Pathogenesis of Pachyvein Formation in Central Serous Chorioretinopathy: A Hydrodynamic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Examinations
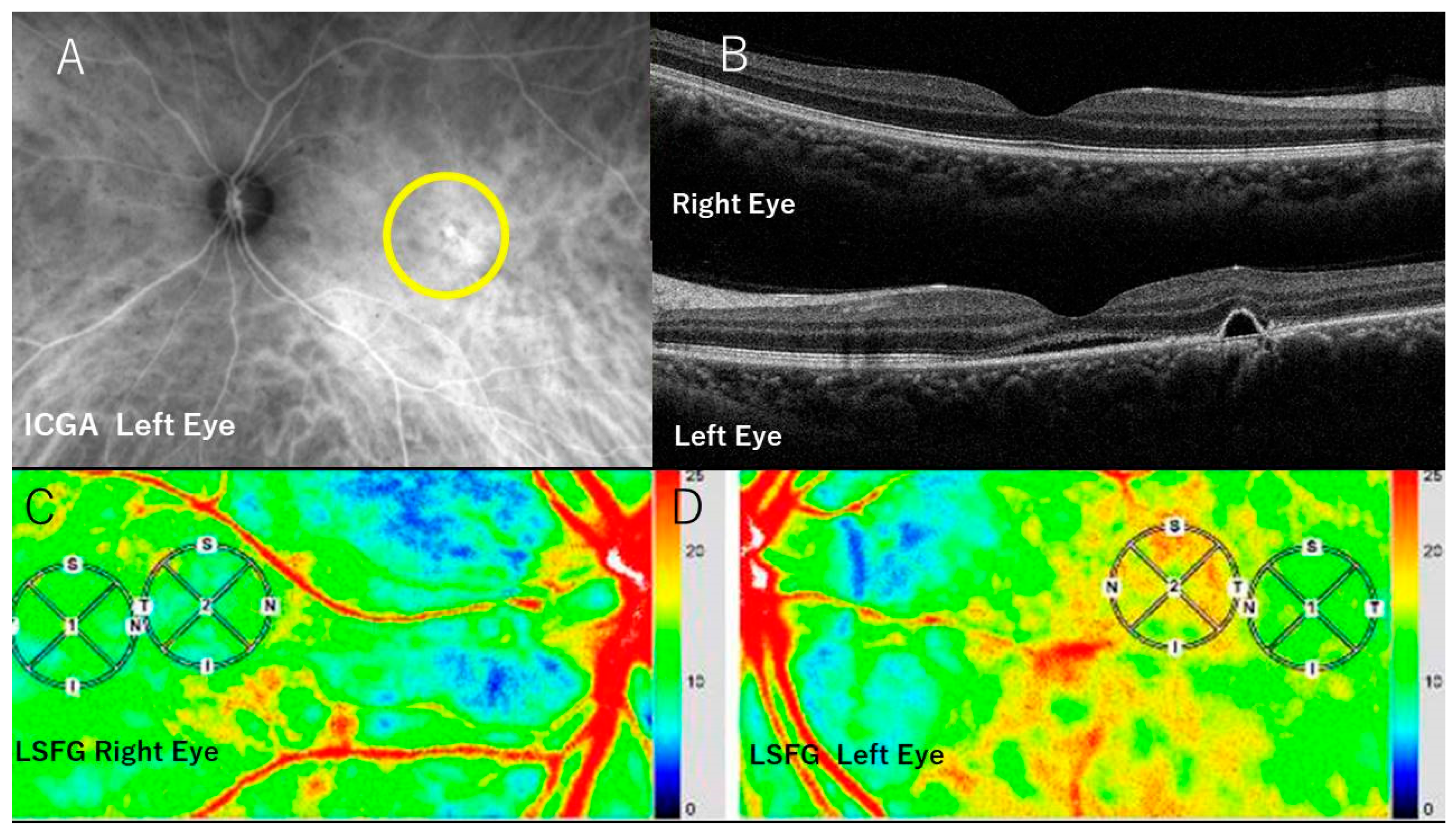
2.2. FA, ICGA, and Optical Coherence Tomography (OCT)
2.3. LSFG
2.4. Statistical Analysis
2.5. Simulated Blood Pressure Calculation in the Choroidal Venules during Choriocapillaris Exudation
- The flow velocity V1 and flow pressure P1, respectively, in the venules, in the physiological condition [17].
- Calculation and conversion of the MBR value of V2 into mm/s.
- Calculation of P2 by applying the V1, V2, and P1 values to Bernoulli’s theorem.
- The P2 value depends on the degree and extent of energy loss, i.e., exudation in the choriocapillaris. The energy loss can be expressed as the percentage of C1, i.e., C2 = C1 − nC1: n = percentage of energy loss. The C1 value is calculated by substituting V1 and P1 values. The relationship between P2 and C2 is then established with the varying n values.
3. Results
3.1. Choroidal Thickness
3.2. MBRs in the CSC Eyes
3.3. MBRs of the Unaffected Contralateral Eyes
3.4. Simulated Blood Pressure Calculation in the Choroidal Venules during Exudation from the Choriocapillaris
- The flow velocity V1 and pressure P1 values in the venules in physiological conditions were 1 cm/s = 10 mm/s and 18 mmHg, respectively [17].
- The mean foveal MBR of the non-CSC eyes was 8.3 (Table 1), and the flow velocity in the venules V1 was 10 mm/s. Therefore, 1 MBR = 10/8.3 mm/s = 1.2 mm/s.
- V2 = 6.4 mean foveal MBR × 1.2 mm/s = 7.7 mm/s.
- = 8 + 21 = 39 mmHg (calculations in Table 2).
- C1 = 67.1, when 10 mm/s and 18 mmHg for V1 and P1, respectively, are substituted into the formula. The results are shown in Table 3.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid diseases. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Prünte, C.; Flammer, J. Choroidal Capillary and Venous Congestion in Central Serous Chorioretinopathy. Am. J. Ophthalmol. 1996, 121, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Fujiwara, T.; Margolis, R.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 2009, 29, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Matsumoto, H.; Sonoda, S.; Hiroe, T.; Sakamoto, T.; Akiyama, H. Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS ONE 2018, 13, e0206646. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, Y.; Kim, J.; Lee, K. Choroidal thickness in patients with central serous chorioretinopathy: Assessment of Haller’s and Sattler’s layers. Acta Ophthalmol. 2015, 93, 1652–1657. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Kikuchi, Y.; Kishi, S.; Akiyama, H. Vortex Vein Anastomosis at the watershed in pachychoroid spectrum diseases. Ophthalmol. Retina 2020, 4, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, N.; Terao, N.; Nakamine, S.; Tamashiro, T.; Wakugawa, S.; Sawaguchi, K.; Koizumi, H. Scleral thickness in central serous chorioretinopathy. Ophthalmol. Retina 2021, 5, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Ryan, E.H., Jr. Loculation of fluid in the posterior choroid in eyes with central serous chorioretinopathy. Am. J. Ophthalmol. 2015, 160, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, N.; Terao, N.; Sawaguchi, S.; Tamashiro, T.; Wakugawa, S.; Yamauchi, Y.; Koizumi, H. Clinical Factors Related to Loculation of Fluid in central serous chorioretinopathy. Am. J. Ophthalmol. 2021, 235, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid pigment epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Nishi, O.; Yasukawa, T. Comment on: Clinical factors related to loculation of fluid in central serous chorioretinopathy. Am. J. Ophthalmol. 2022, 4, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Nishi, O.; Yasukawa, T. Hydrodynamic analysis of the clinical findings in pachychoroid-spectrum diseases. J. Clin. Med. 2022, 11, 5247. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S. Fluid of kinetics: Continuity Equation, Fluid dynamics: Bernoulli’s equation. In Textbook of Fluid Mechanics; CBS Publishers & Distributors Pvt. Ltd.: Delhi, India, 2017; Volume 48, p. 104. [Google Scholar]
- Takahashi, H.; Sugiyama, T.; Tokushige, H.; Maeno, T.; Nakazawa, T.; Ikeda, T.; Araie, M. Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp. Eye Res. 2013, 108, 10–15. [Google Scholar] [CrossRef]
- Wang, L.; Cull, G.A.; Piper, C.; Burgoyne, C.F.; Fortune, B. Anterior and posterior optic nerve head blood flow in non-human primate experimental glaucoma model measured by laser freckle imaging technique and microsphere method. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Thuy, L.; Takahashi, N.; Yasumoto, A.; Fujii, H. Analysis of blood flow in retinal vessels using laser speckle flowgraphy. Atarashii Ganka 2010, 27, 256–259. [Google Scholar]
- Urry, L.; Cain, M.L.; Wasserman, S.; Minorsky, P.V.; Reece, J.P. Chapter 42: Circulation and Gas Exchange. In Campbell Biology, 11th ed.; Pearson: New York, NY, USA, 2016; Volume 928. [Google Scholar]
- Kido, A.; Miyake, M.; Tamura, H.; Hiragi, S.; Kimura, T.; Ohtera, S.; Takahashi, A.; Ooto, S.; Kawakami, K.; Kuroda, T.; et al. Incidence of central serous chorioretinopathy (2011–2018): A nationwide population-based cohort study of Japan. Br. J. Ophthalmol. 2022, 106, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Fujiya, A.; Noda, K.; Ishida, S. Macular choroidal blood flow velocity decreases with regression of acute central serous chorioretinopathy. Br. J. Ophthalmol. 2013, 97, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Saito, W.; Hirooka, K.; Hashimoto, Y.; Mori, S.; Noda, K.; Ishida, S. Pulse waveform changes in macular choroidal hemodynamics with regression of acute central chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M.R. Hyperemia. J. Tissue Viability 1998, 4, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Gill, P.; Jones, C.; Taylor, J.A.; Van den Bruel, A.; Heneghan, C.; Roberts, N.; Thomson, M. The diagnostic value of capillary refill time for detecting serious illness in children: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138155. [Google Scholar] [CrossRef] [PubMed]
| Case | Age | Sex | Choroidal Thickness | MBR (CSC-Eyes) | MBR (Contralateral Eyes) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSC-Eyes | Contra-Lateral Eyes | Fovea | Peri-Fovea | MBR-Difference | Fovea | Peri-Fovea | MBR-Difference | ||||
| 1 | H.T. | 36 | M | 310μm | 314μm | 12.8 | 16.6 | −3.8 | 10.9 | 10.4 | +0.5 |
| 2 | Y.K. | 55 | M | 431 | 324 | 7.7 | 11.3 | −3.6 | 7.8 | 8.8 | −1.0 |
| 3 | T.S. | 52 | M | 343 | 334 | 6.0 | 9.9 | −3.9 | 10.3 | 11.0 | −0.7 |
| 4 | S.S. | 51 | M | 435 | 339 | 3.2 | 7.0 | −3.8 | 9.6 | 10.4 | −0.8 |
| 5 | H.N. | 38 | M | 348 | 245 | 8.2 | 9.6 | −1.4 | 8.9 | 8.4 | +0.5 |
| 6 | Y.I. | 35 | M | 454 | 367 | 11.6 | 13.8 | −2.2 | 12.0 | 9.0 | +3.0 |
| 7 | H.M. | 59 | F | 505 | 415 | 11.5 | 12.5 | −1.0 | 13.1 | 8.7 | +4.4 |
| 8 | A.Y. | 43 | M | 322 | 241 | 7.2 | 13.8 | −5.6 | 9.7 | 8.6 | +1.1 |
| 9 | Y.Y. | 67 | M | 325 | 312 | 0.9 | 5.2 | −4.3 | 3.5 | 5.6 | −2.1 |
| 10 | T.M. | 44 | M | 203 | 168 | 4.5 | 6.9 | −2.4 | 3.4 | 3.7 | −0.3 |
| 11 | H.G. | 58 | M | 458 | 399 | 3.4 | 7.0 | −3.6 | 6.2 | 6.0 | +0.2 |
| 12 | K.Y. | 52 | F | 320 | 276 | 4.2 | 7.1 | −2.9 | 3.6 | 3.0 | +0.6 |
| 13 | R.H. | 83 | M | 357 | 165 | 4.4 | 7.0 | −2.6 | 10.5 | 8.0 | +2.5 |
| 14 | K.I. | 41 | M | 415 | 328 | 7.7 | 9.0 | −1.3 | 11.2 | 9.5 | +2.0 |
| 15 | M.F. | 73 | F | 614 | 571 | 3.9 | 10.0 | −6.1 | 3.6 | 5.1 | −1.5 |
| 16 | Y.F. | 50 | M | 397 | 340 | 4.8 | 7.4 | −2.6 | 4.4 | 5.2 | −0.8 |
| 17 | K.K. | 45 | M | 495 | 409 | 5.9 | 7.3 | −1.4 | 10.9 | 9.8 | +1.1 |
| 18 | H.G. | 59 | M | 443 | 380 | 4.7 | 6.7 | −2.0 | 9.5 | 9.5 | 0 |
| 19 | K.T. | 44 | M | 467 | 376 | 9.6 | 13.9 | −4.3 | 8.7 | 8.6 | +0.1 |
| 51.8 ± 12.7 | 402.2 ± 92.4 | 331.7 ± 92.7 | 6.4 ± 3.2 | 9.6 ± 3.2 | −3.1 ± 1.4 | 8.3 ± 3.2 | 7.9 ± 2.4 | 0.4 ± 1.4 | |||
 | |||||||||||
| V1 | Blood flow velocity of venules in normal state: 10 mm/s |
| P1 | Blood pressure of venules in normal state: 18 mmHg |
| V2 | Blood flow velocity of venules during exudation (6.4 mean macular MBR in CSC eyes): see the calculation below. |
| P2 | Blood pressure of venules during exudation: the pressure looked for. |
| ρ | Blood density: 1.05 |
| The unit of MBR value for blood flow velocity is converted into mm/s using the mean macular MBR of the non-CSC eyes, which is 8.3 MBR, corresponding to 10 mm for V1: 1 MBR = 10/8.3 mm/s = 1.2 mm/s | |
| Hence, V2 = 6.4 mean macular MBR = 6.4 × 1.2 mm/s = 7.7 mm/s | |
| Thus, in simulation, there would be a nearly 216% blood pressure rise from 18 mmHg to 39 mmHg according to Bernoulli’s theorem, when the velocity decreased from 10 mm/sec to 7.7 mm/s. | |
| Energy Loss (%) | C2 | P2 (mmHg) |
|---|---|---|
| (=100 × (C1 − C2)/C1) | ||
| 0 | 67.1 | 39.4 |
| 5 | 63.6 | 38.2 |
| 10 | 60.4 | 35.3 |
| 15 | 57.0 | 27 |
| 20 | 53.7 | 23.7 |
| 30 | 47.0 | 17 |
| 40 | 40.0 | 10.5 |
| 50 | 34.0 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, O.; Nishi, Y.; Tatsumichi, M.; Yasukawa, T. Pathogenesis of Pachyvein Formation in Central Serous Chorioretinopathy: A Hydrodynamic Analysis. J. Clin. Med. 2024, 13, 4777. https://doi.org/10.3390/jcm13164777
Nishi O, Nishi Y, Tatsumichi M, Yasukawa T. Pathogenesis of Pachyvein Formation in Central Serous Chorioretinopathy: A Hydrodynamic Analysis. Journal of Clinical Medicine. 2024; 13(16):4777. https://doi.org/10.3390/jcm13164777
Chicago/Turabian StyleNishi, Okihiro, Yutaro Nishi, Miki Tatsumichi, and Tsutomu Yasukawa. 2024. "Pathogenesis of Pachyvein Formation in Central Serous Chorioretinopathy: A Hydrodynamic Analysis" Journal of Clinical Medicine 13, no. 16: 4777. https://doi.org/10.3390/jcm13164777





