Exploring the Potential of Saphenous Vein Grafts Ex Vivo: A Model for Intimal Hyperplasia and Re-Endothelialization
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection
2.2.1. Tissue Samples
2.2.2. Blood Samples
2.3. Cell and Tissue Culture
2.3.1. Tissue Culture
2.3.2. Cell Culture
2.4. Histological Analysis
2.5. Immunofluorescence (IF)
2.6. Image Processing
2.7. Statistical Analysis
3. Results
3.1. Demographic, Clinical Characteristics and Outcome
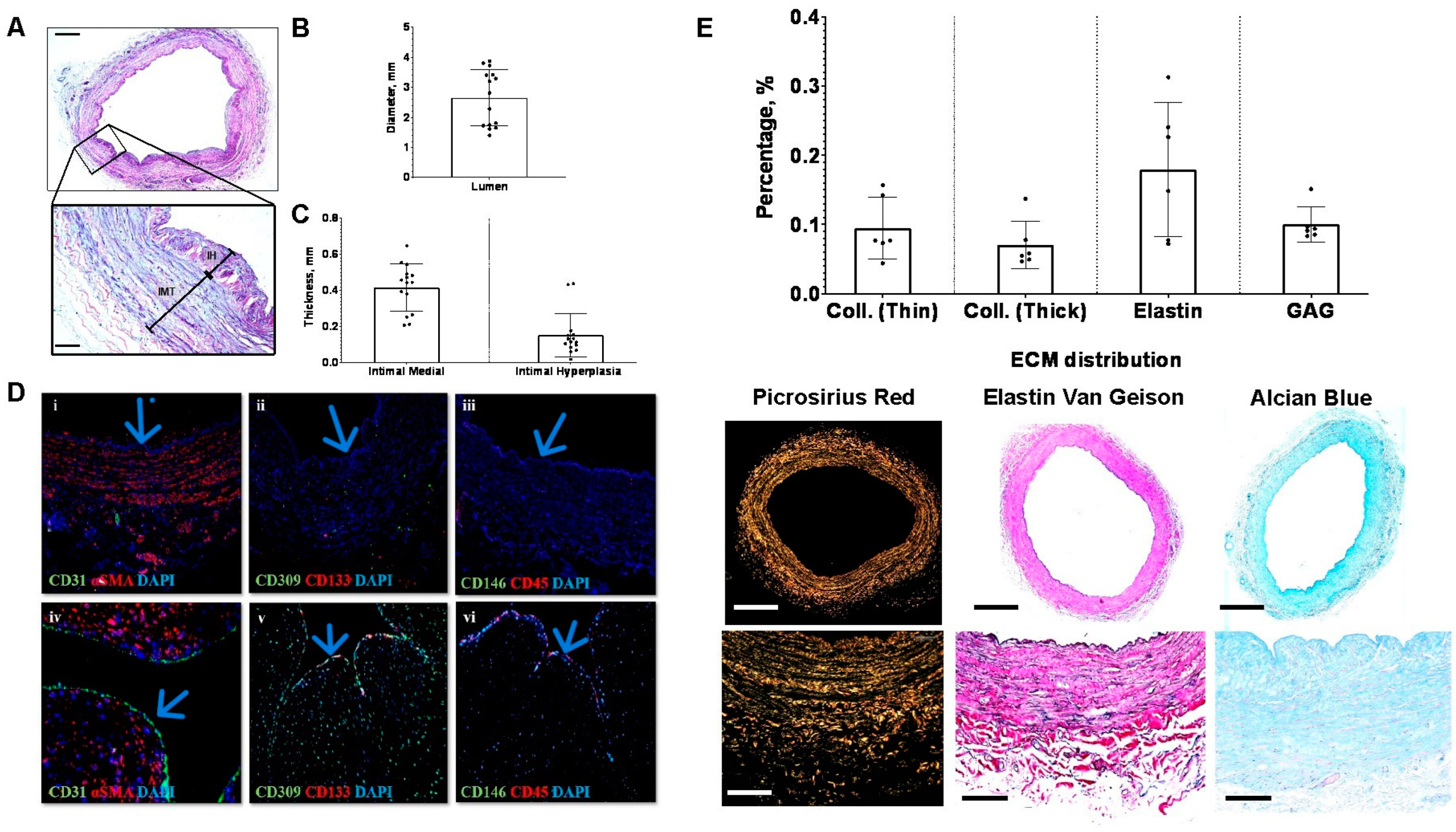
3.2. Variability in Lumen Diameter, Medial Thickness, and Intimal Hyperplasia
3.3. Surplus Saphenous Vein Grafts Endothelial Cells Coverage
3.4. Baseline Saphenous Vein Grafts Extracellular Matrix Composition
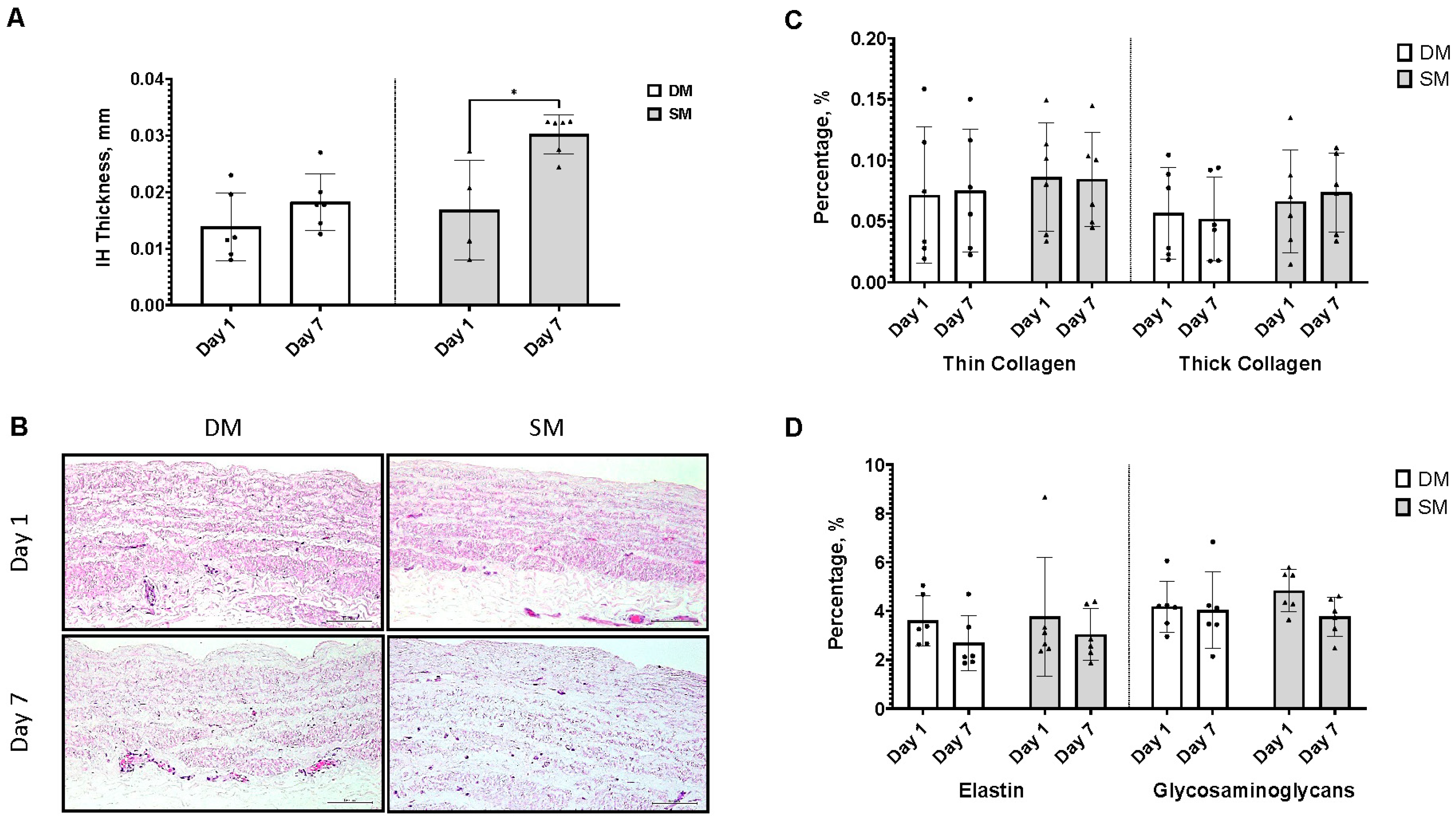
3.5. Surplus Saphenous Vein Grafts Intimal Hyperplasia Ex Vivo Model (DM vs. SM)
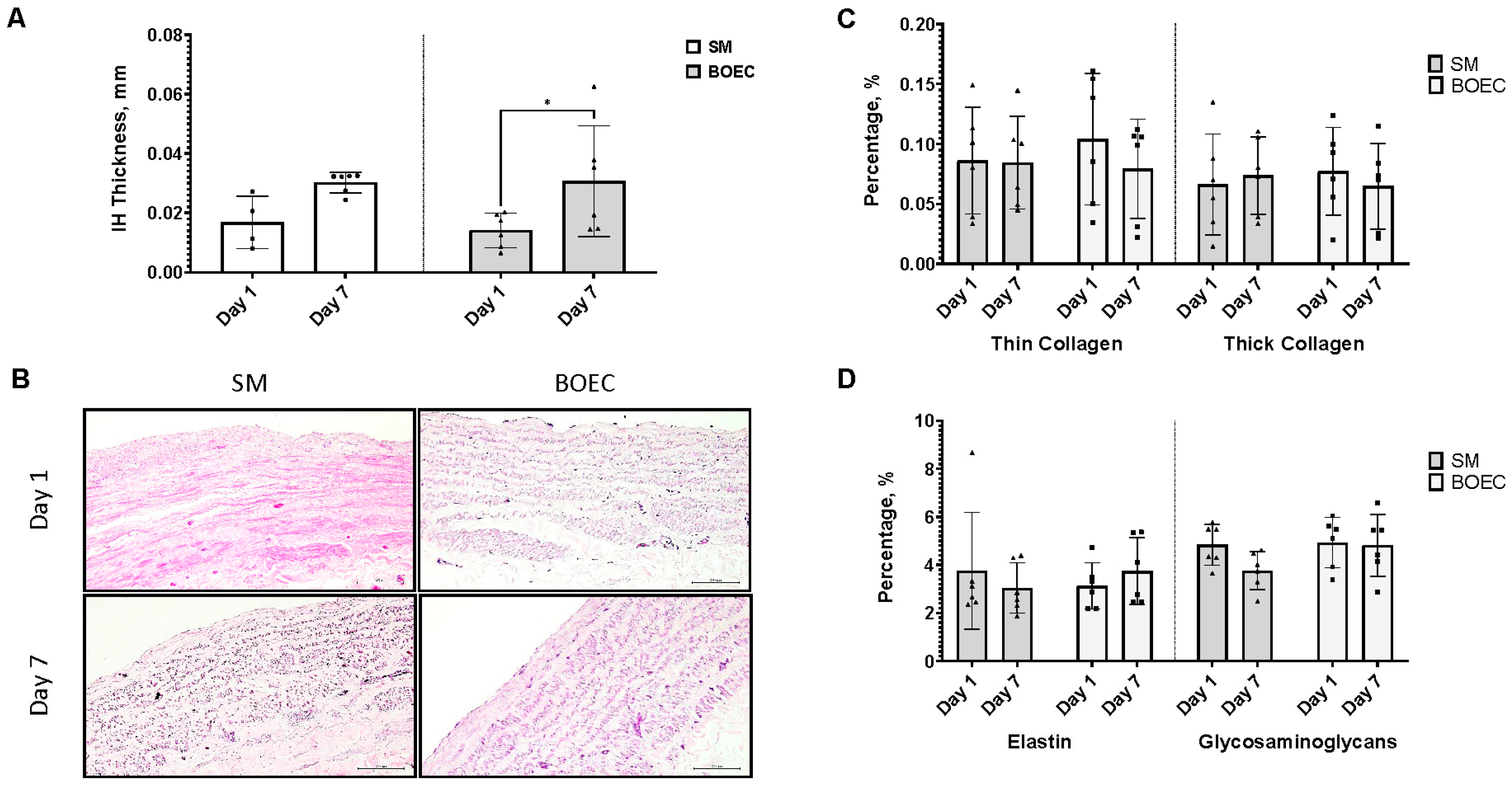
3.6. Re-Endothelialization of Intimal Hyperplasia Ex Vivo Model (SM vs. BOEC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous Vein Grafts in Contemporary Coronary Artery Bypass Graft Surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Chang, C.; Deo, S.V.; Sabik, J.F. Current Role of Saphenous Vein Graft in Coronary Artery Bypass Grafting. Indian J. Thorac. Cardiovasc. Surg. Off. Organ Assoc. Thorac. Cardiovasc. Surg. India 2018, 34, 245. [Google Scholar] [CrossRef]
- Sur, S.; Sugimoto, J.T.; Agrawal, D.K. Coronary Artery Bypass Graft: Why Is the Saphenous Vein Prone to Intimal Hyperplasia? Can. J. Physiol. Pharmacol. 2014, 92, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.N.; Lopes, R.D.; Gibson, C.M.; Hager, R.; Wojdyla, D.M.; Englum, B.R.; Mack, M.J.; Califf, R.M.; Kouchoukos, N.T.; Peterson, E.D.; et al. Saphenous Vein Graft Failure after Coronary Artery Bypass Surgery Insights from PREVENT IV. Circulation 2014, 130, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Déglise, S.; Bechelli, C.; Allagnat, F. Vascular Smooth Muscle Cells in Intimal Hyperplasia, an Update. Front. Physiol. 2022, 13, 1081881. [Google Scholar] [CrossRef] [PubMed]
- Tabbara, M.; Duque, J.C.; Martinez, L.; Escobar, L.A.; Wu, W.; Pan, Y.; Fernandez, N.; Velazquez, O.C.; Jaimes, E.A.; Salman, L.H.; et al. Pre-Existing and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. Am. J. Kidney Dis. 2016, 68, 455. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood Cells and Endothelial Barrier Function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. ENOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Khan, S.; Stewart, D.J.; Courtman, D.W. Engineering Blood Outgrowth Endothelial Cells to Optimize Endothelial Nitric Oxide Synthase and Extracellular Matrix Production for Coating of Blood Contacting Surfaces. Acta Biomater. 2020, 109, 109–120. [Google Scholar] [CrossRef]
- Sánchez, P.F.; Brey, E.M.; Briceño, J.C. Endothelialization Mechanisms in Vascular Grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Y.; Guo, Z. Risk Factors and Possible Mechanisms of Saphenous Vein Graft Failure after Coronary Artery Bypass Surgery. Chin. Med. J. 2020, 133, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Newby, A.C.; Zaltsman, A.B.; Newby, A.C. Molecular Mechanisms in Intimal Hyperplasia. J. Pathol. 2000, 190, 300–309. [Google Scholar] [CrossRef]
- Thyberg, J.; Hedin, U.; Sjöiund, M.; Palmberg, L.; Bottger, B.A. Regulation of Differentiated Properties and Proliferation of Arterial Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 1990, 10, 966–990. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of Smooth Muscle Cells in the Initiation and Early Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Kural, M.H.; Dai, G.; Niklason, L.E.; Gui, L. An Ex Vivo Vessel Injury Model to Study Remodeling. Cell Transplant. 2018, 27, 1375–1389. [Google Scholar] [CrossRef]
- Piola, M.; Prandi, F.; Bono, N.; Soncini, M.; Penza, E.; Agrifoglio, M.; Polvani, G.; Pesce, M.; Fiore, G.B. A Compact and Automated Ex Vivo Vessel Culture System for the Pulsatile Pressure Conditioning of Human Saphenous Veins. J. Tissue Eng. Regen. Med. 2016, 10, E204–E215. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Allagnat, F.; Berard, X.; Alonso, F.; Haefliger, J.A.; Deglise, S.; Corpataux, J.M. Procedure for Human Saphenous Veins Ex Vivo Perfusion and External Reinforcement. J. Vis. Exp. 2014, 92, e52079. [Google Scholar] [CrossRef]
- Dummler, S.; Eichhorn, S.; Tesche, C.; Schreiber, U.; Voss, B.; Deutsch, M.A.; Hauner, H.; Lahm, H.; Lange, R.; Krane, M. Pulsatile Ex Vivo Perfusion of Human Saphenous Vein Grafts under Controlled Pressure Conditions Increases MMP-2 Expression. Biomed. Eng. Online 2011, 10, 1–14. [Google Scholar] [CrossRef]
- McQueen, L.W.; Ladak, S.S.; Zakkar, M. Acute Shear Stress and Vein Graft Disease. Int. J. Biochem. Cell Biol. 2022, 144, 106173. [Google Scholar] [CrossRef] [PubMed]
- Gusic, R.J.; Myung, R.; Petko, M.; Gaynor, J.W.; Gooch, K.J. Shear Stress and Pressure Modulate Saphenous Vein Remodeling Ex Vivo. J. Biomech. 2005, 38, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Devillard, C.D.; Marquette, C.A. Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel. Front. Bioeng. Biotechnol. 2021, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Bajada, S.; Mazakova, I.; Richardson, J.B.; Ashammakhi, N. Updates on Stem Cells and Their Applications in Regenerative Medicine. J. Tissue Eng. Regen. Med. 2008, 2, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.A.; Shi, J.; Guo, J.Y.; Wang, S.F. Recent Strategies for Improving Hemocompatibility and Endothelialization of Cardiovascular Devices and Inhibition of Intimal Hyperplasia. J. Mater. Chem. B 2022, 10, 3781–3792. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Wu, Z. Strategies in Cell-Free Tissue-Engineered Vascular Grafts. J. Biomed. Mater. Res. A 2020, 108, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Roszita, I.; Nur, A.M.; AR, S.Z.; Aljunid, S.M. Estimation Of Cost Of Diagnostic Laboratory Services Using Activity Based Costing (ABC) For Implementation Of Malaysia Diagnosis Related Group (My-Drg®) In A Teaching Hospital. Malays. J. Public Health Med. 2017, 17, 1–8. [Google Scholar]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Firus Khan, A.Y.; Ramli, A.S.; Abdul Razak, S.; Mohd Kasim, N.A.; Chua, Y.A.; Ul-Saufie, A.Z.; Jalaludin, M.A.; Nawawi, H. The Malaysian HEalth and WellBeing AssessmenT (MyHEBAT) Study Protocol: An Initiation of a National Registry for Extended Cardiovascular Risk Evaluation in the Community. Int. J. Environ. Res. Public. Health 2022, 19, 11789. [Google Scholar] [CrossRef]
- Cheong, S.S.; Chin, K.Y.; Ugusman, A.; Aminuddin, A. Cardiovascular Profiles of Younger and Older Coronary Artery Disease Patients in Asian and Western Regions. Curr. Epidemiol. Rep. 2023, 10, 85–114. [Google Scholar] [CrossRef]
- Polak, J.F.; Pencina, M.J.; Meisner, A.; Pencina, K.M.; Brown, L.S.; Wolf, P.A.; D’Agostino, R.B. Associations of Carotid Artery Intima-Media Thickness (IMT) with Risk Factors and Prevalent Cardiovascular Disease: Comparison of Mean Common Carotid Artery IMT with Maximum Internal Carotid Artery IMT. J. Ultrasound Med. 2010, 29, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, F.; Qi, L.; Jia, J.; Yang, Y.; Li, J.; Zhang, Y. The Association between Carotid Intima-Media Thickness and New-Onset Hypertension in a Chinese Community-Based Population. BMC Cardiovasc. Disord. 2019, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Kon, Z.N.; White, C.; Kwon, M.H.; Judy, J.; Brown, E.N.; Gu, J.; Burris, N.S.; Laird, P.C.; Brown, T.; Brazio, P.S.; et al. The Role of Preexisting Pathology in the Development of Neointimal Hyperplasia in Coronary Artery Bypass Grafts. J. Surg. Res. 2007, 142, 351–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashmi, S.F.; Krishnamoorthy, B.; Critchley, W.R.; Walker, P.; Bishop, P.W.; Venkateswaran, R.V.; Fildes, J.E.; Yonan, N. Histological and Immunohistochemical Evaluation of Human Saphenous Vein Harvested by Endoscopic and Open Conventional Methods. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 178–185. [Google Scholar] [CrossRef]
- Ferdinand, F.D.; MacDonald, J.K.; Balkhy, H.H.; Bisleri, G.; Hwang, H.Y.; Northrup, P.; Trimlett, R.H.J.; Wei, L.; Kiaii, B.B. Endoscopic Conduit Harvest in Coronary Artery Bypass Grafting Surgery: An ISMICS Systematic Review and Consensus Conference Statements. Innovations 2017, 12, 301–319. [Google Scholar] [CrossRef]
- Raja, S.G.; Sarang, Z. Endoscopic Vein Harvesting: Technique, Outcomes, Concerns & Controversies. J. Thorac. Dis. 2013, 5, S630. [Google Scholar] [CrossRef]
- Rousou, L.J.; Taylor, K.B.; Lu, X.G.; Healey, N.; Crittenden, M.D.; Khuri, S.F.; Thatte, H.S. Saphenous Vein Conduits Harvested by Endoscopic Technique Exhibit Structural and Functional Damage. Ann. Thorac. Surg. 2009, 87, 62–70. [Google Scholar] [CrossRef]
- Kaplan, S.; Bisleri, G.; Kilinç, K.; Çobanoǧlu, A.; Öz, M.C. Effects of Harvesting Technique on Endothelial Inflammation and Nitric Oxide Production in Saphenous Vein Grafts. Turk. J. Thorac. Cardiovasc. Surg. 2013, 21, 31–36. [Google Scholar] [CrossRef]
- Siddiqi, M.S. Saphenous Vein Harvest Wound Complications: Risk Factors, Identification, Prevention, and Management. Chronic Wound Care Manag. Res. 2016, 3, 147–156. [Google Scholar] [CrossRef]
- Hochberg, C.P.; Botnaru, I.; Carrozza, J.P. Saphenous Vein Grafts. In Cardiovascular Catheterization and Intervention: A Textbook of Coronary, Peripheral, and Structural Heart Disease, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 567–581. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The Extracellular Matrix and Blood Vessel Formation: Not Just a Scaffold. J. Cell Mol. Med. 2007, 11, 176. [Google Scholar] [CrossRef]
- Osgood, M.J.; Hocking, K.M.; Voskresensky, I.V.; Li, F.D.; Komalavilas, P.; Cheung-Flynn, J.; Brophy, C.M. Surgical Vein Graft Preparation Promotes Cellular Dysfunction, Oxidative Stress, and Intimal Hyperplasia in Human Saphenous Vein. J. Vasc. Surg. 2014, 60, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Kawamura, K.; Takebayashi, S. Effects of Collagen Matrix on Proliferation and Differentiation of Vascular Smooth Muscle Cells in Vitro. Exp. Mol. Pathol. 1990, 52, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, Z.T.; Xu, L.H.; Su, T.Y.; Han, Y.; Bao, M.; Liu, Z.; Fan, Y.J.; Lou, Y.; Chen, Y.; et al. Platelet-Derived Extracellular Vesicles Increase Col8a1 Secretion and Vascular Stiffness in Intimal Injury. Front. Cell Dev. Biol. 2021, 9, 404. [Google Scholar] [CrossRef]
- Arun Gopinathan, P.; Kokila, G.; Jyothi, M.; Ananjan, C.; Pradeep, L.; Humaira Nazir, S. Study of Collagen Birefringence in Different Grades of Oral Squamous Cell Carcinoma Using Picrosirius Red and Polarized Light Microscopy. Scientifica 2015, 2015, 802980. [Google Scholar] [CrossRef] [PubMed]
- Sansilvestri-Morel, P.; Fioretti, F.; Rupin, A.; Senni, K.; Fabiani, J.N.; Godeau, G.; Verbeuren, T.J. Comparison of Extracellular Matrix in Skin and Saphenous Veins from Patients with Varicose Veins: Does the Skin Reflect Venous Matrix Changes? Clin. Sci. 2007, 112, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.; Niculescu, I.; Bona-Casas, C.; Merks, R.M.H.; Hoekstra, A.G. An in Silico Study on the Role of Smooth Muscle Cell Migration in Neointimal Formation after Coronary Stenting. J. R. Soc. Interface 2015, 12, 20150358. [Google Scholar] [CrossRef]
- Mammoto, A.; Matus, K.; Mammoto, T. Extracellular Matrix in Aging Aorta. Front. Cell Dev. Biol. 2022, 10, 822561. [Google Scholar] [CrossRef]
- Patel, S.D.; Waltham, M.; Wadoodi, A.; Burnand, K.G.; Smith, A. The Role of Endothelial Cells and Their Progenitors in Intimal Hyperplasia. Ther. Adv. Cardiovasc. Dis. 2010, 4, 129–141. [Google Scholar] [CrossRef]
- Banerjee, S.; Mwangi, J.G.; Stanley, T.K.; Mitra, R.; Ebong, E.E. Regeneration and Assessment of the Endothelial Glycocalyx to Address Cardiovascular Disease. Ind. Eng. Chem. Res. 2021, 60, 17328–17347. [Google Scholar] [CrossRef]
- Krishnamoorthy, B.; Critchley, W.; Barnard, J.; Waterworth, P.; Caress, A.; Fildes, J.; Yonan, N. Validation of the Endothelial Staining Markers CD31 and CD34 in Immunohistochemistry of the Long Saphenous Vein. J. Cardiothorac. Surg. 2015, 10, A321. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Zhang, Y.; Hui, H.; Tong, W.; Wei, Z.; Li, Z.; Zhang, S.; Yang, X.; Tian, J.; Chen, Y. The Effect of Endothelial Progenitor Cell Transplantation on Neointimal Hyperplasia and Reendothelialisation after Balloon Catheter Injury in Rat Carotid Arteries. Stem Cell Res. Ther. 2021, 12, 99. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Popova, P.I.; Avdonin, P.P.; Kudryavtsev, I.V.; Serebryakova, M.K.; Korf, E.A.; Avdonin, P.V. Markers of Endothelial Cells in Normal and Pathological Conditions. Biochem. (Mosc) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Cao, X.; Yu, B.; Qu, A. Vascular Stem/Progenitor Cells in Vessel Injury and Repair. Front. Cardiovasc. Med. 2022, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Ofstein, R.H.; Rapp, B.; Saadatzadeh, M.R.; Bhavsar, J.R.; Fajardo, A.; Dalsing, M.C.; Ingram, D.A.; Murphy, M.P. Adult Venous Endothelium Is a Niche for Highly Proliferative and Vasculogenic Endothelial Colony-Forming Cells. J. Vasc. Surg. 2017, 66, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Malinska, A.; Podemska, Z.; Perek, B.; Jemielity, M.; Buczkowski, P.; Grzymislawska, M.; Sujka-Kordowska, P.; Nowicki, M. Preoperative Factors Predicting Saphenous Vein Graft Occlusion in Coronary Artery Bypass Grafting: A Multivariate Analysis. Histochem. Cell Biol. 2017, 148, 417–424. [Google Scholar] [CrossRef]
- Brzoska, E.; Ciemerych, M.A.; Przewozniak, M.; Zimowska, M. Regulation of Muscle Stem Cells Activation: The Role of Growth Factors and Extracellular Matrix. Vitam. Horm. 2011, 87, 239–276. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.D.; Jeremy, J.Y. Towards the Treatment of Saphenous Vein Bypass Graft Failure—A Perspective of the Bristol Heart Institute. Biorheology 2002, 39, 491–499. [Google Scholar]
- Wali, M.A.; Eid, R.A.; Dewan, M.; Al-Homrany, M.A. Pre-Existing Histopathological Changes in the Cephalic Vein of Renal Failure Patients before Arterio-Venous Fistula (AVF) Construction. Ann. Thorac. Cardiovasc. Surg. 2006, 12, 341–348. [Google Scholar]
- Corpataux, J.M.; Naik, J.; Porter, K.E.; London, N.J.M. A Comparison of Six Statins on the Development of Intimal Hyperplasia in a Human Vein Culture Model. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 177–181. [Google Scholar] [CrossRef]
- Samah, N.; Ugusman, A.; Hamid, A.A.; Sulaiman, N.; Aminuddin, A. Role of Matrix Metalloproteinase-2 in the Development of Atherosclerosis among Patients with Coronary Artery Disease. Mediat. Inflamm. 2023, 2023, 9715114. [Google Scholar] [CrossRef]
- Kulik, A.; Brookhart, M.A.; Levin, R.; Ruel, M.; Solomon, D.H.; Choudhry, N.K. Impact of Statin Use on Outcomes after Coronary Artery Bypass Graft Surgery. Circulation 2008, 118, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Wadey, K.; Lopes, J.; Bendeck, M.; George, S. Role of Smooth Muscle Cells in Coronary Artery Bypass Grafting Failure. Cardiovasc. Res. 2018, 114, 601–610. [Google Scholar] [CrossRef]
- Hildebrand, S.; Ibrahim, M.; Schlitzer, A.; Maegdefessel, L.; Röll, W.; Pfeifer, A. PDGF Regulates Guanylate Cyclase Expression and CGMP Signaling in Vascular Smooth Muscle. Commun. Biol. 2022, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial Dysfunction Due to ENOS Uncoupling: Molecular Mechanisms as Potential Therapeutic Targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Webb, R.C. New Insights into RhoA/Rho-Kinase Signaling: A Key Regulator of Vascular Contraction. Small GTPases 2021, 12, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci.—Landmark 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Gangahar, D.M.; Agrawal, D.K. Cellular, Molecular and Immunological Mechanisms in the Pathophysiology of Vein Graft Intimal Hyperplasia. Immunol. Cell Biol. 2006, 84, 115–124. [Google Scholar] [CrossRef]
- Sarah Azman, S.; Dain Yazid, M.; Azurah Abdul Ghani, N.; Zahratul Azma Raja Sabudin, R.; Ramzisham Abdul Rahman, M.; Sulaiman, N.; Canselor Tuanku Mukhriz, H.; Yaacob Latif, J.; Tun Razak, B.; Lumpur, K. Generation of a Novel Ex-Vivo Model to Study Re-Endothelialization. Artif. Cells Nanomed. Biotechnol. 2023, 51, 408–416. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef]

| Patient Characteristics | Patient (n = 35) |
|---|---|
| Mean age in years ± standard deviation | 59 ± 9.2 |
| CVD risk factors: Diabetes mellitus, type II, n (%) | 13 (37%) |
| Hypertension, n (%) | 28 (80%) |
| Hyperlipidemia, n (%) | 22 (63%) |
| Obesity, n (%) | 2 (6%) |
| History of smoking, n (%) | 14 (40%) |
| Past medical history: Chronic kidney disease, n (%) | 4 (11%) |
| Fatty liver disease, n (%) | 2 (6%) |
| Transient ischemic attack (TIA), or mini stroke, n (%) | 3 (9%) |
| LVEF%: Reduced (≤40%), n (%) | 4 (11%) |
| Borderline (41–49%), n (%) | 10 (29%) |
| Normal (50–70%), n (%) | 21 (60%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haron, N.A.; Ishak, M.F.; Yazid, M.D.; Vijakumaran, U.; Ibrahim, R.; Raja Sabudin, R.Z.A.; Alauddin, H.; Md Ali, N.A.; Haron, H.; Ismail, M.I.; et al. Exploring the Potential of Saphenous Vein Grafts Ex Vivo: A Model for Intimal Hyperplasia and Re-Endothelialization. J. Clin. Med. 2024, 13, 4774. https://doi.org/10.3390/jcm13164774
Haron NA, Ishak MF, Yazid MD, Vijakumaran U, Ibrahim R, Raja Sabudin RZA, Alauddin H, Md Ali NA, Haron H, Ismail MI, et al. Exploring the Potential of Saphenous Vein Grafts Ex Vivo: A Model for Intimal Hyperplasia and Re-Endothelialization. Journal of Clinical Medicine. 2024; 13(16):4774. https://doi.org/10.3390/jcm13164774
Chicago/Turabian StyleHaron, Nur A’tiqah, Mohamad Fikeri Ishak, Muhammad Dain Yazid, Ubashini Vijakumaran, Roszita Ibrahim, Raja Zahratul Azma Raja Sabudin, Hafiza Alauddin, Nur Ayub Md Ali, Hairulfaizi Haron, Muhammad Ishamuddin Ismail, and et al. 2024. "Exploring the Potential of Saphenous Vein Grafts Ex Vivo: A Model for Intimal Hyperplasia and Re-Endothelialization" Journal of Clinical Medicine 13, no. 16: 4774. https://doi.org/10.3390/jcm13164774
APA StyleHaron, N. A., Ishak, M. F., Yazid, M. D., Vijakumaran, U., Ibrahim, R., Raja Sabudin, R. Z. A., Alauddin, H., Md Ali, N. A., Haron, H., Ismail, M. I., Abdul Rahman, M. R., & Sulaiman, N. (2024). Exploring the Potential of Saphenous Vein Grafts Ex Vivo: A Model for Intimal Hyperplasia and Re-Endothelialization. Journal of Clinical Medicine, 13(16), 4774. https://doi.org/10.3390/jcm13164774








