The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration and PICO Strategy
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Selection Process
2.5. Data Collection Process
2.6. Risk of Bias
- Random sequence generation;
- Allocation concealment;
- Blinding of participants and personnel;
- Blinding of outcome assessment (patient-reported outcomes);
- Blinding of outcome assessment (all-cause mortality);
- Incomplete outcomes (short-term [2–6 weeks]);
- Incomplete outcomes (long-term [>6 weeks]);
- Selective outcome reporting.
2.7. Data Synthesis and Meta-Analysis
2.8. Certainty of Evidence
3. Results
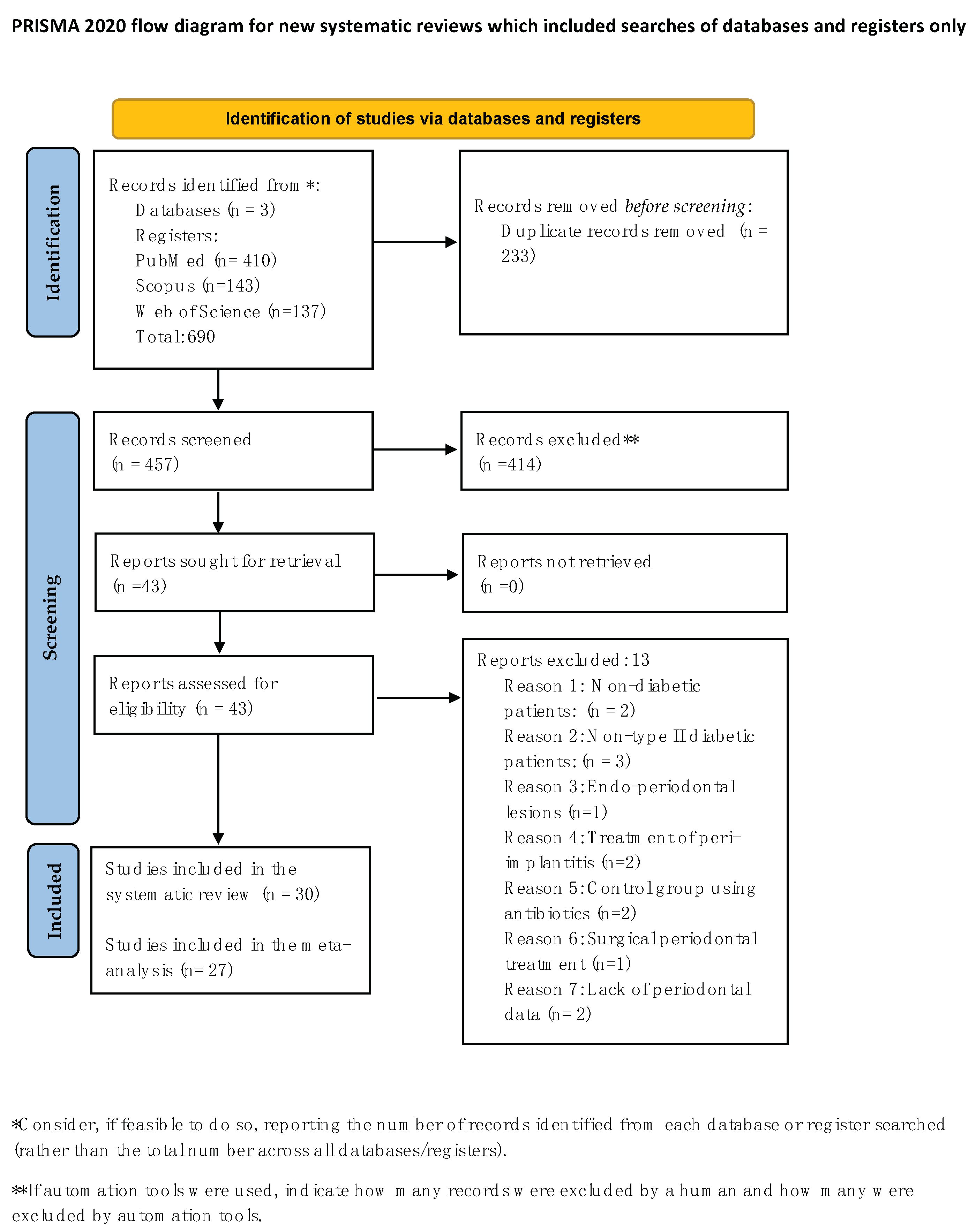
3.1. Selection of Studies
3.2. General Characteristics of the Included Studies
3.3. Description and Results of Studies with the Combination of Topical Antibiotics
3.4. Description and Results of Studies with the Combination of Systemic Antibiotics
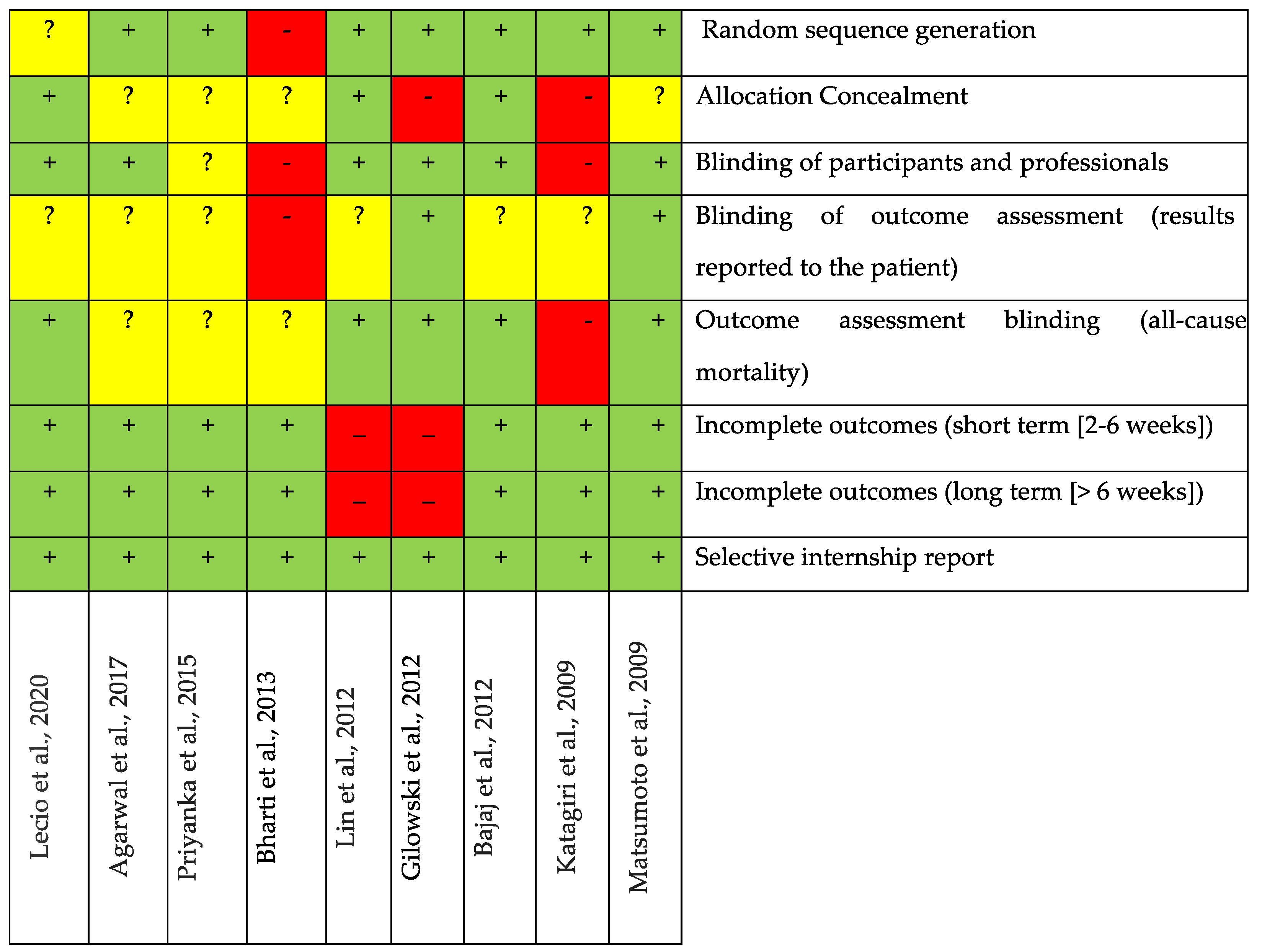
3.5. Bias Analysis
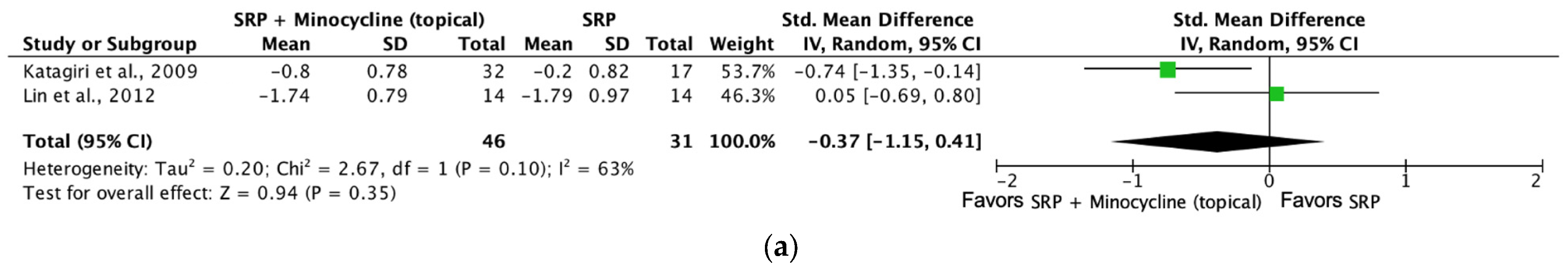
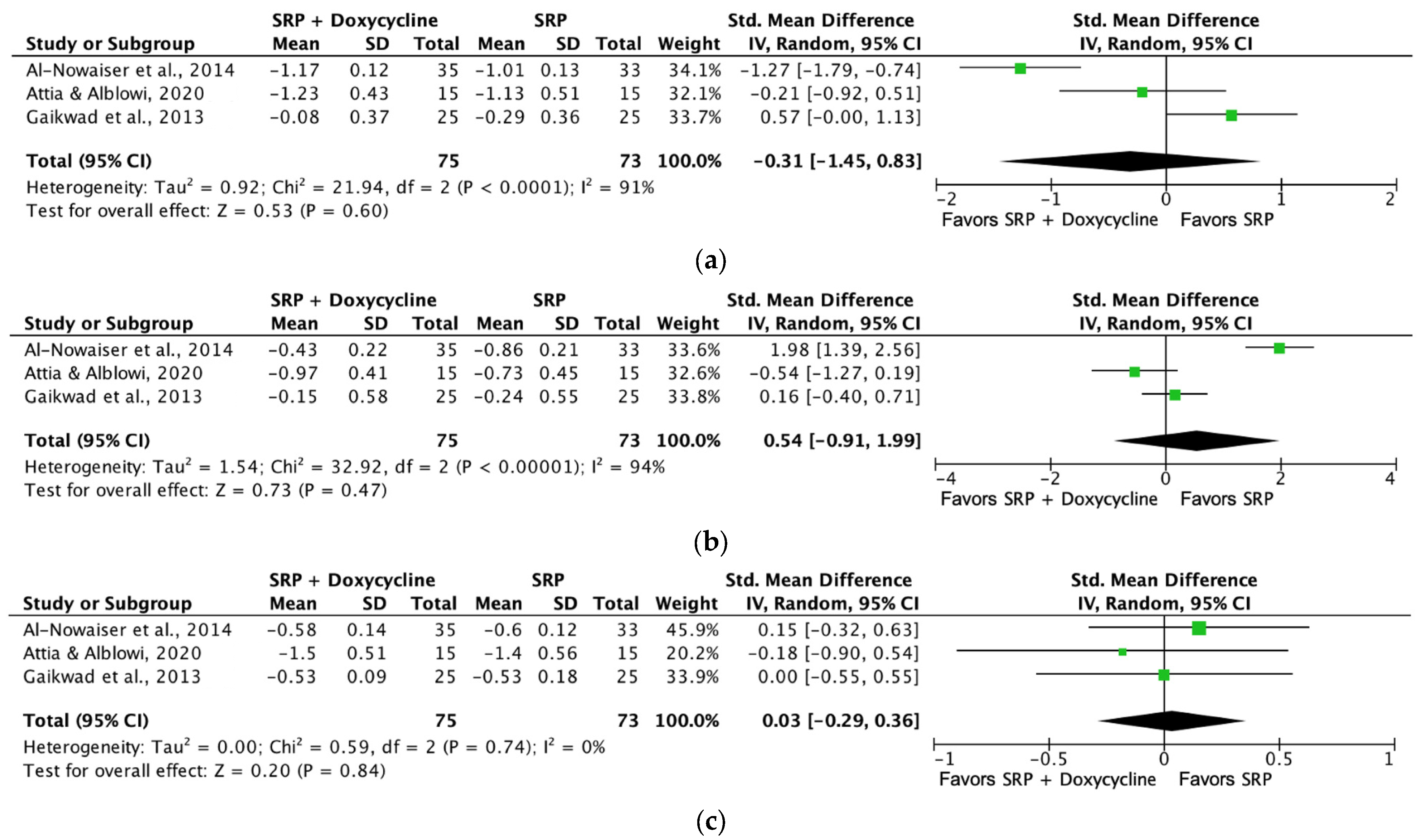
3.6. Main Results and Meta-Analysis
3.7. Certainty of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, A.D. Classification and Diagnosis of Diabetes. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; de Avila, E.D.; Cirelli, J.A.; Steffens, J.P. Periodontal research contributions to basic sciences: From cell communication and host-parasite interactions to inflammation and bone biology. Biocell 2022, 46, 633–638. [Google Scholar] [CrossRef]
- Rosa, R.A.C.; Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Mulinari-Santos, G.; Cirelli, T.; de Molon, R.S.; Gouveia Garcia, V.; Theodoro, L.H. The Relationship between Hypertension and Periodontitis: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 5140. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.V.S.; Claudio, M.M.; Franciscon, J.P.S.; Rosa, R.A.C.; Cirelli, T.; de Molon, R.S.; Figueredo, C.M.S.; Garcia, V.G.; Theodoro, L.H. The Effect of Non-Surgical Periodontal Treatment on Patients with Combined Refractory Arterial Hypertension and Stage III, Grade B Periodontitis: A Preliminary Prospective Clinical Study. J. Clin. Med. 2023, 12, 4277. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Suvan, J.E.; D’Aiuto, F. Periodontitis and metabolic diseases (diabetes and obesity): Tackling multimorbidity. Periodontol. 2000 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rios-Carrasco, B.; Monteiro, L.; Lopez-Jarana, P.; Carneiro, F.; Relvas, M. Association between Type 1 Diabetes Mellitus and Periodontal Diseases. J. Clin. Med. 2023, 12, 1147. [Google Scholar] [CrossRef]
- Tamiya, H.; Mitani, A.; Abe, M.; Nagase, T. Putative Bidirectionality of Chronic Obstructive Pulmonary Disease and Periodontal Disease: A Review of the Literature. J. Clin. Med. 2023, 12, 5935. [Google Scholar] [CrossRef]
- de Aquino, S.G.; Abdollahi-Roodsaz, S.; Koenders, M.I.; van de Loo, F.A.; Pruijn, G.J.; Marijnissen, R.J.; Walgreen, B.; Helsen, M.M.; van den Bersselaar, L.A.; de Molon, R.S.; et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J. Immunol. 2014, 192, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Liu, R.; Alikhani, M.; Al-Mashat, H.; Trackman, P.C. Diabetes-enhanced inflammation and apoptosis—Impact on periodontal pathology. J. Dent. Res. 2006, 85, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Qorri, E.; Converti, I.; Lorusso, F.; Delvecchio, M.; Gnoni, A.; Scacco, S.; Scarano, A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhan, Q.; Wu, C.Z.; Yuan, Y.H.; Chen, W.; Yu, F.Y.; Li, Y.; Li, L.J. Baseline HbA1c Level Influences the Effect of Periodontal Therapy on Glycemic Control in People with Type 2 Diabetes and Periodontitis: A Systematic Review on Randomized Controlled Trails. Diabetes Ther. 2021, 12, 1249–1278. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Xue, M.; Al-Motawa, M.; Thornalley, P.J.; Rabbani, N. Glycolytic overload-driven dysfunction of periodontal ligament fibroblasts in high glucose concentration, corrected by glyoxalase 1 inducer. BMJ Open Diabetes Res. Care 2020, 8, e001458. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Karatas, O.; Tulu, F.; Altan, A.; Gevrek, F. Effect of diabetes on collagen metabolism and hypoxia in human gingival tissue: A stereological, histopathological, and immunohistochemical study. Biotech. Histochem. 2019, 94, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.Y.; Wang, Q.X.; Chen, Q.M. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Negrini, T.C.; Carlos, I.Z.; Duque, C.; Caiaffa, K.S.; Arthur, R.A. Interplay Among the Oral Microbiome, Oral Cavity Conditions, the Host Immune Response, Diabetes Mellitus, and Its Associated-Risk Factors-An Overview. Front. Oral Health 2021, 2, 697428. [Google Scholar] [CrossRef]
- Genco, R.J.; Graziani, F.; Hasturk, H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol. 2000 2020, 83, 59–65. [Google Scholar] [CrossRef]
- Pavanelli, A.L.R.; de Menezes, B.S.; Pereira, E.B.B.; de Souza Morais, F.A.; Cirelli, J.A.; de Molon, R.S. Pharmacological Therapies for the Management of Inflammatory Bone Resorption in Periodontal Disease: A Review of Preclinical Studies. Biomed. Res. Int. 2022, 2022, 5832009. [Google Scholar] [CrossRef]
- Da Ponte Leguizamon, N.; de Molon, R.S.; Coletto-Nunes, G.; Nogueira, A.V.B.; Rocha, S.V.; Neo-Justino, D.M.; Soares-Costa, A.; Cerri, P.S.; Lerner, U.H.; Souza, P.P.C.; et al. Phytocystatin CsinCPI-2 Reduces Osteoclastogenesis and Alveolar Bone Loss. J. Dent. Res. 2022, 101, 216–225. [Google Scholar] [CrossRef]
- Sahni, V.; Van Dyke, T.E. Immunomodulation of periodontitis with SPMs. Front. Oral Health 2023, 4, 1288722. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Methodol, E.W.P. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60, Erratum in J. Clin. Periodontol. 2021, 48, 164–165. [Google Scholar] [CrossRef]
- Grossi, S.G.; Skrepcinksi, F.B.; DeCaro, T.; Robertson, D.C.; Ho, A.W.; Dunford, R.G.; Genco, R.J. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J. Periodontol. 1997, 68, 713–719. [Google Scholar] [CrossRef]
- Das, A.C.; Das, S.J.; Panda, S.; Sharma, D.; Taschieri, S.; Fabbro, M.D. Adjunctive Effect of Doxycycline with Conventional Periodontal Therapy on Glycemic Level for Chronic Periodontitis with Type 2 Diabetes Mellitus Subjects. J. Contemp. Dent. Pract. 2019, 20, 1417–1423. [Google Scholar]
- El-Makaky, Y.; Shalaby, H.K. The effects of non-surgical periodontal therapy on glycemic control in diabetic patients: A randomized controlled trial. Oral Dis. 2020, 26, 822–829. [Google Scholar] [CrossRef]
- Vergnes, J.N.; Canceill, T.; Vinel, A.; Laurencin-Dalicieux, S.; Maupas-Schwalm, F.; Blasco-Baqué, V.; Hanaire, H.; Arrivé, E.; Rigalleau, V.; Nabet, C.; et al. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. J. Clin. Periodontol. 2018, 45, 1150–1163. [Google Scholar] [CrossRef]
- Szulc, M.; Zakrzewska, A.; Zborowski, J. Local drug delivery in periodontitis treatment: A review of contemporary literature. Dent. Med. Probl. 2018, 55, 333–342. [Google Scholar] [CrossRef]
- Mahuli, S.A.; Zorair, A.M.; Jafer, M.A.; Sultan, A.; Sarode, G.; Baeshen, H.A.; Raj, A.T.; Sarode, S.; Patil, S. Antibiotics for Periodontal Infections: Biological and Clinical Perspectives. J. Contemp. Dent. Pract. 2020, 21, 372–376. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. 2021. Available online: https://training.cochrane.org/handbook/current (accessed on 1 September 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Grp, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- GDT, G. GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.). 2020. Available online: https://www.gradepro.org/ (accessed on 1 September 2022).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of recommendations. Updated October 2013. The GRADE Working Group. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 September 2022).
- Murad, M.H.; Mustafa, R.A.; Schunemann, H.J.; Sultan, S.; Santesso, N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid. Based Med. 2017, 22, 85–87. [Google Scholar] [CrossRef]
- Santesso, N.; Glenton, C.; Dahm, P.; Garner, P.; Akl, E.A.; Alper, B.; Brignardello-Petersen, R.; Carrasco-Labra, A.; De Beer, H.; Hultcrantz, M.; et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020, 119, 126–135. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Agarwal, E.; Bajaj, P.; Naik, S.B.; Pradeep, A.R. Locally Delivered 0.5% Azithromycin as an Adjunct to Non-Surgical Treatment in Patients With Chronic Periodontitis With Type 2 Diabetes: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1281–1287. [Google Scholar] [CrossRef]
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Kumari, M.; Naik, S.B. Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well-controlled type 2 diabetes: A randomized controlled clinical trial. J. Investig. Clin. Dent. 2012, 3, 276–283. [Google Scholar] [CrossRef]
- Priyanka, N.; Kalra, N.; Saquib, S.; Malgaonkar, N.; Tarakji, B.; Varsha, J.; Pradeep, A.R. Efficacy of Subgingivally Delivered Satranidazole in the Treatment of Type 2 Diabetes Subjects with Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Int. Acad. Periodontol. 2015, 17, 42–48. [Google Scholar]
- Lin, S.J.; Tu, Y.K.; Tsai, S.C.; Lai, S.M.; Lu, H.K. Non-surgical periodontal therapy with and without subgingival minocycline administration in patients with poorly controlled type II diabetes: A randomized controlled clinical trial. Clin. Oral Investig. 2012, 16, 599–609. [Google Scholar] [CrossRef]
- Bharti, P.; Katagiri, S.; Nitta, H.; Nagasawa, T.; Kobayashi, H.; Takeuchi, Y.; Izumiyama, H.; Uchimura, I.; Inoue, S.; Izumi, Y. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes. Res. Clin. Pract. 2013, 7, e129–e138. [Google Scholar] [CrossRef]
- Katagiri, S.; Nitta, H.; Nagasawa, T.; Uchimura, I.; Izumiyama, H.; Inagaki, K.; Kikuchi, T.; Noquchi, T.; Kanazawa, M.; Matsuo, A.; et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res. Clin. Pract. 2009, 83, 308–315. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ogawa, H.; Soda, S.; Hirayama, S.; Amarasena, N.; Aizawa, Y.; Miyazaki, H. Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J. Clin. Periodontol. 2009, 36, 142–148. [Google Scholar] [CrossRef]
- Gilowski, L.; Kondzielnik, P.; Wiench, R.; Plocica, I.; Strojek, K.; Krzeminski, T.F. Efficacy of short-term adjunctive subantimicrobial dose doxycycline in diabetic patients-randomized study. Oral Dis. 2012, 18, 763–770. [Google Scholar] [CrossRef]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.V.C.; Nociti, F.; Moura, L.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Al-Nowaiser, A.M.; Al-Zoman, H.; Baskaradoss, J.K.; Robert, A.A.; Al-Zoman, K.H.; Al-Sohail, A.M.; Al-Suwyed, A.S.; Ciancio, S.G.; Al-Mubarak, S.A. Evaluation of adjunctive systemic doxycycline with non-surgical periodontal therapy within type 2 diabetic patients. Saudi Med. J. 2014, 35, 1203–1209. [Google Scholar]
- Al-Zahrani, M.S.; Bamshmous, S.O.; Alhassani, A.A.; Al-Sherbini, M.M. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J. Periodontol. 2009, 80, 1568–1573. [Google Scholar] [CrossRef]
- Attia, M.S.; Alblowi, J.A. Effect of Subantimicrobial Dose Doxycycline Treatment on Gingival Crevicular Fluid Levels of MMP-9 and MMP-13 in Periodontitis Stage 2, Grade B in Subjects with Type 2 Diabetes Mellitus. J. Immunol. Res. 2020, 2020, 2807259. [Google Scholar] [CrossRef]
- Botero, J.E.; Yepes, F.L.; Ochoa, S.P.; Hincapie, J.P.; Roldan, N.; Ospina, C.A.; Castrillon, C.A.; Becerra, M.A. Effects of periodontal non-surgical therapy plus azithromycin on glycemic control in patients with diabetes: A randomized clinical trial. J. Periodontal Res. 2013, 48, 706–712. [Google Scholar] [CrossRef]
- Engebretson, S.P.; Hey-Hadavi, J. Sub-antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: A pilot study. Pharmacol. Res. 2011, 64, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Hincapie, J.P.; Castrillon, C.A.; Yepes, F.L.; Roldan, N.; Becerra, M.A.; Moreno, S.M.; Consuegra, J.; Contreras, A.; Botero, J.E. Microbiological effects of periodontal therapy plus azithromycin in patients with diabetes: Results from a randomized clinical trial. Acta Odontol. Latinoam. 2014, 27, 89–95. [Google Scholar] [PubMed]
- Gaikwad, S.P.; Gurav, A.N.; Shete, A.R.; Desarda, H.M. Effect of scaling and root planing combined with systemic doxycycline therapy on glycemic control in diabetes mellitus subjects with chronic generalized periodontitis: A clinical study. J. Periodontal Implant. Sci. 2013, 43, 79–86. [Google Scholar] [CrossRef]
- Promsudthi, A.; Pimapansri, S.; Deerochanawong, C.; Kanchanavasita, W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005, 11, 293–298. [Google Scholar] [CrossRef]
- Tsalikis, L.; Sakellari, D.; Dagalis, P.; Boura, P.; Konstantinidis, A. Effects of doxycycline on clinical, microbiological and immunological parameters in well-controlled diabetes type-2 patients with periodontal disease: A randomized, controlled clinical trial. J. Clin. Periodontol. 2014, 41, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, H.; Huo, P.; Jin, D.; Zhu, Y.; Meng, H. Effects of amoxicillin and metronidazole as an adjunct to scaling and root planing on glycemic control in patients with periodontitis and type 2 diabetes: A short-term randomized controlled trial. J. Periodontal Res. 2024, 59, 249–258. [Google Scholar] [CrossRef]
- Miranda, T.S.; Feres, M.; Perez-Chaparro, P.J.; Faveri, M.; Figueiredo, L.C.; Tamashiro, N.S.; Bastos, M.F.; Duarte, P.M. Metronidazole and amoxicillin as adjuncts to scaling and root planing for the treatment of type 2 diabetic subjects with periodontitis: 1-year outcomes of a randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2014, 41, 890–899. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.A.A.; Taba, M.; Nomizo, A.; Freitas, M.C.F.; Suaid, F.A.; Uyemura, S.A.; Trevisan, G.L.; Novaes, A.B.; Souza, S.L.S.; Palioto, D.B.; et al. Effects of periodontal therapy on glycemic control and inflammatory markers. J. Periodontol. 2008, 79, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.C.; Taba, M.; Novaes, A.B.; Souza, S.L.S.; Grisi, M.F.M. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J. Periodontol. 2004, 75, 780. [Google Scholar] [CrossRef]
- Tamashiro, N.S.; Duarte, P.M.; Miranda, T.S.; Maciel, S.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Amoxicillin Plus Metronidazole Therapy for Patients with Periodontitis and Type 2 Diabetes: A 2-year Randomized Controlled Trial. J. Dent. Res. 2016, 95, 829–836. [Google Scholar] [CrossRef]
- Cruz, D.F.D.; Duarte, P.M.; Figueiredo, L.C.; da Silva, H.D.P.; Retamal-Valdes, B.; Feres, M.; Miranda, T.S. Metronidazole and amoxicillin for patients with periodontitis and diabetes mellitus: 5-year secondary analysis of a randomized controlled trial. J. Periodontol. 2021, 92, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Mrag, M.; Khalji, Y.; Alhodhodi, A.; Shadia, A.E.; Ayed, Y.; Kassab, A. Adjunctive systemic antibiotic effect on periodontal state, salivary enzyme activity, and glycemia imbalance in type-2 diabetics after non-surgical periodontal management. Libyan J. Med. 2023, 18, 2222449. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Oshikiri, S.; Nagano, T.; Yashima, A.; Matsushima, Y.; Shirakawa, S.; Komatsu, K.; Mokubo, A.; Gomi, K. Effects of One-Stage Full-Mouth Scaling and Root Planing with Azithromycin on Diabetes and Periodontal Disease: A Randomized Controlled Trial. Antibiotics 2022, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Bokhari, S.A.H.; Haque, Z.; Baloch, A.A.; Zaheer, S. Clinical efficacy of scaling and root planing with and without metronidazole on glycemic control: Three-arm randomized controlled trial. BMC Oral Health 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; Feres, M.; Yassine, L.L.S.; Soares, G.M.S.; Miranda, T.S.; Faveri, M.; Retamal-Valdes, B.; Figueiredo, L.C. Clinical and microbiological effects of scaling and root planing, metronidazole and amoxicillin in the treatment of diabetic and non-diabetic subjects with periodontitis: A cohort study. J. Clin. Periodontol. 2018, 45, 1326–1335. [Google Scholar] [CrossRef]
- Gómez-Sandoval, J.R.; Robles-Cervantes, J.A.; Hernández-González, S.O.; Espinel-Bermudez, M.C.; Mariaud-Schmidt, R.; Martínez-Rodríguez, V.; Morgado-Castillo, K.C.; Mercado-Sesma, A.R. Efficacy of clindamycin compared with amoxicillin-metronidazole after a 7-day regimen in the treatment of periodontitis in patients with diabetes: A randomized clinical trial. BMJ Open Diab Res. Care 2020, 8, e000665. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Sculean, A. Nonsurgical therapy for teeth and implants-When and why? Periodontol. 2000 2019, 79, 15–21. [Google Scholar] [CrossRef]
- Liew, A.K.C.; Punnanithinont, N.; Lee, Y.C.; Yang, J. Effect of non-surgical periodontal treatment on HbA1c: A meta-analysis of randomized controlled trials. Aust. Dent. J. 2013, 58, 350–357. [Google Scholar] [CrossRef]
- Sakellari, D.; Goodson, J.M.; Socransky, S.S.; Kolokotronis, A.; Konstantinidis, A. Concentration of 3 tetracyclines in plasma, gingival crevice fluid and saliva. J. Clin. Periodontol. 2000, 27, 53–60. [Google Scholar] [CrossRef]
- Bastendorf, K.D.; Strafela-Bastendorf, N.; Lussi, A. Mechanical Removal of the Biofilm: Is the Curette Still the Gold Standard? Monogr. Oral Sci. 2021, 29, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.L.S.; Rovai, E.S.; Ganhito, J.A.; Holzhausen, M.; Chambrone, L.; Pannuti, C.M. Efficacy of systemic antibiotics in nonsurgical periodontal therapy for diabetic subjects: A systematic review and meta-analysis. Int. Dent. J. 2018, 68, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Mugri, M.H. Efficacy of Systemic Amoxicillin-Metronidazole in Periodontitis Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Medicina 2022, 58, 1605. [Google Scholar] [CrossRef] [PubMed]
- Grellmann, A.P.; Sfreddo, C.S.; Maier, J.; Lenzi, T.L.; Zanatta, F.B. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: A meta-analysis. J. Clin. Periodontol. 2016, 43, 250–260. [Google Scholar] [CrossRef]
- Rovai, E.S.; Souto, M.L.; Ganhito, J.A.; Holzhausen, M.; Chambrone, L.; Pannuti, C.M. Efficacy of Local Antimicrobials in the Non-Surgical Treatment of Patients With Periodontitis and Diabetes: A Systematic Review. J. Periodontol. 2016, 87, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.C.H.; Pulikkotil, S.J. Systemic doxycycline as an adjunct to scaling and root planing in diabetic patients with periodontitis: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Skaleric, U.; Schara, R.; Medvescek, M.; Hanlon, A.; Doherty, F.; Lessem, J. Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J. Int. Acad. Periodontol. 2004, 6, 160–165. [Google Scholar]
- Kanwar, I.; Sah, A.K.; Suresh, P.K. Biofilm-mediated Antibiotic-resistant Oral Bacterial Infections: Mechanism and Combat Strategies. Curr. Pharm. Des. 2017, 23, 2084–2095. [Google Scholar] [CrossRef]
- Mombelli, A. Heresy? Treatment of chronic periodontitis with systemic antibiotics only. J. Clin. Periodontol. 2006, 33, 661–662. [Google Scholar] [CrossRef]



| Study Country | Type of Study | Number of Patients | Interventions | Study Duration | Results |
|---|---|---|---|---|---|
| Matsumoto et al. (2009) [47] Japan | Randomized clinical trial | 21 individuals with DM2:
|
| 9 months | Clinical parameters of the test group turned out to be better. The % of sites with PPD ≥ 4 mm showed a reduction of 8.3% and 9.3% after the administration of the gel, compared to just 5% in the control group. |
| Katagiri et al. (2009) [46] Japan | Parallel to control | 49 individuals with DM2:
|
| 6 months | PPD and BoP improved in the 1st month compared to the baseline, and these improvements were maintained during the study period for the test group. In the control group, the parameters were somewhat reduced at 6 months compared to the beginning of treatment. |
| Lin et al. (2012) [44] Taiwan | Randomized controlled clinical trial | 28 individuals with DM2:
|
| 6 months | Both groups showed improvements in the periodontal levels of PPD, BoP, and CAL, as well as benefits in DM2 control, but without significant differences between them. |
| Bajaj et al. (2012) [42] India | Randomized controlled clinical trial | 56 individuals with DM2:
|
| 6 months | Both therapies resulted in significant clinical improvements. Test group demonstrated improved reductions in PI, GI, SBI, and PPD and gains in CAL after 6 months as compared to control. |
| Gilowski et al. (2012) [48] Poland | Randomized study | 34 individuals with DM2:
|
| 3 months | CAL, PPD, and BOP improved significantly in both groups after therapy. Difference between the two groups after therapy was seen in PPD in tooth sites with initial PPD ≥ 4 mm (SI + placebo: 3.41 ± 0.6 mm vs SI + doxycycline: 2.92 ± 0.5 mm, p < 0.05) |
| Bharti et al. (2013) [45] Japan | 29 individuals with DM2:
|
| 6 months | The test group demonstrated improvements in PPD and BoP starting at 2 months and continuing for 6 months. In the control group, PPD and BoP were not changed. BoP was significantly lower in the test group compared to the control group at baseline and after 6 months. | |
| Priyanka et al. (2015) [43] India | Randomized controlled clinical trial | 57 individuals with DM2:
|
| 6 months | PI and GI were reduced, albeit without significant difference between the two groups. GI and PPD were significantly reduced at 3 and 6 months compared to baseline. CAL gain was significantly greater in the test group compared to the control group at all periods. |
| Agarwal et al. (2017) [41] India | Randomized controlled clinical trial | 56 individuals with DM2:
|
| 9 months | Both therapies resulted in significant clinical improvements. Patients in the test group showed enhanced reductions in PI, GI, SBI, and PPD and gains in CAL over 9 months compared with the control group. |
| Lecio et al. (2020) [49] Brazil | Parallel, double-blind, placebo-controlled clinical trial | 40 individuals with DM2:
|
| 6 months | Both groups showed clinical improvement in all parameters after treatment (p < 0.05). Deep pockets showed improvements in BoP (3 and 6 months), PPD (at 3 months), and CAL gain (at 1 and 3 months). The percentage of sites presenting PPD reduction and CAL gain ≥ 2 mm was higher in the test group at 3 months. |
| Study Country | Type of Study | Number of Patients | Interventions | Study Duration | Results |
|---|---|---|---|---|---|
| Rodrigues et al. (2004) [62] Brazil | Parallel, double blind, control | 30 individuals with DM2:
|
| 3 months | Both groups presented clinical periodontal improvements. There was a reduction in PPD of 0.8 ± 0.6 mm in the test and 0.9 ± 0.4 mm in the control group. No significant changes in CAL were observed. At the 3-month mark, an improvement in PPD was recorded for both treatment groups. The test group showed a mean PPD reduction from 2.7 ± 0.7 mm to 1.9 ± 0.4 mm and the control group from 3.2 ± 0.8 mm to 2.3 ± 0.5 mm. No changes in CAL levels were recorded. Both groups presented an improvement in BoP. The test group showed a reduction from 38 ± 13% to 15 ± 9% and the control group from 32 ± 15% to 11 ± 7%. |
| Promsudth i et al. (2005) [57] Thailand | Parallel, double blind, placebo control | 52 individuals with DM2:
|
| 3 months | PPD and CAL were not different between the groups. However, the mean PPD and BoP in the test group were increased compared to the control. At 3 months, the clinical parameters of test group improved. All included subjects had decreased PPD, BoP, and PPD. CAL gain was observed in the control group with no significant changes in PPD, and BoP. The test group showed significantly shallower PPD and CAL than the control group. |
| O’Connell et al. (2008) [61] Brazil | Parallel, double blind, placebo control | 30 individuals with DM2:
|
| 3 months | PPD reductions of 0.8 mm for the control group and 1.1 mm for the test group were noted. Mean PPD reduction of 0.9 mm and a mean CAL gain of 0.7 mm were evidenced. PPD decreased 23%, and BoP decreased 38% in the test group. PPD and CAL reductions were 0.8 and 0.5 mm in the control group. BoP was not significant between the groups (34.9% for the control and 42.4% for the test group) after 3 months. The test and control groups evidenced significant reductions in PI scores (22.2% and 23.8% for control and test groups). |
| Al-Zahrani et al. (2009) [51] Saudi Arabia | Single-masked, randomized, controlled trial | 45 individuals with DM2:
|
| 12 weeks | Statistically significant differences in mean PPD, CAL, PI, and BoP were found between baseline and 12 weeks post-treatment for all groups. The groups were balanced (no statistically significant differences) regarding the levels of PPD, CAL, PI, and BoP (p > 0.05). The mean CAL were similar between males and females (p > 0.05). |
| Engebretson and Hey-Hadavi (2011) [54] Colombia | Randomized placebo-controlled pilot clinical trial | 45 individuals with DM2:
|
| 3 months | At one and three months’ follow-up, clinical periodontal parameters decreased in all groups. However, there were no statistical differences in clinical periodontal parameters between the groups. At three months, average HbA1c levels in the SDD group showed a 12.5% improvement. |
| Gaikwad et al. (2013) [56] India | Clinical study | 50 individuals with DM2:
|
| 4 months | Both the test group and control group showed significant improvement in periodontal parameters over the experimental periods. The mean PPD for the test group and control group at baseline, 1, 2, 3, and 4 months were reduced. PPD between the two groups was significantly different at 4 months (2.67 ± 0.32 and 2.82 ± 0.21). There was a greater reduction in CAL in the test group (2.69 ± 0.42) than in the control group (2.99 ± 0.49). PI showed a statistically significant difference between the two groups. The differences in GI between the test and control group were not significant at any interval. |
| Botero et al. (2013) [53] Colombia | Randomized clinical trial | 90 individuals with DM1 or DM2:
|
| 9 months | Periodontal parameters were improved in the test and control group when compared to the AZ-Pro group. Mean BoP, PPD, and CAL were improved in the test and control group compared to the AZ-Pro group. However, a decrease in PPD (median Δ0.71 mm; p < 0.05) and number of sites with PPD > 4 mm was observed in the test as compared to the control group (median Δ0.39 mm) at 9 months. Improvement in CAL was similar between the test and control groups. The reduction in PPD was subtle in all groups after 9 months. The AZ-Pro group demonstrated no improvements in PPD and CAL in the evaluated periods. |
| Miranda et al. (2014) [60] Brazil | Randomized placebo-controlled clinical trial | 56 individuals with DM2:
|
| 1 year | Clinical periodontal parameters were not different between groups at baseline. The test group showed lower mean PPD, mean number of sites with PPD ≥ 5 mm, decreased % of BoP, and suppuration compared to the control group. The test group presented a significant reduction in PPD and gain in CAL compared to placebo for moderate and deep sites. Reductions in the number of sites with PPD ≥ 5–6 mm were significantly greater in the test group compared to the control group. |
| Al-Nowaiser et al. (2014) [50] Saudi Arabia | Multi-center, randomized, parallel, single-blinded study | 76 individuals with DM2:
|
| 6 months | PPD was reduced by 0.93 mm in the test and 0.88 mm in the control group in the follow-up periods. CAL was reduced by 0.84 mm and by 0.96 mm in the period from baseline to 6 months in the test and control group, respectively. CAL was reduced by 0.39 mm in the test group and by 0.74 mm in the control group after 6 months’ follow-up. GI was not statistically significant between the test and control group after 1, 3, and 6 months after systemic doxycycline administration. |
| Hincapie et al. (2014) [55] Colombia | Randomized clinical trial | 90 individuals with DM2:
|
| 9 months | Periodontal status in all the allocated groups was not significantly different between groups. An increase in CAL gain was observed in the test and control group, while no improvement was observed in the AZPRO group. |
| Tsalikis et al. (2014) [58] Greece | Randomized controlled clinical trial | 66 individuals with DM2:
|
| 6 months | No differences were noted between the groups for the clinical parameters evaluated. A statistically significant improvement was observed from baseline to 3 months in both groups. However, only patients in the test group showed differences between 3 and 6 months. The numbers of sites displaying PPD > 5 mm with BoP were significantly decreased in the test group in the 6-month period. |
| Tamashiro et al. (2016) [63] Brazil | Randomized controlled clinical trial | 56 individuals with DM2:
|
| 2 years | The % of BoP, suppuration, and mean PPD were significantly lower in the test group at one and two years. The test group had significantly fewer sites with PPD ≥ 5 mm than the control at one and two years’ follow-up (control group = 14.7 ± 13.1, test group = 3.5 ± 3.4, p < 0.05). A greater reduction in mean PPD and CAL gain at moderate and deep sites were evidenced in the test group. |
| Vergnes et al. (2018) [28] France | Randomized controlled trial | 76 individuals with DM1 or DM2:
|
| 3 months | Clinical periodontal status improved significantly after the treatment. PPD, CAL, and BoP improved significantly 3 months after SRP for type 1 and type 2 DM patients. No differences were noted in periodontal improvements according to diabetes duration and treatment (p > 0.05). |
| Das et al. (2019) [26] India | Randomized, parallel-arm, double-centered clinical trial | 51 individuals with DM2:
| Group 1: OHI + SRP
| 3 months | Periodontal parameters were significantly higher in groups 1 and 2 compared to group 3 at 90 days. Mean PI decreased by 47.3% on day 90 (1.04 ± 0.28). The mean difference in PI in group 2 was 1.33 (53.20%). PI in group 3 increased by 0.06 (2.46%). GI in group 1 decreased by 45.0%. GI reduction in group 2 was 46.08%. GI in group 3 increased by 0.03 (2.46%). PPD in group 1 decreased by 24.67% (3.08 ± 0.30 and 2.32 ± 0.28). Mean PPD in group 2 decreased by 30.51%. PPD in group 3 increased by 0.10 from day 0 to 90 (2.53%). CAL reduced by 0.73 (19.26%) and 0.93 mm (24.73%) in groups 1 and 2, respectively. CAL in group 3 increased by 0.07 mm. |
| El-Makaky and Shalaby (2020) [27] Egypt | Randomized controlled trial | 88 individuals with DM2:
|
| 3 months | PI, PPD, BoP, and CAL were significantly decreased from baseline to 3 months in the test group. PI, PPD, BoP, and CAL were significantly increased from baseline to the end of the follow-up period in the control. Differences between the studied groups at 3 months were statistically significant. |
| Attia and Alblowi (2020) [52] Saudi Arabia | 30 individuals with DM2:
|
| 3 months | The mean GI reduction in the control group was −0:73 ± 0:45 and −0:67 ± 0:55 at 1 and 3 months, respectively. The test group showed a decrease in GI by −0:97 ± 0:41 and − 1:27 ± 0:74 at 1 and 3 months, respectively. These differences were significant between the groups. PI within the control and test groups showed significant reductions between baseline, 1, and 3 months. Mean PPD was reduced throughout the study period in the control (−1:37 ± 0:61) and test group (−1:40 ± 0:50), without differences between them. | |
| Cruz et al. (2021) [64] Brazil | Randomized controlled trial | 58 individuals with DM2:
|
| 5 years | PI, BoP, PPD, CAL, and sites with PPD ≥ 5 mm in the test group showed decreased values at 5 years’ follow-up compared to baseline. The percentage of sites with BoP was reduced at 5 years in the control group. The mean number and percentage of sites with PPD≥ 5 mm were significantly lower in the test group than in the control group at 2 years, but not at 5 years. |
| Qureshi et al. (2021) [67] Pakistan | Three-arm randomized controlled trial | 150 individuals with DM2:
|
| 6 months | BoP, PPD, and CAL were significantly reduced after 6 months. No differences between the groups were observed. Mean PPD significantly increased in the control group, whereas BoP and mean CAL remained unaffected. There was a significant reduction observed in all periodontal variables in the test groups with respect to the control. However, there were no significant differences in periodontal variables between the two test groups. |
| Komatsu et al. (2022) [66] Japan | Randomized controlled trial | 46 individuals with DM2:
|
| 9 months | The control group exhibited no substantial alterations in comparison to the initial values across all the clinical parameters assessed (PPD, BoP, and GI). Conversely, the test group manifested noteworthy enhancements in clinical parameters in relation to the baseline measurements at 1, 3, 6, and 9 months (p < 0.001). Moreover, the test group demonstrated significant progress when compared to the control group at every time interval. |
| Mrag et al. (2023) [65] Egypt | Single-center cross-sectional study | 125 individuals with DM2:
|
| 3 months | A significant decrease in periodontal indices was observed in the test group. Compared to baseline, the control group did not show a significant decrease in periodontal indices. The test group showed a significant reduction in periodontal indices. Furthermore, pairwise comparison between the control group and test group showed a significant decrease in periodontal indices following antibiotic therapy. |
| Xu et al. (2024) [59] China | Short-term randomized controlled trial | 49 individuals with DM2:
|
| 3 months | Periodontal parameters improved significantly and similarly in both groups after treatment. The test group had more sites of improvement than the control group when the initial PPD was >6 mm. After treatment, the mean PPD decreased from 4.85 ± 0.97 mm to 3.74 ± 0.98 mm in the control group and from 4.86 ± 0.93 mm to 3.65 ± 0.96 mm in the test group. Additionally, the mean GI and PI values decreased and CAL significantly increased in both groups after treatment. When the initial PPD was >6 mm, the test group had more sites of improvement than the control group (698 sites [78.96%] vs. 545 sites [73.35%]). |
| Outcomes | Certainty Assessment | Summary of Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants (Studies) | Risk of Bias | Inconsistency | Indirect Evidence | Imprecision | Overall Certainty of Evidence | Intervention | Comparator | Standard Mean Difference (CI95%) | |
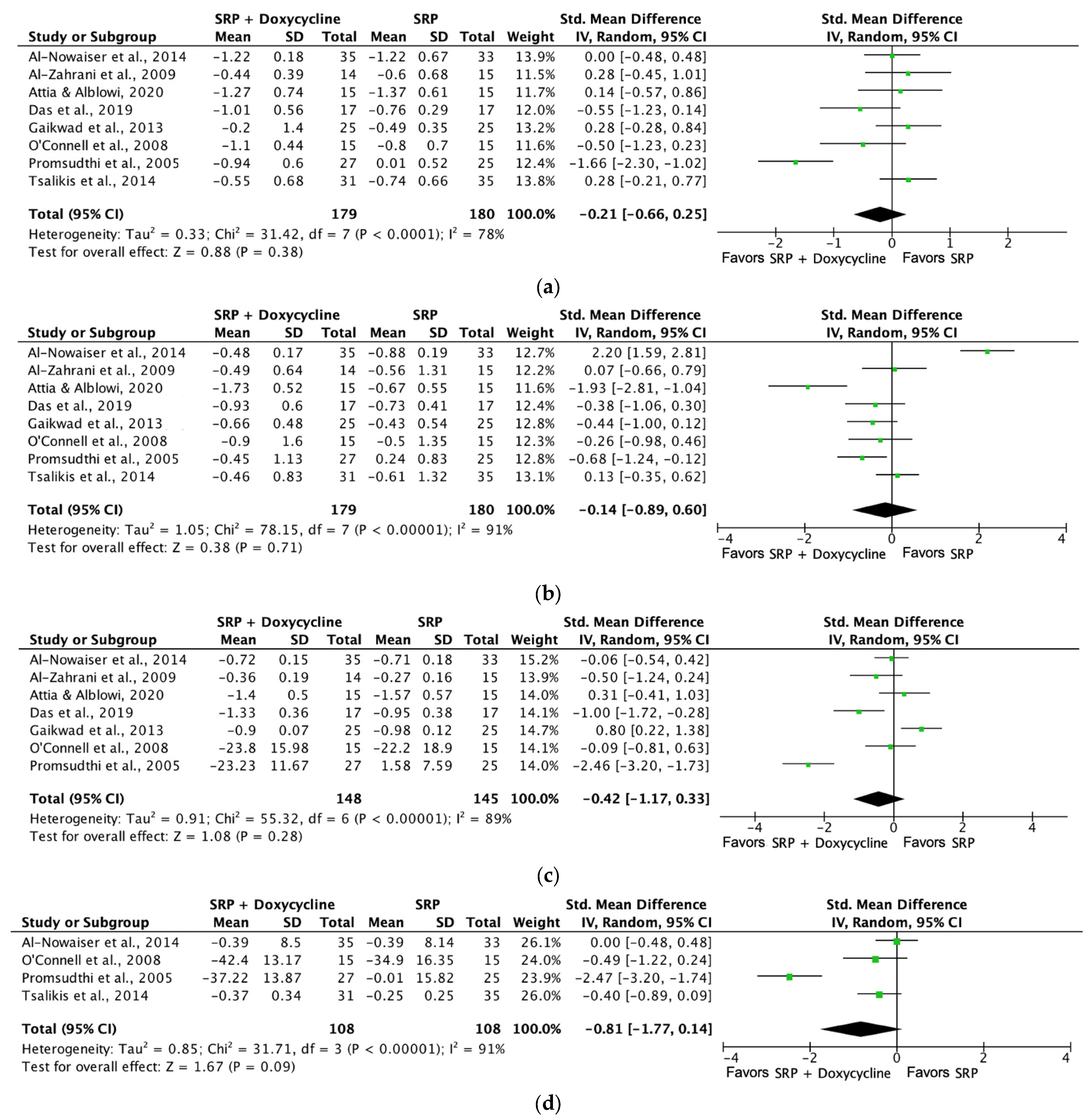
| Plaque index (SRP + doxycycline [1 month]) | 148 (3 RCTs) | Serious a | Not serious | Not serious | Not serious | ⨁⨁⨁◯ Moderate a | 75 | 73 | SMD 0.3 SD (−0.29 to 0.36) |
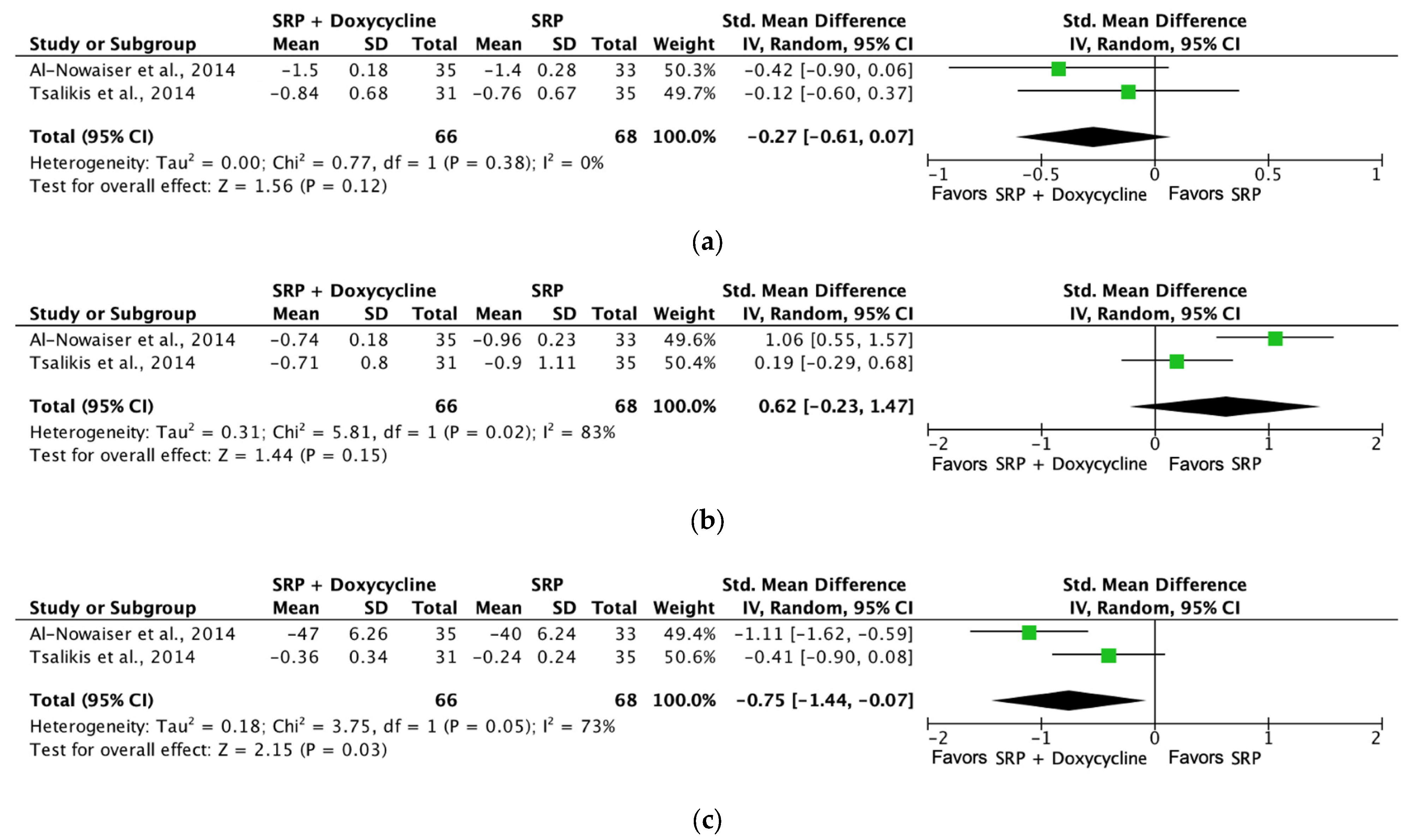
| Probing depth (SRP + doxycycline [6 months]) | 134 (2 RCTs) | Not serious | Not serious | Not serious | Not serious | ⨁⨁⨁⨁ High | 66 | 68 | SMD −0.27 SD (−0.61 to 0.07) |
| Bleeding on probing (SRP + doxycycline [6 months]) | 134 (2 RCTs) | Not serious | Not serious | Not serious | Not serious | ⨁⨁⨁⨁ High | 66 | 68 | SMD −0.75 SD (−1.44 to −0.07) |
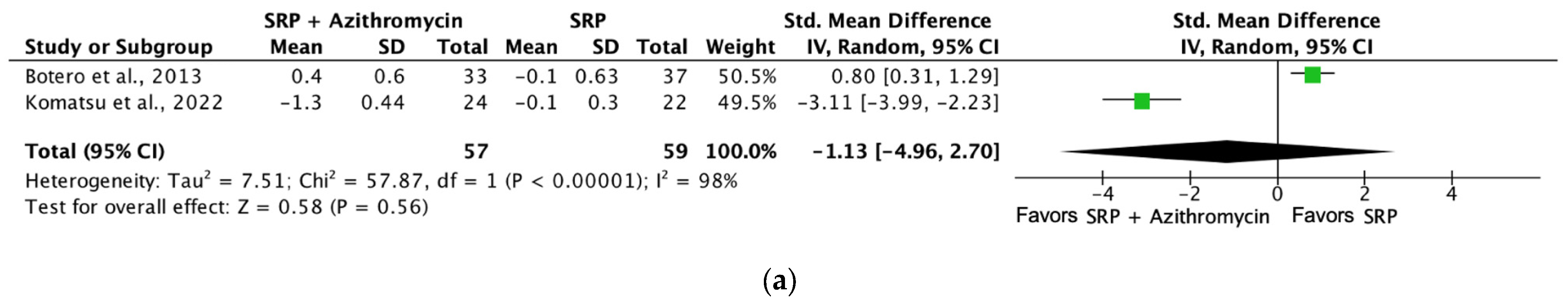
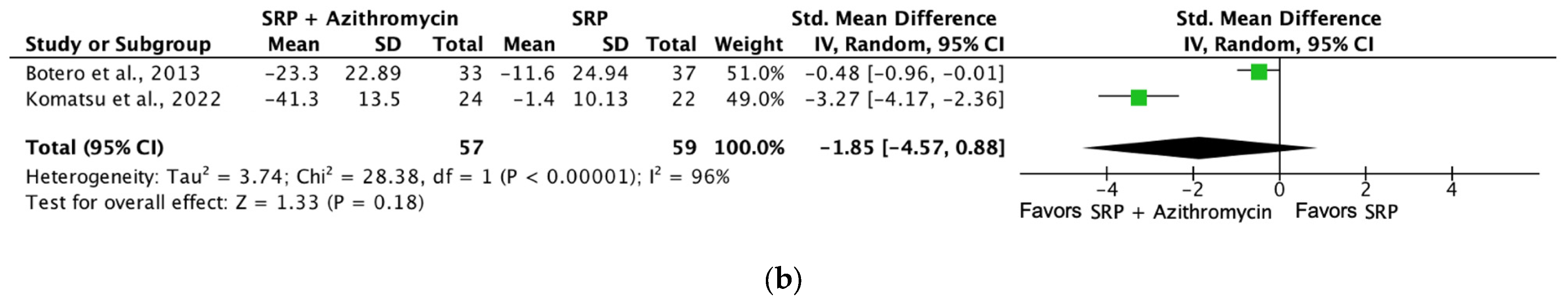
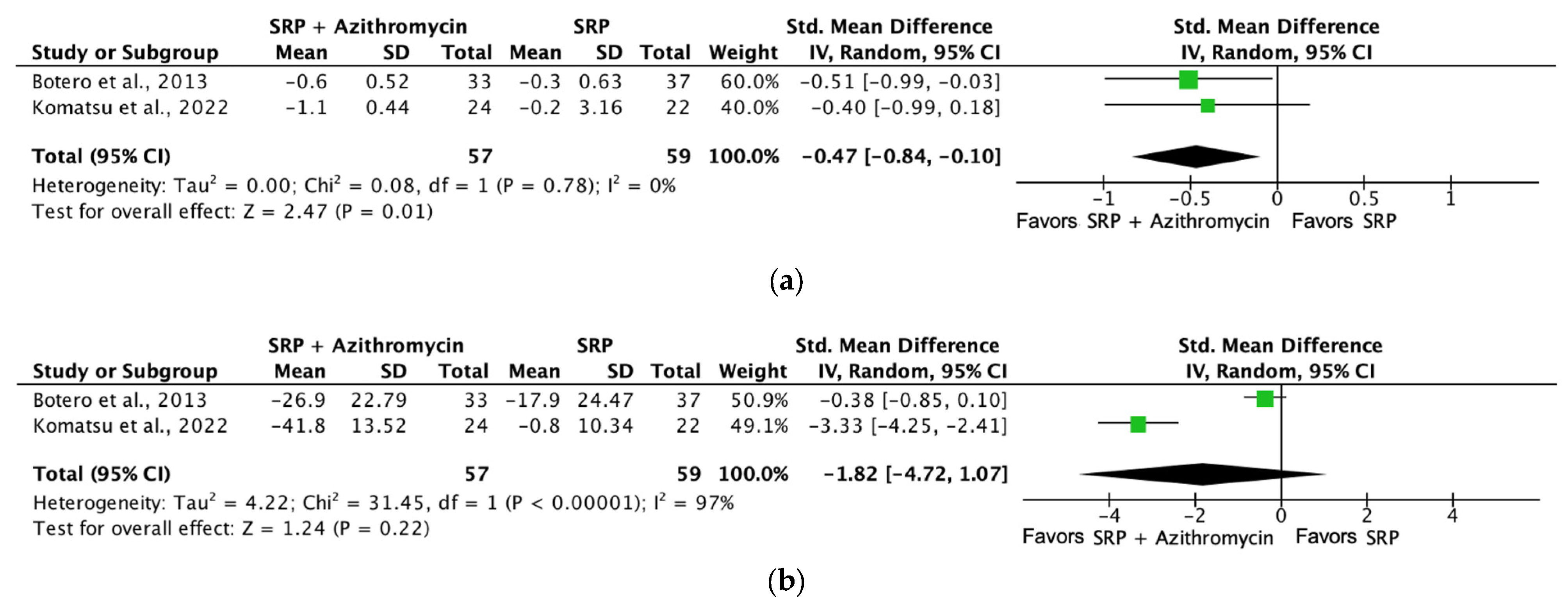
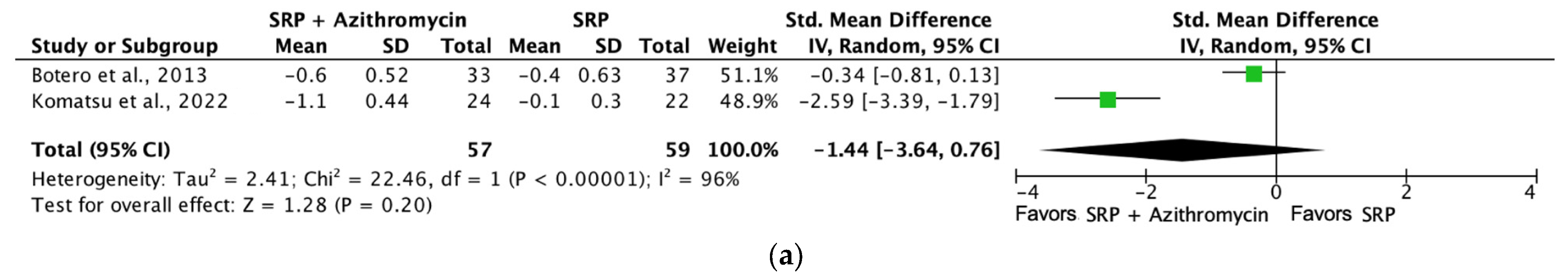
| Probing depth (SRP + azithromycin [6 months]) | 116 (2 RCTs) | Serious b | Not serious | Not serious | Not serious | ⨁⨁⨁◯ Moderate a | 57 | 59 | SMD −0.47 SD (−0.84 to −0.1) |
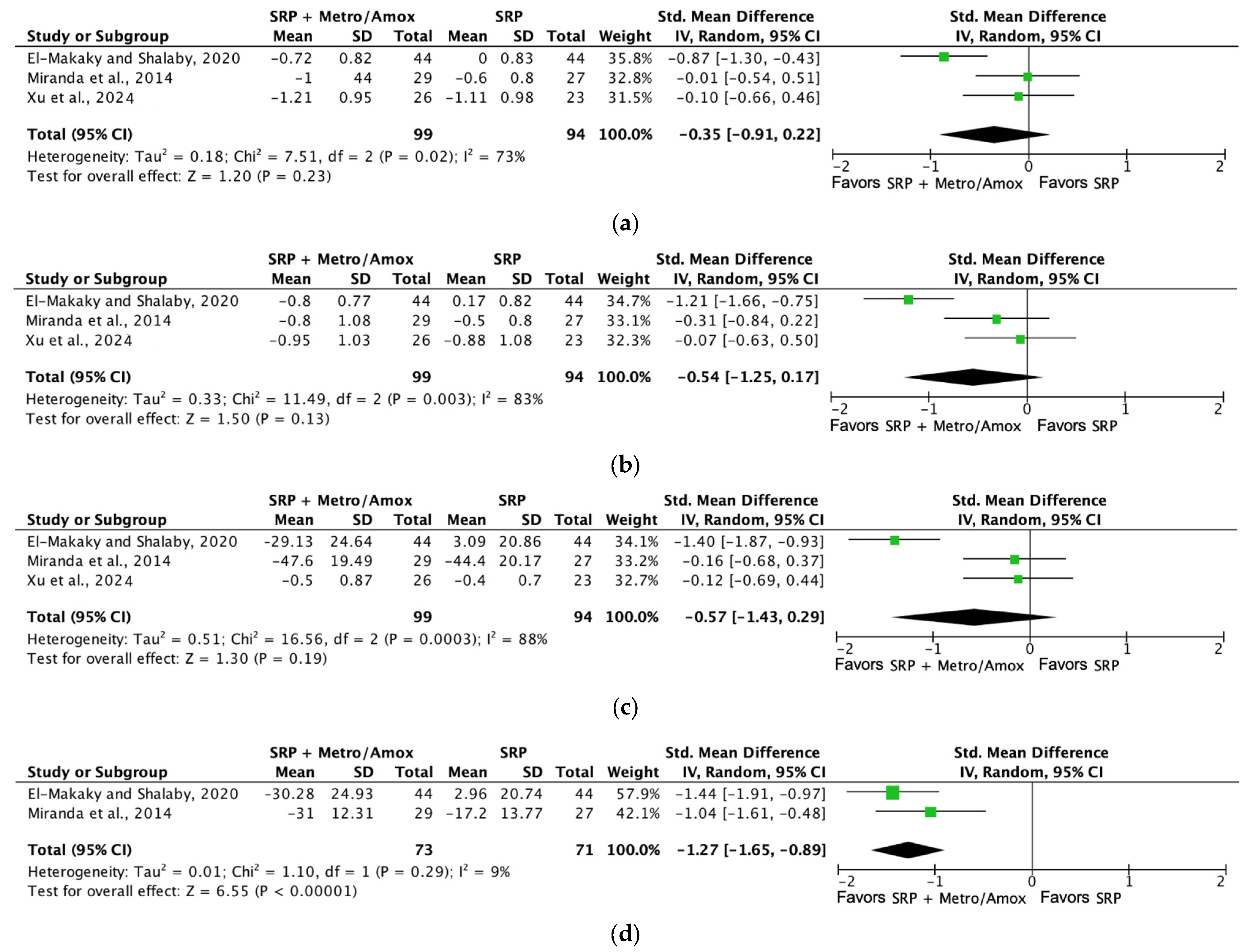
| Probing depth (SRP + Metro/Amox [3 months]) | 193 (3 RCTs) | Not serious | Not serious | Not serious | Not serious | ⨁⨁⨁⨁ High | 99 | 94 | SMD −0.35 SD (−0.91 to 0.22) |
| Bleeding on probing (SRP + Metro/Amox [3 months]) | 144 (2 RCTs) | Not serious | Not serious | Not serious | Not serious | ⨁⨁⨁⨁ High | 73 | 71 | SMD −1.27 SD (−1.65 to −0.89) |
| Probing depth (SRP + minocycline—topical application route [3 months]) | 77 (2 RCTs) | Serious a | Not serious | Not serious | Not serious | ⨁⨁⨁◯ Moderate a | 46 | 31 | SMD −0.37 SD (−1.15 to 0.41) |
| Bleeding on probing (SRP + minocycline—topical application route [3 months]) | 77 (2 RCTs) | Serious a | Not serious | Not serious | Not serious | ⨁⨁⨁◯ Moderate a | 46 | 31 | SMD −0.93 SD (−1.42 to −0.44) |
GRADE Working Group grades of evidence
| |||||||||
| Explanations a. Methodological limitations related to allocation concealment, blinding processes, and incomplete outcomes. b. Methodological limitations related to blinding of outcome assessment and incomplete outcomes. | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Molon, R.S.; Rodrigues, J.V.S.; Deroide, M.B.; da Silva Barbirato, D.; Garcia, V.G.; Theodoro, L.H. The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2024, 13, 4763. https://doi.org/10.3390/jcm13164763
de Molon RS, Rodrigues JVS, Deroide MB, da Silva Barbirato D, Garcia VG, Theodoro LH. The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine. 2024; 13(16):4763. https://doi.org/10.3390/jcm13164763
Chicago/Turabian Stylede Molon, Rafael Scaf, Joao Victor Soares Rodrigues, Mariella Boaretti Deroide, Davi da Silva Barbirato, Valdir Gouveia Garcia, and Leticia Helena Theodoro. 2024. "The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Journal of Clinical Medicine 13, no. 16: 4763. https://doi.org/10.3390/jcm13164763
APA Stylede Molon, R. S., Rodrigues, J. V. S., Deroide, M. B., da Silva Barbirato, D., Garcia, V. G., & Theodoro, L. H. (2024). The Efficacy of Topical or Systemic Antibiotics as Adjuvants to Non-Surgical Periodontal Treatment in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine, 13(16), 4763. https://doi.org/10.3390/jcm13164763






