Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
HRV and ECG Recording
2.3. Statistical Analysis
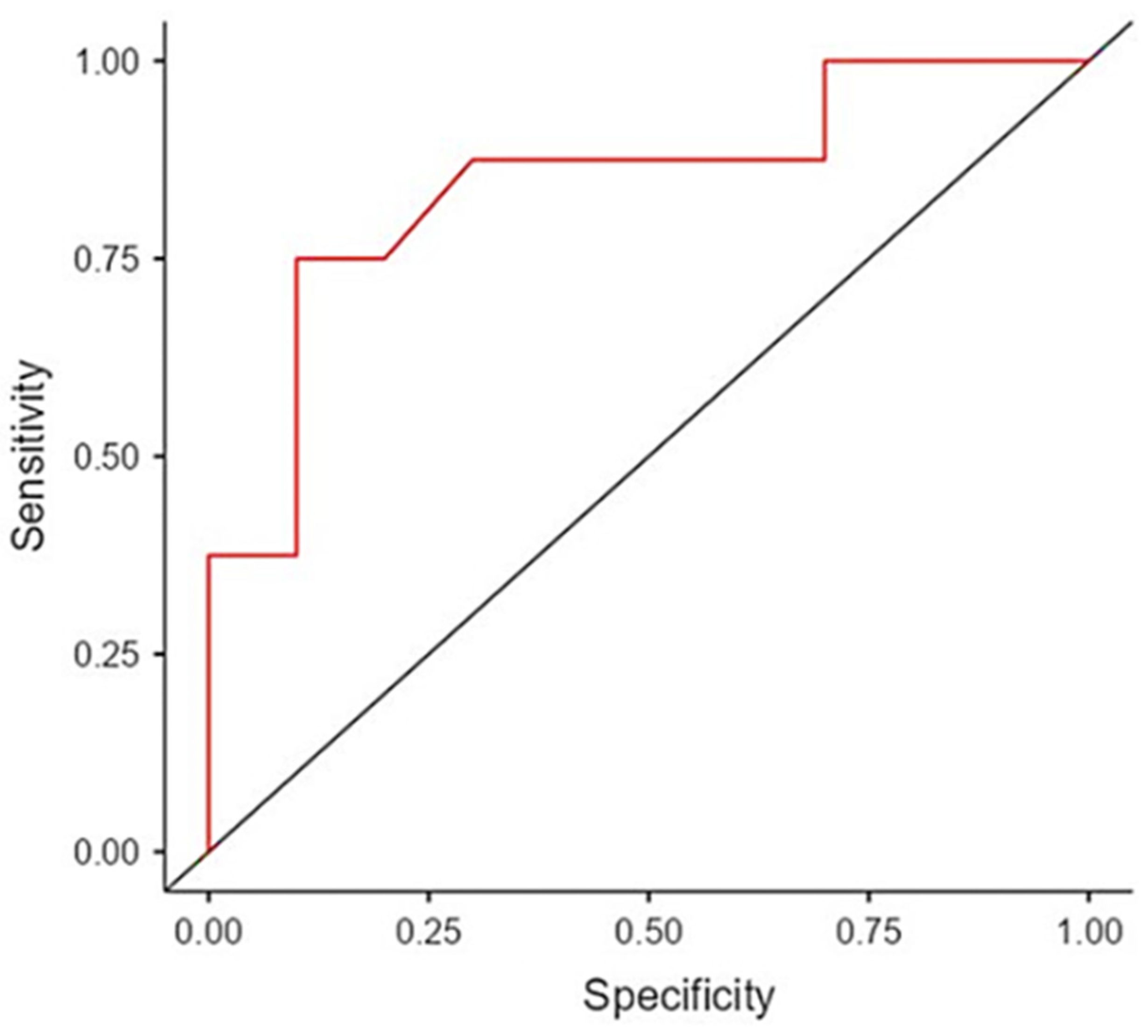
3. Results
| Characteristics (n, %; M ± SD) | Overall | Responders | Non-Responders | t/χ2/F | p |
|---|---|---|---|---|---|
| Overall | 18 | 8 (44.5) | 10 (55.5) | ||
| Sociodemographic features | |||||
| Age (years) | 55.6 ± 9.39 | 51 ± 9.4 | 59.2 ± 8.05 | 1.99 | 0.06 |
| Gender | 0.18 | 0.67 | |||
| Male | 10 (55.6) | 4 (50) | 6 (60) | ||
| Female | 8 (44.4) | 4 (50) | 4 (40) | ||
| Education (years) | 14.7 ± 2.97 | 14.9 ± 2.6 | 14.5 ± 3.4 | −0.26 | 0.79 |
| Occupation (unemployed) | 10 (55.6) | 4 (50) | 6 (60) | 0.18 | 0.67 |
| Marital status (unmarried) | 8 (44.4) | 3 (37.5) | 5 (50) | 0.28 | 0.59 |
| Clinical data | |||||
| BMI | 26.9 ± 4.43 | 26.8 ± 5.21 | 26.9 ± 3.99 | 0.02 | 0.99 |
| Smoking | 8 (44.4) | 4 (40) | 4 (50) | 0.18 | 0.67 |
| Lifetime MDE (number) | 3.78 ± 2.26 | 3.75 ± 2.92 | 3.8 ± 1.75 | 0.05 | 0.96 |
| FLX equivalents (mg) | 56.5 ± 23.1 | 60.6 ± 23.8 | 54.5 ± 24.1 | −0.41 | 0.69 |
| BDI | 35.7 ± 9.1 | 37.1 ± 10.5 | 34.4 ± 8.02 | −0.59 | 0.56 |
| HRV (lnmsec)2 | 6.01 ± 1.1 | 5.37 ± 0.93 | 6.52 ± 0.91 | 2.62 | 0.02 |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhdanava, M.; Pilon, D.; Ghelerter, I.; Chow, W.; Joshi, K.; Lefebvre, P.; Sheehan, J.J. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J. Clin. Psychiatry 2021, 82, 20m13699. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; Guidotti, R.; D’Andrea, G.; De Risio, L.; D’Andrea, A.; Chiappini, S.; Carullo, R.; Barlati, S.; Zanardi, R.; REAL-ESK Study Group; et al. Predicting outcome with Intranasal Esketamine treatment: A machine-learning, three-month study in Treatment-Resistant Depression (ESK-LEARNING). Psychiatry Res. 2023, 327, 115378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, T.E.; Lee, S.H.; Koo, J.W. The Role of Glutamate Underlying Treatment-resistant Depression. Clin. Psychopharmacol. Neurosci. 2023, 21, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; Lanzotti, P.; Pepe, M.; Palumbo, L.; Janiri, D.; Camardese, G.; Bentivoglio, A.R.; Di Nicola, M.; Calabresi, P.; Sani, G. Remission of functional motor symptoms following esketamine administration in a patient with treatment-resistant depression: A single-case report. Int. Clin. Psychopharmacol. 2022, 37, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; di Luzio, M.; Conte, E.; Modica, M.; Ambrosecchia, M.; Ardizzi, M.; Lanzotti, P.; Kotzalidis, G.D.; Janiri, D.; Di Nicola, M.; et al. Sense of agency and its disturbances: A systematic review targeting the intentional binding effect in neuropsychiatric disorders. Psychiatry Clin. Neurosci. 2024, 78, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Elfving, B.; Rafati, A.H.; Mansouri, M.; Zarate, C.A., Jr.; Mathe, A.A.; Wegener, G. Rapid effects of S-ketamine on the morphology of hippocampal astrocytes and BDNF serum levels in a sex-dependent manner. Eur. Neuropsychopharmacol. 2020, 32, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, G.; Vita, A.; Fagiolini, A.; Maina, G.; Bertolino, A.; Dell’Osso, B.; Siracusano, A.; Clerici, M.; Bellomo, A.; REAL-ESK Study Group; et al. Real-world experience of esketamine use to manage treatment-resistant depression: A multicentric study on safety and effectiveness (REAL-ESK study). J. Affect. Disord. 2022, 319, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Bartolucci, G.; Marcelli, I.; Simonetti, A.; Camardese, G.; Di Nicola, M.; Sani, G. Reduction in Cognitive Symptoms Following Intranasal Esketamine Administration in Patients with Chronic Treatment-resistant Depression: A 12-Week Case Series. J. Psychiatr. Pract. 2023, 29, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined with a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 2019, 176, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; d’Andrea, G.; De Filippis, S.; Di Nicola, M.; Andriola, I.; Bassetti, R.; Barlati, S.; Pettorruso, M.; Sensi, S.; Clerici, M.; et al. Esketamine in treatment-resistant depression patients comorbid with substance-use disorder: A viewpoint on its safety and effectiveness in a subsample of patients from the REAL-ESK study. Eur. Neuropsychopharmacol. 2023, 74, 15–21. [Google Scholar] [CrossRef] [PubMed]
- d’Andrea, G.; Chiappini, S.; McIntyre, R.S.; Stefanelli, G.; Carullo, R.; Andriola, I.; Zanardi, R.; Martiadis, V.; Sensi, S.L.; Sani, G.; et al. Investigating the Effectiveness and Tolerability of Intranasal Esketamine Among Older Adults With Treatment-Resistant Depression (TRD): A Post-hoc Analysis from the REAL-ESK Study Group. Am. J. Geriatr. Psychiatry. 2023, 31, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Bartolucci, G.; Marcelli, I.; Pesaresi, F.; Brugnami, A.; Caso, R.; Fischetti, A.; Grisoni, F.; Mazza, M.; Camardese, G.; et al. The Patient’s Perspective on the Effects of Intranasal Esketamine in Treatment-Resistant Depression. Brain Sci. 2023, 13, 1494. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D. A literature review of heart rate variability in depressive and bipolar disorders. Aust. N. Z. J. Psychiatry 2016, 50, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Izuno, T.; Tsuchiya, Y.; Hayasaka, S.; Matsumoto, K.; Murakami, H.; Ito, A.; Shinse, Y.; Suzuki, A.; Nakamura, M. Acupuncture-induced changes of vagal function in patients with depression: A preliminary sham-controlled study with press needles. Complement. Ther. Clin. Pract. 2015, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Carnevali, L.; Alfonso Mde, L.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Leistedt, S.J.; Linkowski, P.; Lanquart, J.P.; Mietus, J.E.; Davis, R.B.; Goldberger, A.L.; Costa, M.D. Decreased neuroautonomic complexity in men during an acute major depressive episode: Analysis of heart rate dynamics. Transl. Psychiatry 2011, 1, e27. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Wilhelm, M.; Salzmann, S.; Rief, W.; Euteneuer, F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 2019, 49, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus Am. Psychiatry Publ. 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Giurgi-Oncu, C.; Tudoran, C.; Enatescu, V.R.; Tudoran, M.; Pop, G.N.; Bredicean, C. Evolution of Heart Rate Variability and Heart Rate Turbulence in Patients with Depressive Illness Treated with Selective Serotonin Reuptake Inhibitors. Medicina 2020, 56, 590. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.W.; Jeon, H.J. Heart Rate Variability for the Prediction of Treatment Response in Major Depressive Disorder. Front. Psychiatry 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Terhardt, J.; Lederbogen, F.; Feuerhack, A.; Hamann-Weber, B.; Gilles, M.; Schilling, C.; Lecei, O.; Deuschle, M. Heart rate variability during antidepressant treatment with venlafaxine and mirtazapine. Clin. Neuropharmacol. 2013, 36, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, R., Jr.; Marci, C.; Fava, M.; Iosifescu, D.V.; Bankier, B.; Loh, R.; Dougherty, D.D. Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Res. 2007, 151, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Schmidt, F.M.; Sander, C.; Hegerl, U. Heart Rate Variability as Indicator of Clinical State in Depression. Front. Psychiatry 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Kemp, A.H.; Dantas, E.M.; Goulart, A.C.; Nunes, M.A.; Boggio, P.S.; Mill, J.G.; Lotufo, P.A.; Fregni, F.; Benseñor, I.M. Heart rate variability is a trait marker of major depressive disorder: Evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int. J. Neuropsychopharmacol. 2013, 16, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.P.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Foderaro, G.; Isella, V.; Mazzone, A.; Biglia, E.; Di Gangi, M.; Pasotti, F.; Sansotera, F.; Grobberio, M.; Raimondi, V.; Mapelli, C.; et al. Brand new norms for a good old test: Northern Italy normative study of MiniMental State Examination. Neurol. Sci. 2022, 43, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; Quintigliano, M.; Janiri, D.; De Martin, V.; Rogier, G.; Sani, G.; Janiri, L.; Velotti, P.; Gallese, V.; Speranza, A.M.; et al. Heart rate variability and interoceptive accuracy predict impaired decision-making in Gambling Disorder. J. Behav. Addict. 2021, 10, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, Y.; Muraoka, I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J. Appl. Physiol. 1993, 74, 875–881. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV); Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- First, M.; Williams, J.; Benjamin, L.; Spitzer, R. Structured Clinical Interview for DSM-5 Personality Disorders: SCID-5-PD; American Psychiatric Publishing: Arlington, TX, USA, 2016. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II, Beck Depression Inventory: Manual, 2nd ed.; Harcourt Brace: Boston, MA, USA, 1996. [Google Scholar]
- Meyer, T.; Brunovsky, M.; Horacek, J.; Novak, T.; Andrashko, V.; Seifritz, E.; Olbrich, S. Predictive value of heart rate in treatment of major depression with ketamine in two controlled trials. Clin. Neurophysiol. 2021, 132, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Quigley, K.S.; Gianaros, P.J.; Norman, G.J.; Jennings, J.R.; Berntson, G.G.; de Geus, E.J.C. Publication guidelines for human heart rate and heart rate variability studies in psychophysiology-Part 1: Physiological underpinnings and foundations of measurement. Psychophysiology 2024, e14604. [Google Scholar] [CrossRef]
- Porges, S.W.; Bohrer, R.E. The analysis of periodic processes in psychophysiological research. In Principles of Psychophysiology: Physical, Social, and Inferential Elements; Cacioppo, J.T., Tassinary, L.G., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 708–753. [Google Scholar]
- Lewis, G.F.; Furman, S.A.; McCool, M.F.; Porges, S.W. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biol. Psychol. 2012, 89, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, A.S.; Weinberg, A.; Klonsky, E.D. Field validity of heart rate variability metrics produced by QRSTool and CMetX. Psychol. Assess. 2012, 24, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.J.; Chambers, A.S.; Towers, D.N. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biol. Psychol. 2007, 74, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Ardizzi, M.; Ambrosecchia, M.; Gallese, V. Closing the gap between the inside and the outside: Interoceptive sensitivity and social distances. PLoS ONE 2013, 8, e75758. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Purgato, M.; Magni, L.R.; Ogawa, Y.; Takeshima, N.; Cipriani, A.; Barbui, C.; Leucht, S.; Furukawa, T.A. Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials. J. Affect. Disord. 2015, 180, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ngampramuan, S.; Tungtong, P.; Mukda, S.; Jariyavilas, A.; Sakulisariyaporn, C. Evaluation of Autonomic Nervous System, Saliva Cortisol Levels, and Cognitive Function in Major Depressive Disorder Patients. Depress. Res. Treat. 2018, 2018, 7343592. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Andrack, C.; Bär, K.J. Differences of sympathetic and parasympathetic modulation in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Koenig, J.; Thayer, J.F. From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci. Biobehav. Rev. 2017, 83, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, T.; Thayer, J.F.; Pohlack, S.; Nees, F.; Grimm, O.; Flor, H. Structural brain correlates of heart rate variability in a healthy young adult population. Brain Struct. Funct. 2017, 222, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, H.; Wu, G.R. Structural Covariance of the Prefrontal-Amygdala Pathways Associated with Heart Rate Variability. Front. Hum. Neurosci. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Meshkat, S.; Ho, R.C.; Cao, B.; Teopiz, K.M.; Rosenblat, J.D.; Rhee, T.G.; Di Vincenzo, J.D.; Ceban, F.; Jawad, M.Y.; McIntyre, R.S. Biomarkers of ketamine’s antidepressant effect: An umbrella review. J. Affect. Disord. 2023, 323, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Danyeli, L.V.; Sen, Z.D.; Colic, L.; Kurzweil, L.; Gensberger-Reigl, S.; Macharadze, T.; Götting, F.; Refisch, A.; Liebe, T.; Chand, T.; et al. Correction: Association of the delayed changes in glutamate levels and functional connectivity with the immediate network effects of S-ketamine. Transl. Psychiatry 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Balogh, S.; Fitzpatrick, D.F.; Hendricks, S.E.; Paige, S.R. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacol. Bull. 1993, 29, 201–206. [Google Scholar] [PubMed]
- Glassman, A.H.; Bigger, J.T.; Gaffney, M.; Van Zyl, L.T. Heart rate variability in acute coronary syndrome patients with major depression: Influence of sertraline and mood improvement. Arch. Gen. Psychiatry 2007, 64, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Kircanski, K.; Williams, L.M.; Gotlib, I.H. Heart rate variability as a biomarker of anxious depression response to antidepressant medication. Depress. Anxiety 2019, 36, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.; Kemp, A.H. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Cosmo, C.; Seligowski, A.V.; Aiken, E.M.; Van’t Wout-Frank, M.; Philip, N.S. Heart Rate Variability Features as Predictors of Intermittent Theta-Burst Stimulation Response in Posttraumatic Stress Disorder. Neuromodulation 2022, 25, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, B.; Qiu, J.; Zhang, L.; Zou, Z. Heart rate variability changes in patients with panic disorder. J. Affect. Disord. 2020, 267, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.; Kimmel, M.C.; Furmark, T.; Wikman, A.; Grueschow, M.; Skalkidou, A.; Frick, A.; Fransson, E. Investigating heart rate variability measures during pregnancy as predictors of postpartum depression and anxiety: An exploratory study. Transl Psychiatry 2024, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Shinba, T.; Kirimoto, T.; Matsui, T. An Objective Screening Method for Major Depressive Disorder Using Logistic Regression Analysis of Heart Rate Variability Data Obtained in a Mental Task Paradigm. Front. Psychiatry 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zheng, J.; Wang, Y.; Su, Z.; Zhu, R.; Liu, R.; Wei, Y.; Zhang, X.; Wang, F. Prediction of the efficacy of group cognitive behavioral therapy using heart rate variability based smart wearable devices: A randomized controlled study. BMC Psychiatry 2024, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, M.; Harati, S.; Paredes, P.; Khan, Y.; Foudeh, A.; Kim, J.; Bao, Z.; Williams, L.M. Stress Markers for Mental States and Biotypes of Depression and Anxiety: A Scoping Review and Preliminary Illustrative Analysis. Chronic Stress 2021, 5, 24705470211000338. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, M.; Weidner, K.; Petrowski, K.; Siepmann, T. Heart Rate Variability: A Measure of Cardiovascular Health and Possible Therapeutic Target in Dysautonomic Mental and Neurological Disorders. Appl. Psychophysiol Biofeedback 2022, 47, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Blase, K.; Vermetten, E.; Lehrer, P.; Gevirtz, R. Neurophysiological approach by self-control of your stress-related autonomic nervous system with depression, stress and anxiety patients. Int. J. Environ. Res. Public Health 2021, 18, 3329. [Google Scholar] [CrossRef] [PubMed]
- Pizzoli, S.F.M.; Marzorati, C.; Gatti, D.; Monzani, D.; Mazzocco, K.; Pravettoni, G. A meta-analysis on heart rate variability biofeedback and depressive symptoms. Sci. Rep. 2021, 11, 6650. [Google Scholar] [CrossRef] [PubMed]

| F | p | η2p | |
| Within Subjects Effects | |||
| Time | 0.006 | 0.940 | 0.00 |
| Time × Age | 0.663 | 0.429 | 0.04 |
| Time × Gender | 3.421 | 0.086 | 0.19 |
| Time × Group | 15.24 | 0.002 | 0.52 |
| Between Subjects Effects | |||
| Age | 7.91 | 0.014 | 0.36 |
| Gender | 2.7 | 0.123 | 0.16 |
| Group | 7.98 | 0.013 | 0.36 |
| HRV | Group | HRV | Group | Mean Change (SE) | p |
|---|---|---|---|---|---|
| Baseline | Non-responders | Baseline | Responders | 1.602 (0.434) | 0.011 |
| Non-responders | 1 month | Non-responders | 0.59 (0.21) | 0.06 | |
| Non-responders | Responders | 0.871 (0.360) | 0.119 | ||
| Responders | Non-responders | −1.016 (0.373) | 0.070 | ||
| Responders | Responders | −0.73 (0.239) | 0.038 | ||
| 1 month | Non-responders | Responders | 0.285 (0.303) | 0.784 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, L.; Bartolucci, G.; Pepe, M.; Marcelli, I.; Grisoni, F.; Brugnami, A.; Caso, R.; Bardi, F.; Calderoni, C.; Giannico, A.M.; et al. Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report. J. Clin. Med. 2024, 13, 4767. https://doi.org/10.3390/jcm13164767
Moccia L, Bartolucci G, Pepe M, Marcelli I, Grisoni F, Brugnami A, Caso R, Bardi F, Calderoni C, Giannico AM, et al. Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report. Journal of Clinical Medicine. 2024; 13(16):4767. https://doi.org/10.3390/jcm13164767
Chicago/Turabian StyleMoccia, Lorenzo, Giovanni Bartolucci, Maria Pepe, Ilaria Marcelli, Flavia Grisoni, Andrea Brugnami, Romina Caso, Francesca Bardi, Claudia Calderoni, Alessandro Michele Giannico, and et al. 2024. "Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report" Journal of Clinical Medicine 13, no. 16: 4767. https://doi.org/10.3390/jcm13164767
APA StyleMoccia, L., Bartolucci, G., Pepe, M., Marcelli, I., Grisoni, F., Brugnami, A., Caso, R., Bardi, F., Calderoni, C., Giannico, A. M., Benini, E., Di Nicola, M., & Sani, G. (2024). Heart Rate Variability as a Potential Predictor of Response to Intranasal Esketamine in Patients with Treatment-Resistant Depression: A Preliminary Report. Journal of Clinical Medicine, 13(16), 4767. https://doi.org/10.3390/jcm13164767







