Novel Flowable Hemostatic Agent ActiClot: Efficacy and Safety Assessment in Rat and Porcine Models
Abstract
1. Introduction
2. Materials and Methods
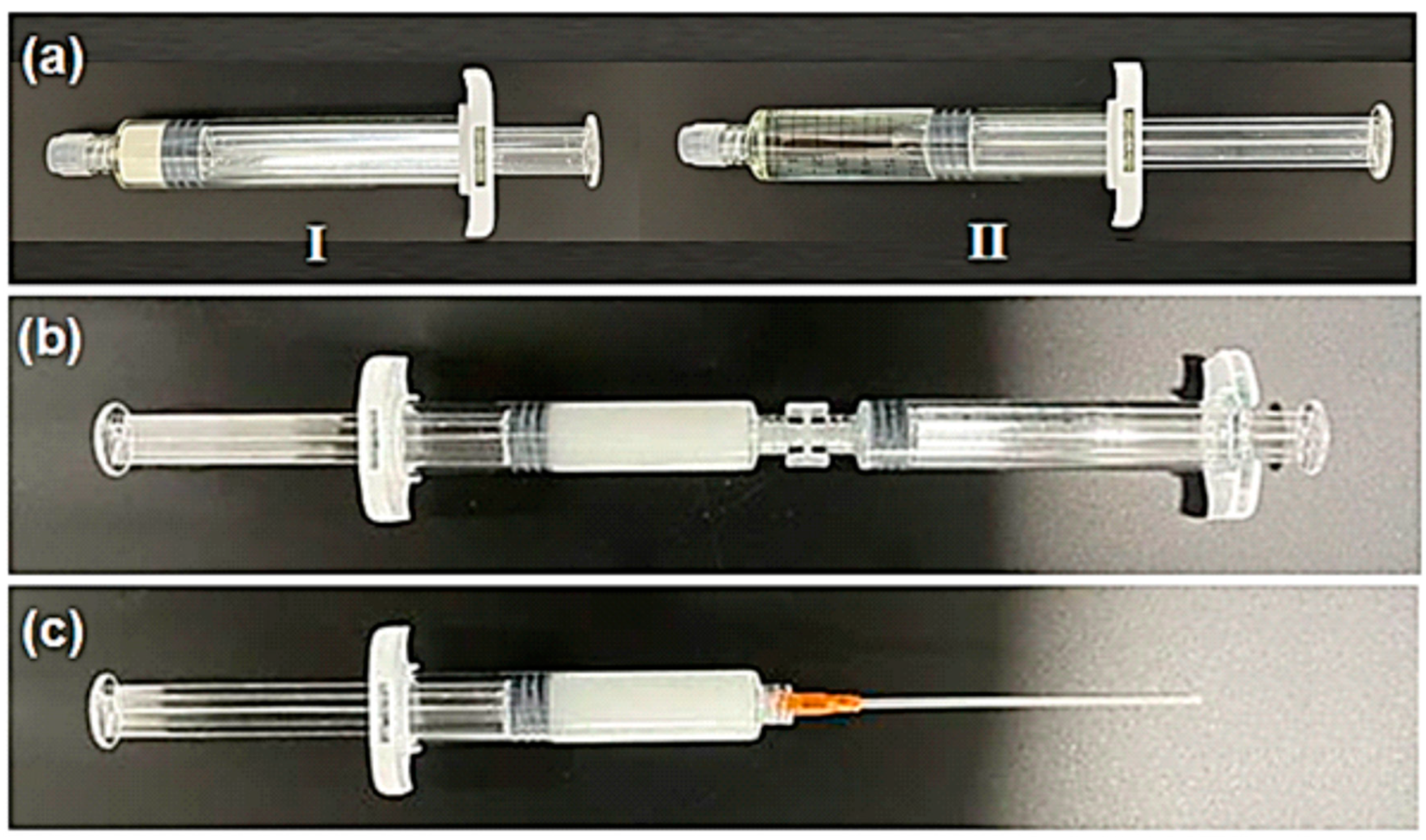
2.1. Hemostatic Agent
2.2. In Vivo Evaluation of Hemostasis
- (1)
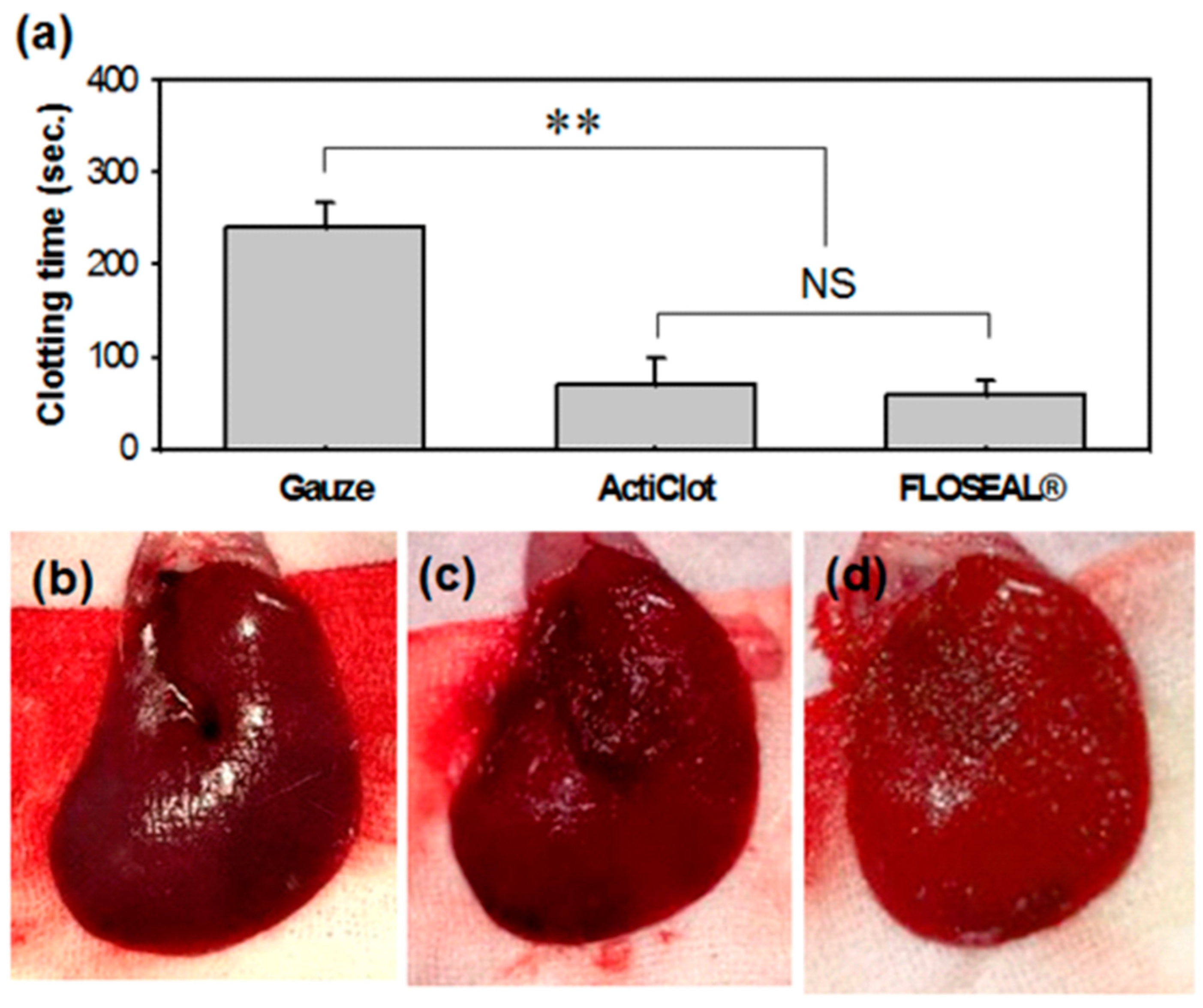
- Rat liver bleeding wound model test
- (2)
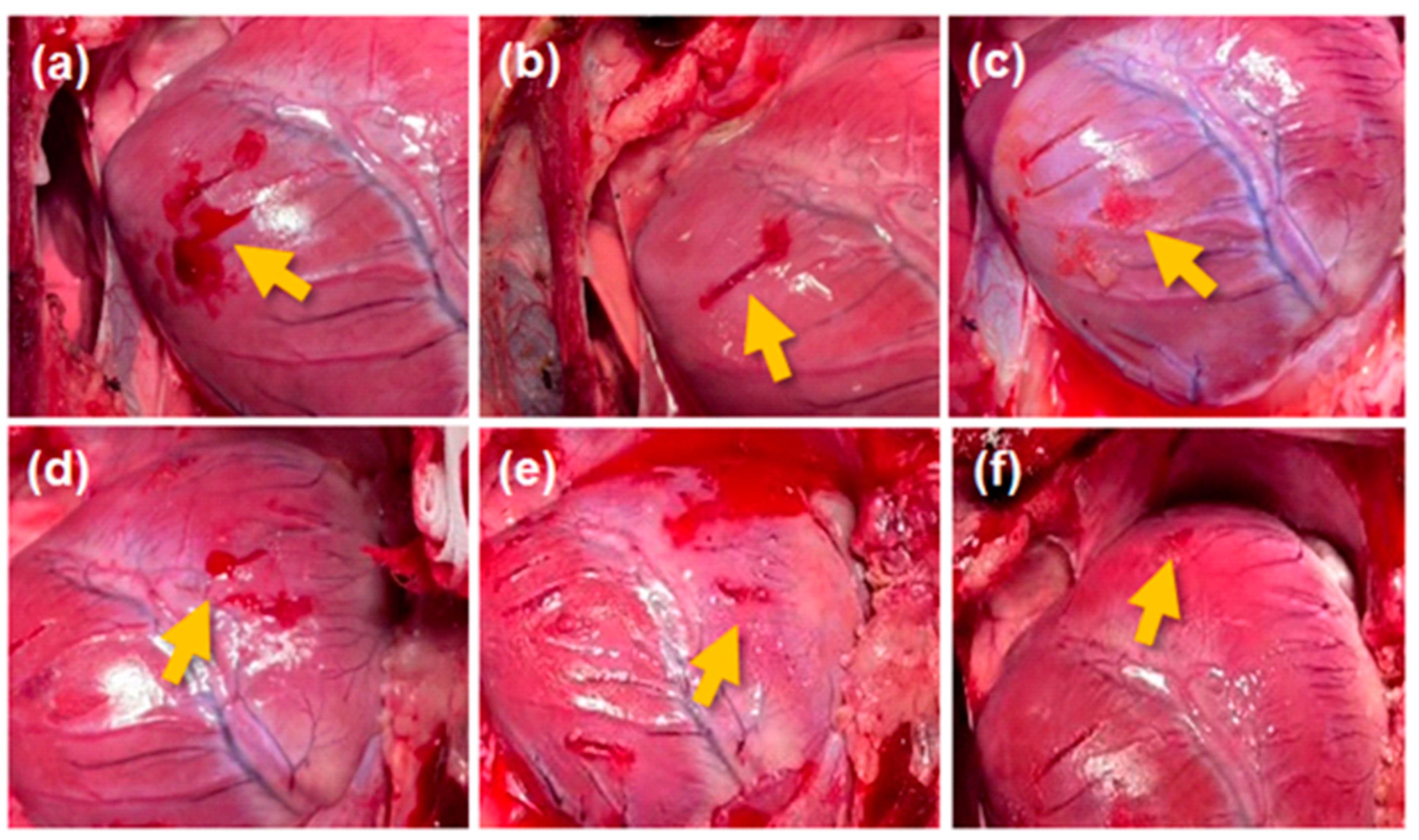
- Porcine heart bleeding model test
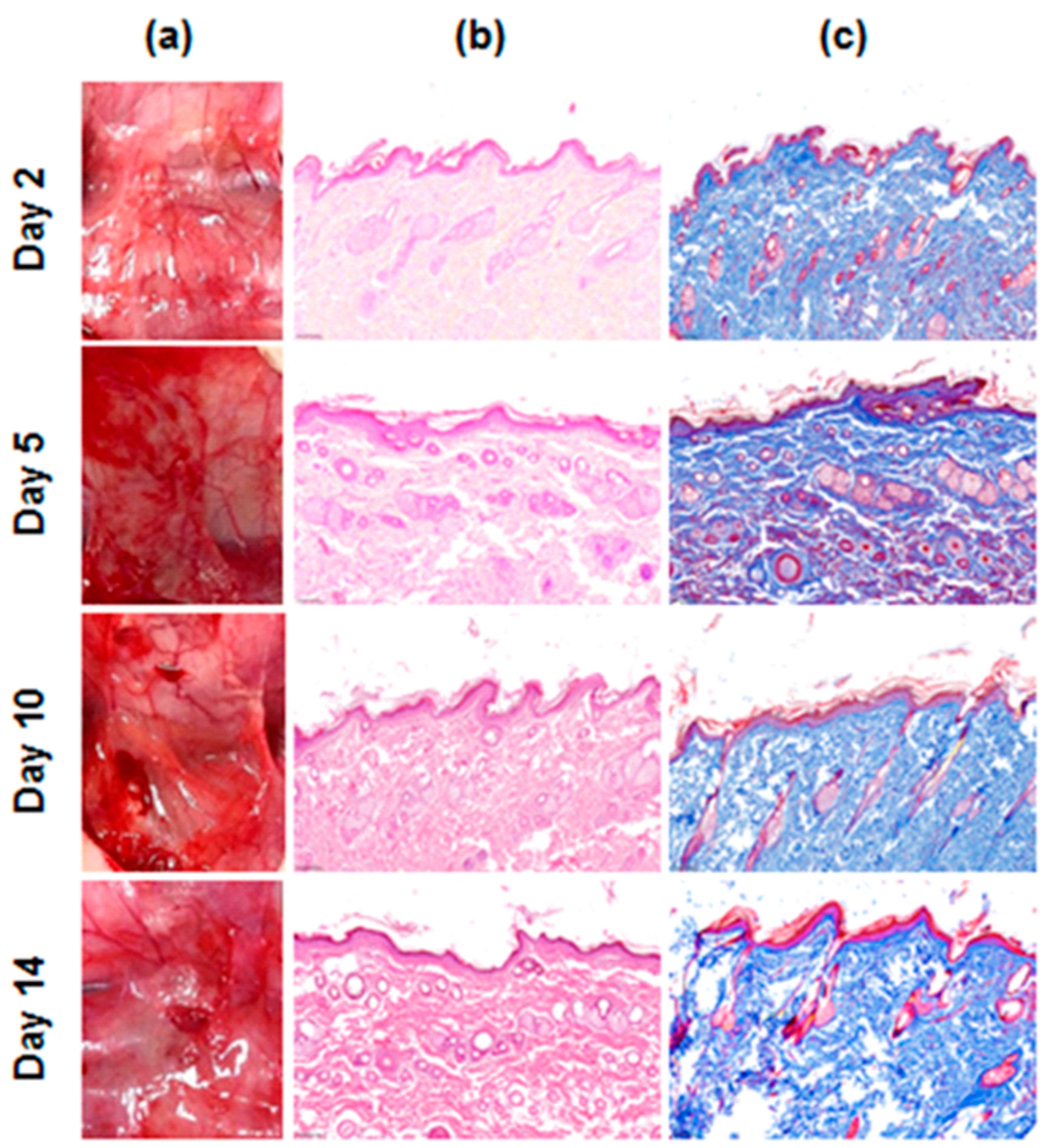
2.3. In Vivo Evaluation of Safety by Rat Subcutaneous Implantation Model
Techniques for Inflammation Evaluation
- (1)
- Histological evaluation: Surrounding tissues, including the implant, were collected at specified intervals (2, 4, 10, and 14 days) post implantation.
- (2)
- Tissue fixation: The collected tissue was fixed by immersing it in a 10% formalin solution.
- (3)
- Tissue processing: The fixed tissue was embedded in paraffin and processed following standard protocols.
- (4)
- Tissue sectioning: The paraffin-embedded tissue was sectioned into 5 µm-thick slices using a microtome.
- (5)
- Staining and observation: Hematoxylin and eosin (H&E) staining and Masson’s trichrome staining were performed on the tissue sections.
2.4. Statistical Analysis
3. Results
3.1. In Vivo Hemostatic Performance in Rat Model
3.2. In Vivo Hemostatic Performance in Porcine Model
3.3. In Vivo Evaluation of Safety by Rat Subcutaneous Implantation Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danker, W., III; Aggarwal, J.; Kelkar, S.S.; Marston, X.L.; Gao, X.; Johnston, S.S. Real-World Clinical and Economic Outcomes Associated with Surgiflo® vs Floseal in Cardiovascular Surgeries in the US. Clin. Outcomes Res. 2022, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Price, J.S.; Tackett, S.; Patel, V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J. Med. Econ. 2015, 18, 777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tackett, S.M.; Calcaterra, D.; Magee, G.; Lattouf, O.M. Real-world outcomes of hemostatic matrices in cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1558. [Google Scholar] [CrossRef] [PubMed]
- Tackett, S.M.; Sugarman, R.; Kreuwel, H.T.; Alvarez, P.; Nasso, G. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J. Med. Econ. 2014, 17, 670. [Google Scholar] [CrossRef][Green Version]
- Clapp, B.; Santillan, A. Small bowel obstruction after FloSeal use. J. Soc. Laparosc. Robot. Surg. 2011, 15, 361. [Google Scholar] [CrossRef][Green Version]
- Kim, J.E.; Yoo, H.S.; Choi, D.J.; Park, E.J.; Hwang, J.H.; Suh, J.D.; Yoo, J.H. Effectiveness of Gelatin-Thrombin Matrix Sealants (Floseal(R)) on Postoperative Spinal Epidural Hematoma during Single-Level Lumbar Decompression Using Biportal Endoscopic Spine Surgery: Clinical and Magnetic Resonance Image Study. Biomed. Res. Int. 2020, 2020, 4801641. [Google Scholar] [CrossRef] [PubMed]
- Nasso, G.; Piancone, F.; Bonifazi, R.; Romano, V.; Visicchio, G.; De Filippo, C.M.; Impiombato, B.; Fiore, F.; Bartolomucci, F.; Alessandrini, F.; et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann. Thorac. Surg. 2009, 88, 1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, X.; Li, Z.; Wang, B. Efficacy, safety, and physicochemical properties of a flowable hemostatic agent made from absorbable gelatin sponge via vacuum pressure steam sterilization. J. Biomater. Appl. 2021, 35, 776. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.C.; Silva, E.; Bartrom, J.; Young, K.; Bass, E.J.; Potter, D.; Bieber, T. Improved bleeding scores using Gelfoam® Powder with incremental concentrations of bovine thrombin in a swine liver lesion model. J. Thromb. Thrombolysis 2016, 42, 352. [Google Scholar] [CrossRef]
- Chiara, O.; Cimbanassi, S.; Bellanova, G.; Chiarugi, M.; Mingoli, A.; Olivero, G.; Ribaldi, S.; Tugnoli, G.; Basilico, S.; Bindi, F.; et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018, 18, 68. [Google Scholar] [CrossRef]
- Ko, Y.G.; Kim, B.N.; Kim, E.J.; Chung, H.Y.; Park, S.Y.; Kim, Y.J.; Kwon, O.H. Bioabsorbable Carboxymethyl Starch-Calcium Ionic Assembly Powder as a Hemostatic Agent. Polymers 2022, 14, 3909. [Google Scholar] [CrossRef]
- Singh, R.K.; Baumgartner, B.; Mantei, J.R.; Parreno, R.N.; Sanders, P.J.; Lewis, K.M.; Barry, J.J. Hemostatic Comparison of a Polysaccharide Powder and a Gelatin Powder. J. Investig. Surg. 2019, 32, 393. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.H.; Jeon, C.S.; Ko, J.H.; Park, S.N.; Lee, Y.T. Evaluation of a novel collagen hemostatic matrix in a porcine heart and cardiac vessel injury model. J. Thorac. Dis. 2019, 11, 2722. [Google Scholar] [CrossRef]
- MacDonald, M.H.; Zhang, G.; Tasse, L.; Wang, D.; De Leon, H.; Kocharian, R. Hemostatic efficacy of two topical adjunctive hemostats in a porcine spleen biopsy punch model of moderate bleeding. J. Mater. Sci. Mater. Med. 2021, 32, 127. [Google Scholar] [CrossRef]
- Gallet, P.; Phulpin, B.; Merlin, J.L.; Leroux, A.; Bravetti, P.; Mecellem, H.; Tran, N.; Dolivet, G. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS ONE 2011, 6, e29399. [Google Scholar] [CrossRef]
- ISO/DIS 10993-6; Biological Evaluation of Medical Devices–Part 6: Tests for Local Effects after Implantation, 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2016.
- Andrade, T.A.; Iyer, A.; Das, P.K.; Foss, N.T.; Garcia, S.B.; Coutinho-Netto, J.; Jordao, A.A., Jr.; Frade, M.A. The inflammatory stimulus of a natural latex biomembrane improves healing in mice. Braz. J. Med. Biol. Res. 2011, 44, 1036. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Yu, Y.; Wang, X.-Y.; Yi, J. Hemostatic effect of a medical polysaccharide hemostatic healing sponge on hemostasis of bleeding from femoral artery in pigs. Med. J. Chin. People’s Lib. Army 2015, 40, 245. [Google Scholar]
- Hu, Z.; Ouyang, Q.Q.; Cheng, Y.; Hong, P.Z.; Liao, M.N.; Chen, F.J.; Li, S.D. Optimization of preparation process and characterization of carboxymethyl chitosan/sodium alginate hemostatic sponge. In Proceedings of the 2017 Global Conference on Polymer and Composite Materials (PCM 2017), Guangzhou, China, 23–25 May 2017; Volume 213, p. 012045. [Google Scholar]
- Liu, L.; Lv, Q.; Zhang, Q.; Zhu, H.; Liu, W.; Deng, G.; Wu, Y.; Shi, C.; Li, H.; Li, L. Preparation of Carboxymethyl Chitosan Microspheres and Their Application in Hemostasis. Disaster Med. Public. Health Prep. 2017, 11, 660. [Google Scholar]
- Chen, C.; Li, H.; Pan, J.; Yan, Z.; Yao, Z.; Fan, W.; Guo, C. Biodegradable composite scaffolds of bioactive glass/chitosan/carboxymethyl cellulose for hemostatic and bone regeneration. Biotechnol. Lett. 2015, 37, 457. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Zeng, D.; Xia, Y.; Fan, Y.; Zhang, X.; Li, N.; Liu, X.; Sun, X.; Zhao, S.; et al. Antibacterial and rapidly absorbable hemostatic sponge by aldehyde modification of natural polysaccharide. Commun. Mater. 2024, 5, 129. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Li, N.; Wang, M.; Liang, B.; Ullah, I.; Luis Neve, A.; Feng, Y.; Chen, H.; Shi, C. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater. Sci. 2017, 5, 2357. [Google Scholar] [CrossRef] [PubMed]
| Groups | LV | RV | ||||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #1 | #2 | #3 | #4 | |
| Gauze | A | A | A | A | A | A | A | A |
| ActiClot | C | C | B | C | C | C | C | C |
| FLOSEAL® | C | C | C | C | B | C | B | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Lee, S.-K.; Ko, Y.-J.; Jeon, S.-H.; Kim, E.-J.; Kwon, O.-H.; Cho, Y.-H. Novel Flowable Hemostatic Agent ActiClot: Efficacy and Safety Assessment in Rat and Porcine Models. J. Clin. Med. 2024, 13, 4770. https://doi.org/10.3390/jcm13164770
Kim H-J, Lee S-K, Ko Y-J, Jeon S-H, Kim E-J, Kwon O-H, Cho Y-H. Novel Flowable Hemostatic Agent ActiClot: Efficacy and Safety Assessment in Rat and Porcine Models. Journal of Clinical Medicine. 2024; 13(16):4770. https://doi.org/10.3390/jcm13164770
Chicago/Turabian StyleKim, Hee-Jung, Su-Kyoung Lee, Yun-Jeh Ko, Soo-Hyeon Jeon, Eun-Jin Kim, Oh-Hyeong Kwon, and Yang-Hyun Cho. 2024. "Novel Flowable Hemostatic Agent ActiClot: Efficacy and Safety Assessment in Rat and Porcine Models" Journal of Clinical Medicine 13, no. 16: 4770. https://doi.org/10.3390/jcm13164770
APA StyleKim, H.-J., Lee, S.-K., Ko, Y.-J., Jeon, S.-H., Kim, E.-J., Kwon, O.-H., & Cho, Y.-H. (2024). Novel Flowable Hemostatic Agent ActiClot: Efficacy and Safety Assessment in Rat and Porcine Models. Journal of Clinical Medicine, 13(16), 4770. https://doi.org/10.3390/jcm13164770





