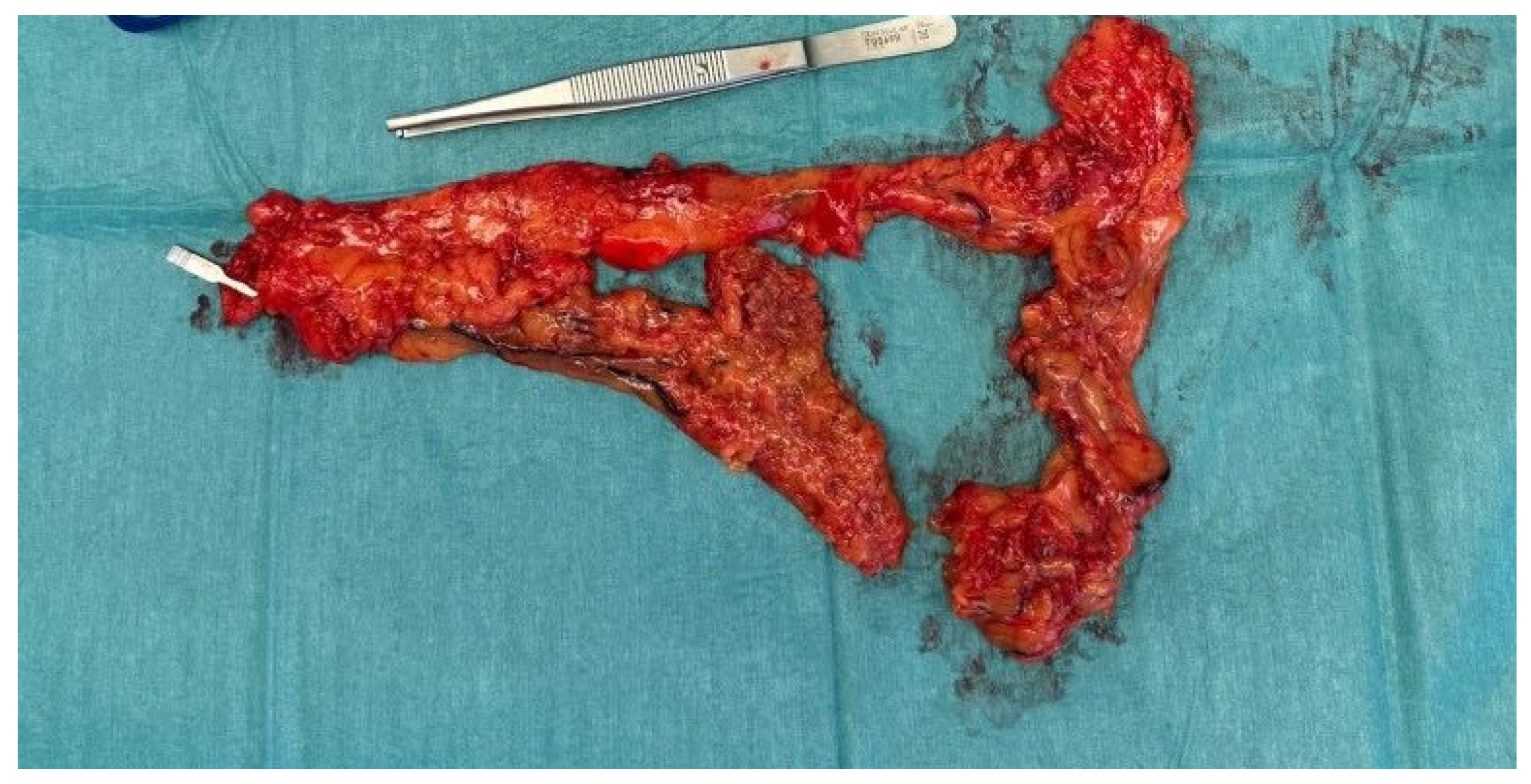
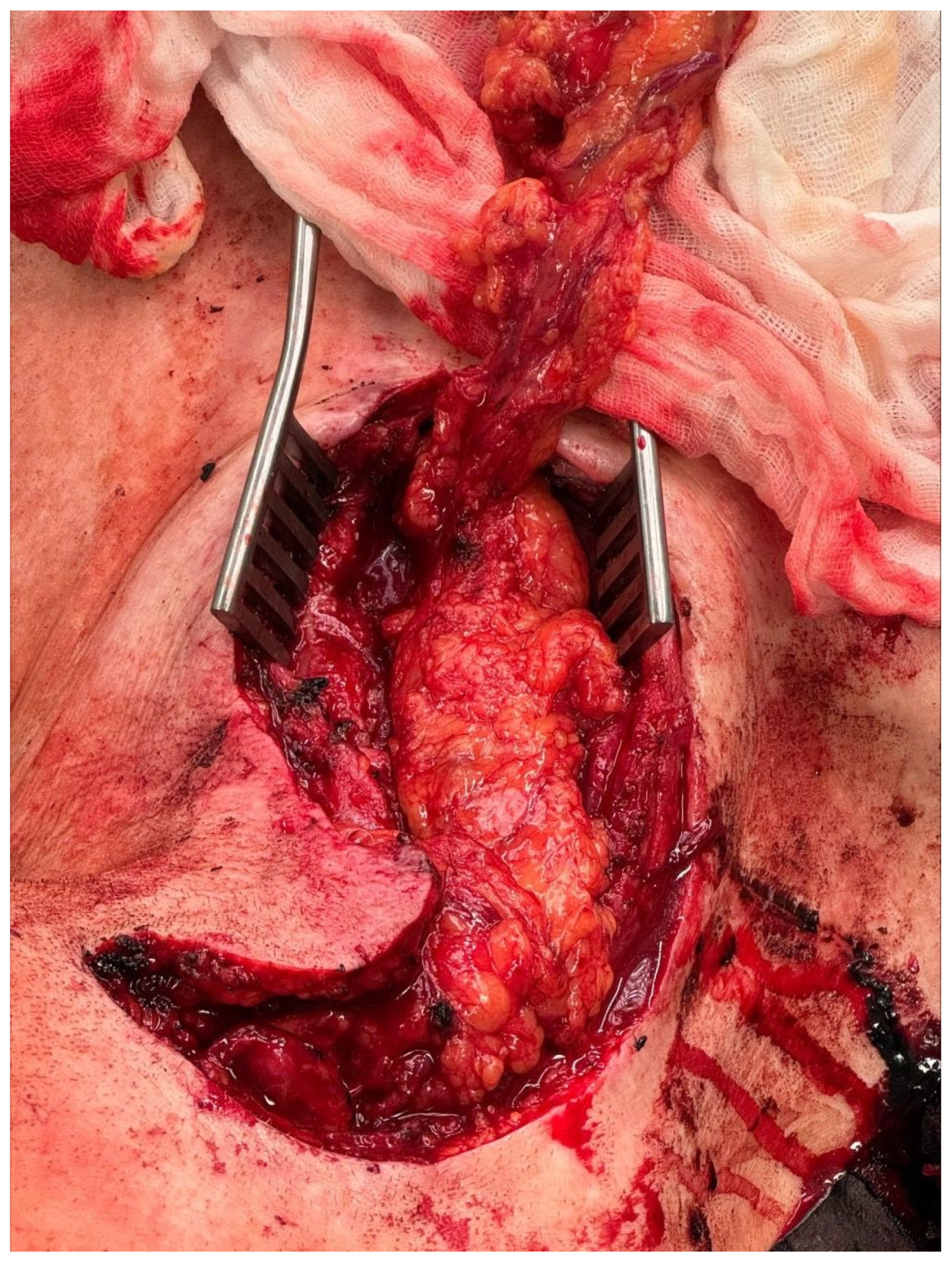
2. Case Report
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reisenauer, J.S.; Puig, C.A.; Reisenauer, C.J.; Allen, M.S.; Bendel, E.; Cassivi, S.D.; Nichols, F.C.; Shen, R.K.; Wigle, D.A.; Blackmon, S.H. Treatment of Postsurgical Chylothorax. Ann. Thorac. Surg. 2018, 105, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Majdalany, B.S.; El-Haddad, G. Contemporary lymphatic interventions for post-operative lymphatic leaks. Transl. Androl. Urol. 2020, 9, S104–S113. [Google Scholar] [CrossRef] [PubMed]
- Spiro, J.D.; Spiro, R.H.; Strong, E.W. The management of chyle fistula. Laryngoscope 1990, 100, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Garrett, H.E.J.; Richardson, J.W.; Howard, H.S.; Garrett, H.E. Retroperitoneal lymphocele after abdominal aortic surgery. J. Vasc. Surg. 1989, 10, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Ninomiya, M.; Kobayashi, J.; Kaneko, Y. VATS thoracic-duct division for aortic surgery-related chylous leakage. Eur. J. Cardio-Thorac. Surg. 2005, 27, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Spyt, T. Chylothorax and wound lymphocele formation as a complication of myocardial revascularization with the internal thoracic artery. J. Thorac. Cardiovasc. Surg. 1994, 108, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Cevese, P.G.; Vecchioni, R.; D’Amico, D.F.; Cordiano, C.; Biasiato, R.; Favia, G.; Farello, G.A. Postoperative chylothorax. Six cases in 2,500 operations, with a survey of the world literature. J. Thorac. Cardiovasc. Surg. 1975, 69, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Borulu, F.; Erkut, B. Successful Conservative Treatment of Chylopericardium after Open-Heart Surgery: A Case Report. J. Tehran Univ. Heart Cent. 2020, 15, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Seelig, M.H.; Klingler, P.J.; Oldenburg, W. Treatment of a postoperative cervical chylous lymphocele by percutaneous sclerosing with povidone-iodine. J. Vasc. Surg. 1998, 27, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Laccourreye, O.; Espinoza, S.; Mukundian, S.; Bonfils, P. Prevention and cure of lymphorrhea and lymphocele after cervical lymph-node surgery. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2013, 130, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Yu, D.; Chen, J.; Shi, X.; Su, L.; Ye, Q.; Ding, Y.-T. Lymphocele: A clinical analysis of 19 cases. Int. J. Clin. Exp. Med. 2015, 8, 7342–7350. [Google Scholar] [PubMed]
- Antao, B.; Croaker, D.; Squire, R. Successful management of congenital chyloperitoneum with fibrin glue. J. Pediatr. Surg. 2003, 38, E7–E8. [Google Scholar] [CrossRef] [PubMed]
- Casler, J.D.; Brietzke, S.E. Repair of a high-output chylous fistula with a free fat graft. Laryngoscope 1998, 108, 938–940. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertel, N.; Dastagir, K.; Schmelzle, M.; Feldbrügge, L.; Helms, F.; Vogt, P.M.; Ruhparwar, A.; Popov, A.-F. Omentoplasty for Cervical Lymphocele after Aortic Arch Replacement. J. Clin. Med. 2024, 13, 4737. https://doi.org/10.3390/jcm13164737
Hertel N, Dastagir K, Schmelzle M, Feldbrügge L, Helms F, Vogt PM, Ruhparwar A, Popov A-F. Omentoplasty for Cervical Lymphocele after Aortic Arch Replacement. Journal of Clinical Medicine. 2024; 13(16):4737. https://doi.org/10.3390/jcm13164737
Chicago/Turabian StyleHertel, Nora, Khaled Dastagir, Moritz Schmelzle, Linda Feldbrügge, Florian Helms, Peter M. Vogt, Arjang Ruhparwar, and Aron-Frederik Popov. 2024. "Omentoplasty for Cervical Lymphocele after Aortic Arch Replacement" Journal of Clinical Medicine 13, no. 16: 4737. https://doi.org/10.3390/jcm13164737




