Prognostic Role of Tissue Iron Deficiency Measured by sTfR Levels in Heart Failure Patients without Systemic Iron Deficiency or Anemia
Abstract
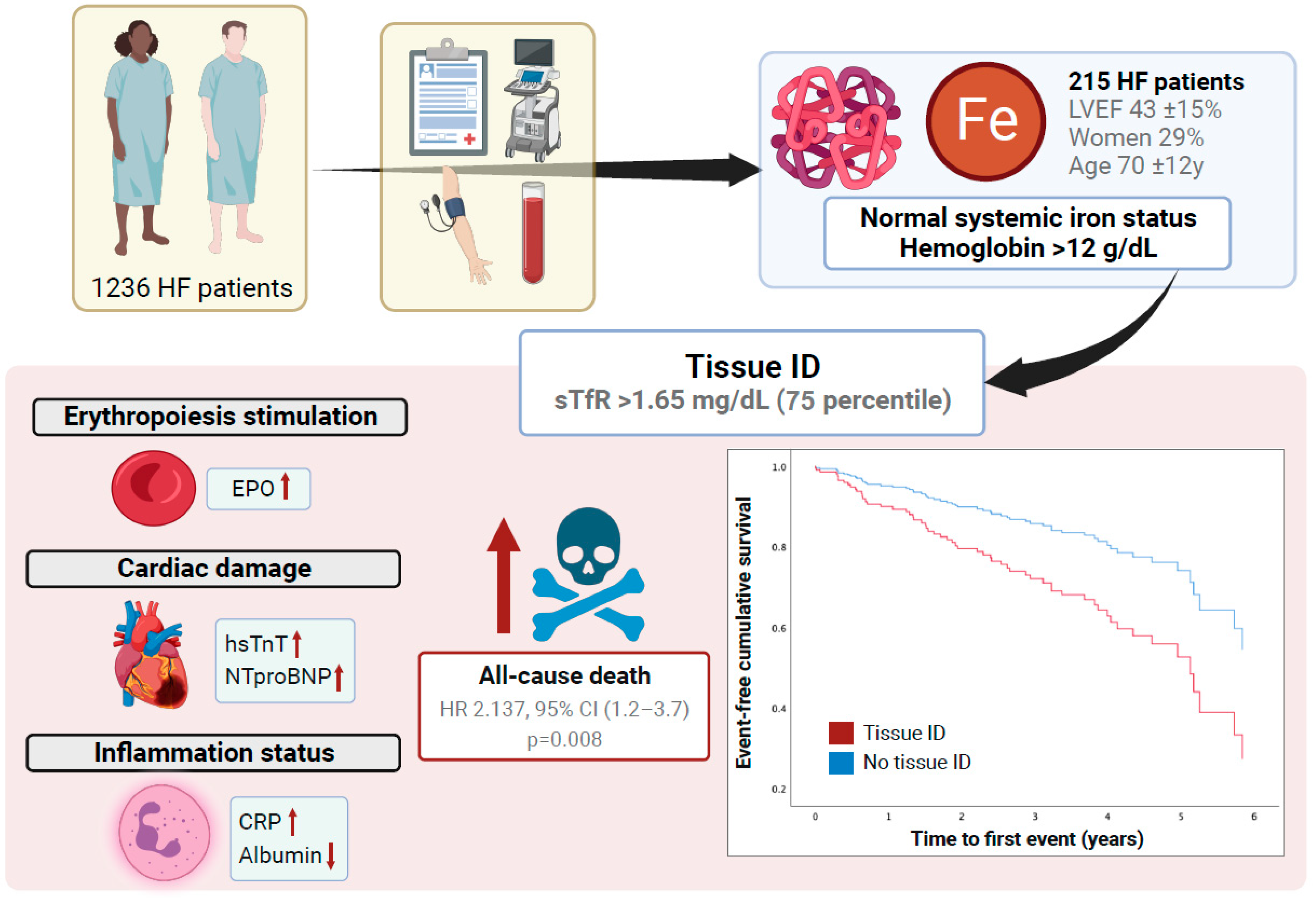
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Patient Characteristics
3.2. sTfR Association with Clinical Outcomes
3.3. sTfr Association with Cardiac, Hematinic, Inflammatory and RAAS Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.; Coats, A.J. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Beverborg, N.G. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Tkaczyszyn, M.; Drozd, M.; Ponikowski, P.; Jankowska, E.A. Iron deficiency in heart failure: A 2020 update. Kardiologia Polska 2019, 77, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2018, 73, 115–123. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ponikowski, P.; Van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Kirwan, B.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Heart Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef]
- Campodonico, J.; Nicoli, F.; Motta, I.; De Amicis, M.M.; Bonomi, A.; Cappellini, M.; Agostoni, P. Prognostic role of transferrin saturation in heart failure patients. Eur. J. Prev. Cardiol. 2021, 28, 1639–1646. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; Klip, I.T.; Meijers, W.C.; Voors, A.A.; Vegter, E.L.; van der Wal, H.H.; Swinkels, D.W.; van Pelt, J.; Mulder, A.B.; Bulstra, S.K.; et al. Definition of Iron Deficiency Based on the Gold Standard of Bone Marrow Iron Staining in Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004519. [Google Scholar] [CrossRef] [PubMed]
- Wish, J.B. Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin. J. Am. Soc. Nephrol. 2006, 1 (Suppl. S1), S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Suominen, P.; Punnonen, K.; Rajamäki, A.; Irjala, K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood 1998, 92, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Moliner, P.; Jankowska, E.A.; van Veldhuisen, D.J.; Farre, N.; Rozentryt, P.; Enjuanes, C.; Polonski, L.; Meroño, O.; Voors, A.A.; Ponikowski, P.; et al. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int. J. Cardiol. 2017, 243, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sierpinski, R.; Josiak, K.; Suchocki, T.; Wojtas-Polc, K.; Mazur, G.; Butrym, A.; Rozentryt, P.; van der Meer, P.; Comin-Colet, J.; von Haehling, S.; et al. High soluble transferrin receptor in patients with heart failure: A measure of iron deficiency and a strong predictor of mortality. Eur. J. Heart Fail. 2021, 23, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Ras-Jiménez, M.D.M.; Ramos-Polo, R.; Francesch Manzano, J.; Corbella Santano, M.; Morillas Climent, H.; Jose-Bazán, N.; Jiménez-Marrero, S.; Garcimartin Cerezo, P.; Yun Viladomat, S.; Moliner Borja, P.; et al. Soluble Transferrin Receptor as Iron Deficiency Biomarker: Impact on Exercise Capacity in Heart Failure Patients. J. Pers. Med. 2023, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Kowska-Florek, W.O.; Ski, R.Z.; Biegus, J.; Owski, P.S.; Banasiak, W.; et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur. Heart J. 2014, 35, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Gallego, M.; Díaz, L.E.; Rondó, P.H. Classic and emergent indicators for the assessment of human iron status. Crit. Rev. Food Sci. Nutr. 2021, 61, 2827–2840. [Google Scholar] [CrossRef]
- Leszek, P.; Sochanowicz, B.; Szperl, M.; Kolsut, P.; Brzóska, K.; Piotrowski, W.; Rywik, T.M.; Danko, B.; Polkowska-Motrenko, H.; Różański, J.M.; et al. Myocardial iron homeostasis in advanced chronic heart failure patients. Int. J. Cardiol. 2012, 159, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, C.; Zhao, C.; Chen, G.; Meng, S.; Hong, M.; Xiang, M.; Xie, Y. Increased Serum Soluble Transferrin Receptor Levels Were Associated With High Prevalence of Cardiovascular Diseases: Insights From the National Health and Nutrition Examination Survey 2017–2018. Front. Cell Dev. Biol. 2022, 10, 874846. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Moreno, J.M.; López-Bermejo, A.; Chico, B.; Vendrell, J.; Ricart, W.; Perseghin, G.; Lattuada, G.; De Cobelli, F.; Ragogna, F.; et al. Circulating soluble transferrin receptor according to glucose tolerance status and insulin sensitivity. Diabetes Care 2007, 30, 604–608. [Google Scholar] [CrossRef] [PubMed]


| Whole Cohort (n = 215) | No Tissue ID (sTfR < 1.65 mg/L) (n = 161) | Tissue ID (sTfR ≥ 1.65 mg/L) (n = 54) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 70 (12) | 69 (12) | 73 (12) | 0.072 |
| Sex (female), n (%) | 62 (29%) | 39 (24%) | 23 (43%) | 0.010 |
| Systolic blood pressure, mmHg | 125 (24) | 126 (24) | 123 (25) | 0.366 |
| Heart rate, bpm | 73 (15) | 72 (14) | 78 (17) | 0.010 |
| NYHA Functional Class, n (%) | 0.020 | |||
| I | 44 (21%) | 39 (24%) | 5 (9%) | |
| II | 112 (53%) | 82 (52%) | 30 (57%) | |
| III | 46 (22%) | 33 (21%) | 13 (24%) | |
| IV | 11 (5%) | 5 (3%) | 6 (11%) | |
| 6 min walking test, meters | 287 (168) | 314 (155) | 206 (179) | <0.001 |
| BMI, Kg/m2 | 28 (6) | 28 (6) | 28 (6) | 0.849 |
| HF hospitalization previous year, n (%) | 172 (80%) | 126 (79%) | 46 (85%) | 0.303 |
| LVEF, % | 43 (15) | 43 (15) | 43 (16) | 0.942 |
| Comorbidities | ||||
| Ischaemic etiology of HF, n (%) | 64 (30%) | 44 (27%) | 20 (37%) | 0.177 |
| Hypertension, n (%) | 156 (73%) | 114 (71%) | 42 (78%) | 0.321 |
| Diabetes Mellitus, n (%) | 68 (32%) | 54 (34%) | 14 (26%) | 0.298 |
| Obesity, n (%) | 58 (27%) | 43 (27%) | 15 (28%) | 0.878 |
| Previous MI, n (%) | 35 (16%) | 24 (15%) | 11 (20%) | 0.347 |
| CKD, n (%) | 90 (42%) | 63 (40%) | 27 (50%) | 0.182 |
| Treatment | ||||
| ACEI or ARBs, n (%) | 185 (86%) | 139 (86%) | 46 (85%) | 0.833 |
| Beta-blockers, n (%) | 191 (89%) | 142 (88%) | 49 (91%) | 0.608 |
| MRA, n (%) | 90 (42%) | 69 (43%) | 21 (39%) | 0.609 |
| Diuretics, n (%) | 195 (91%) | 142 (88%) | 53 (98%) | 0.029 |
| Antiplatelet therapy, n (%) | 79 (37%) | 64 (40%) | 15 (28%) | 0.114 |
| Anticoagulant therapy, n (%) | 110 (51%) | 75 (47%) | 35 (65%) | 0.020 |
| Laboratory | ||||
| Hemoglobin, g/dL | 14.1 (1.4) | 14.2 (1.3) | 14.0 (1.5) | 0.447 |
| Creatinine, mg/dL | 1.2 (0.4) | 1.1 (0.3) | 1.3 (0.5) | 0.047 |
| Estimated glomerular filtration rate, mL/min/kg | 67 (26) | 70 (26) | 60 (25) | 0.018 |
| Serum proteins, g/dL | 6.9 (0.7) | 6.9 (0.7) | 6.8 (0.7) | 0.701 |
| sTFR (mg/L) | 1.42 (0.7) | 1.15 (0.2) | 2.25 (0.82) | 0.000 |
| All-Cause Death | |||
|---|---|---|---|
| Measures of Tissue ID | HR | 95% CI | p-Value |
| sTfR, 1 mg/L | 1.484 | 1.125–1.958 | 0.005 |
| sTfR > 75th percentile (1.63 mg/L) | 2.137 | 1.218–3.749 | 0.008 |
| Heart Failure Hospitalization | |||
| Measures of Tissue ID | HR | 95% CI | p-Value |
| sTfR, 1 mg/L | 1.241 | 0.876–1.759 | 0.225 |
| sTfR > 75th percentile (1.63 mg/L) | 1.436 | 0.762–2.678 | 0.260 |
| Whole Cohort (n = 215) | No Tissue ID (sTfR < 1.65 mg/L) (n = 161) | Tissue ID (sTfR ≥ 1.65 mg/L) (n = 54) | p-Value | |
|---|---|---|---|---|
| Laboratory Values | ||||
| NT-proBNP, pg/mL (median, IQR) | 1125 (587–2668) | 1031 (496–2329) | 1768 (916–4130) | 0.016 |
| Troponin, ng/mL (median, IQR) | 0.010 (0.009–0.034) | 0.010 (0.009–0.031) | 0.012 (0.010–0.012) | 0.190 |
| Serum proteins, g/dL | 6.9 (0.7) | 6.9 (0.7) | 6.8 (0.7) | 0.701 |
| Serum albumin, g/dL | 4.0 (0.6) | 4.1 (0.5) | 3.9 (0.7) | 0.024 |
| Ferritin, ng/mL (median, IQR) | 249 (154–432) | 268 (166–440) | 232 (130–296) | 0.326 |
| TSAT, % | 30 (10) | 30 (9) | 29 (10) | 0.233 |
| Serum iron, ug/dL | 102 (41) | 103 (35) | 98 (57) | 0.509 |
| Erythropoietin, mUI/mL (median, IQR) | 10 (6–17) | 9 (6–16) | 11 (8–19) | 0.010 |
| ACE activity, U/L (median, IQR) | 12 (7.2–21.5) | 12 (6–19) | 12 (11–24) | 0.498 |
| Plasmatic renin activity, ng/mL/h (median, IQR) | 3.7 (1.2–15.7) | 3.3 (1.2–22.1) | 4.8 (1.4–11.5) | 0.844 |
| Aldosterone, pg/mL (median, IQR) | 74.5 (33.0–148.3) | 65 (32–156) | 93 (44–93) | 0.450 |
| C-reactive protein, mg/dL (median, IQR) | 0.42 (0.20–1.10) | 0.40 (0.20–0.90) | 0.72 (0.20–2.15) | 0.429 |
| Cardiac Biomarkers | |||||
|---|---|---|---|---|---|
| Univariate Linear Regression Models | Multivariate Linear Regression Models | ||||
| Measures of Tissue ID | Standardized β Coefficient | p-Value | Standardized β Coefficient | p-Value | R Model |
| Troponin | |||||
| sTfR (1 mg/L) | 0.136 | 0.131 | 0.088 | 0.333 | 0.041 |
| sTfR > 75th percentile (1.65 mg/L) | 0.039 | 0.664 | 0.014 | 0.104 | 0.030 |
| NTproBNP | |||||
| sTfR (1 mg/L) | 0.177 | 0.009 | 0.168 | 0.014 | 0.036 |
| sTfR > 75th percentile (1.65 mg/L) | 0.127 | 0.062 | 0.112 | 0.045 | 0.020 |
| Renin–Angiotensin–Aldosterone System Biomarkers | |||||
| Univariate Linear Regression Models | Multivariate Linear Regression Models | ||||
| Measures of Tissue ID | Standardized β Coefficient | p-Value | Standardized β Coefficient | p-Value | R Model |
| Aldosterone | |||||
| sTfR (1 mg/L) | 0.095 | 0.168 | 0.109 | 0.113 | 0.029 |
| sTfR > 75th percentile (1.65 mg/L) | 0.057 | 0.407 | 0.076 | 0.035 | 0.022 |
| Serum ACE Activity | |||||
| sTfR (1 mg/L) | 0.037 | 0.589 | 0.039 | 0.154 | 0.005 |
| sTfR > 75th percentile (1.65 mg/L) | 0.049 | 0.482 | 0.069 | 0.225 | 0.005 |
| Plasma Renin Activity | |||||
| sTfR (1 mg/L) | −0.014 | 0.845 | −0.015 | 0.421 | 0.003 |
| sTfR > 75th percentile (1.65 mg/L) | 0.026 | 0.705 | 0.009 | 0.784 | −0.011 |
| Inflammation Biomarkers | |||||
| Univariate Linear Regression Models | Multivariate Linear Regression Models | ||||
| Measures of Tissue ID | Standardized β Coefficient | p-Value | Standardized β Coefficient | p-Value | R Model |
| C-reactive Protein | |||||
| sTfR (1 mg/L) | 0.303 | <0.001 | 0.294 | <0.001 | 0.097 |
| sTfR > 75th percentile (1.65 mg/L) | 0.189 | 0.006 | 0.174 | 0.005 | 0.041 |
| Albumin | |||||
| sTfR (1 mg/L) | −0.172 | 0.012 | −0.155 | <0.001 | 0.068 |
| sTfR > 75th percentile (1.65 mg/L) | −0.154 | 0.024 | −0.131 | <0.001 | 0.061 |
| Cellular Response to Hypoxia | |||||
| Univariate Linear Regression Models | Multivariate Linear Regression Models | ||||
| Measures of Tissue ID | Standardized β Coefficient | p-Value | Standardized β Coefficient | p-Value | R Model |
| Erythropoietin | |||||
| sTfR (1 mg/L) | 0.180 | 0.008 | 0.176 | 0.003 | 0.044 |
| sTfR > 75th percentile (1.65 mg/L) | 0.150 | 0.028 | 0.148 | 0.009 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Polo, R.; Ras-Jiménez, M.d.M.; Francesch Manzano, J.; Jovells-Vaqué, S.; Morillas Climent, H.; Pons-Riverola, A.; Yun Viladomat, S.; Moliner Borja, P.; Diez-Lopez, C.; González-Costello, J.; et al. Prognostic Role of Tissue Iron Deficiency Measured by sTfR Levels in Heart Failure Patients without Systemic Iron Deficiency or Anemia. J. Clin. Med. 2024, 13, 4742. https://doi.org/10.3390/jcm13164742
Ramos-Polo R, Ras-Jiménez MdM, Francesch Manzano J, Jovells-Vaqué S, Morillas Climent H, Pons-Riverola A, Yun Viladomat S, Moliner Borja P, Diez-Lopez C, González-Costello J, et al. Prognostic Role of Tissue Iron Deficiency Measured by sTfR Levels in Heart Failure Patients without Systemic Iron Deficiency or Anemia. Journal of Clinical Medicine. 2024; 13(16):4742. https://doi.org/10.3390/jcm13164742
Chicago/Turabian StyleRamos-Polo, Raúl, Maria del Mar Ras-Jiménez, Josep Francesch Manzano, Silvia Jovells-Vaqué, Herminio Morillas Climent, Alexandra Pons-Riverola, Sergi Yun Viladomat, Pedro Moliner Borja, Carles Diez-Lopez, José González-Costello, and et al. 2024. "Prognostic Role of Tissue Iron Deficiency Measured by sTfR Levels in Heart Failure Patients without Systemic Iron Deficiency or Anemia" Journal of Clinical Medicine 13, no. 16: 4742. https://doi.org/10.3390/jcm13164742
APA StyleRamos-Polo, R., Ras-Jiménez, M. d. M., Francesch Manzano, J., Jovells-Vaqué, S., Morillas Climent, H., Pons-Riverola, A., Yun Viladomat, S., Moliner Borja, P., Diez-Lopez, C., González-Costello, J., Garcia-Romero, E., Herrador, L., de Frutos Seminario, F., Enjuanes Grau, C., Tajes Orduña, M., & Comin-Colet, J. (2024). Prognostic Role of Tissue Iron Deficiency Measured by sTfR Levels in Heart Failure Patients without Systemic Iron Deficiency or Anemia. Journal of Clinical Medicine, 13(16), 4742. https://doi.org/10.3390/jcm13164742






