Optimizing Diabetic Macular Edema Treatment: A Meta-Analysis of Subthreshold Micropulse Laser and Anti-Vascular Endothelial Growth Factor Combination Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Collection
2.3. Quality Assessment
2.4. Outcome Measurement
2.5. Subgroup and Sensitivity Analyses
2.6. Statistical Analysis
3. Results
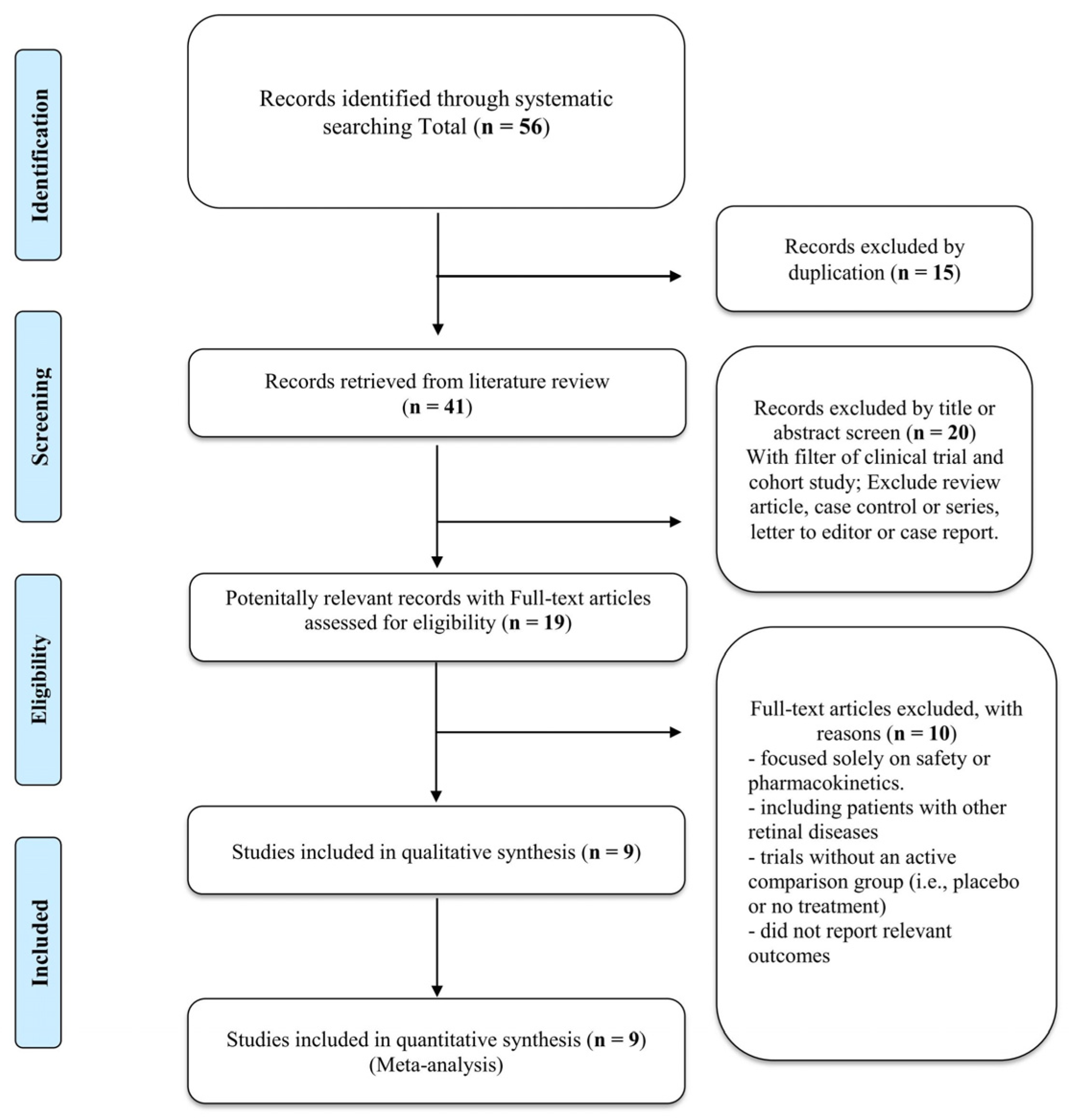
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. BCVA
3.4. CMT
3.5. Total Number of IVIs
3.6. Subgroup Analyses, Sensitivity Analyses, and Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Bahrami, B.; Zhu, M.; Hong, T.; Chang, A. Diabetic macular oedema: Pathophysiology, management challenges and treatment resistance. Diabetology 2016, 59, 1594–1608. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G.; Rangasamy, S. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef]
- Al Shamsi, H.; Ghazi, N.G. Diabetic macular edema: New trends in management. Expert Rev. Clin. Pharmacol. 2012, 5, 55–68. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Hudson, C.; Flanagan, J.G.; Turner, G.S.; Chen, H.C.; Young, L.B.; McLeod, D. Influence of laser photocoagulation for clinically significant diabetic macular oedema (DMO) on short-wavelength and conventional automated perimetry. Diabetologia 1998, 41, 1283–1292. [Google Scholar] [CrossRef]
- Schatz, H.; Madeira, D.; McDonald, H.R.; Johnson, R.N. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch. Ophthalmol. 1991, 109, 1549–1551. [Google Scholar] [CrossRef]
- Lewis, H.; Schachat, A.P.; Haimann, M.H.; Haller, J.A.; Quinlan, P.; von Fricken, M.A.; Fine, S.L.; Murphy, R.P. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology 1990, 97, 503–510, discussion 510. [Google Scholar] [CrossRef]
- Guyer, D.R.; D’Amico, D.J.; Smith, C.W. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am. J. Ophthalmol. 1992, 113, 652–656. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, F.L., 3rd; Friedman, S.M.; Glassman, A.R.; et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010, 117, 1064–1077.e35. [Google Scholar] [CrossRef]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network; Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef]
- Elman, M.J.; Ayala, A.; Bressler, N.M.; Browning, D.; Flaxel, C.J.; Glassman, A.R.; Jampol, L.M.; Stone, T.W.; Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015, 122, 375–381. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Bakri, S.J.; Wolfe, J.D.; Regillo, C.D.; Flynn, H.W.; Wykoff, C.C. Evidence-Based Guidelines for Management of Diabetic Macular Edema. J. Vitr. Dis. 2019, 3, 145–152. [Google Scholar] [CrossRef]
- Chen, J.T.; Chen, L.J.; Chen, S.N.; Chen, W.L.; Cheng, C.K.; Hsu, S.M.; Sheu, S.J.; Wu, W.C.; Yang, C.H.; Yang, C.M.; et al. Management of diabetic macular edema: Experts’ consensus in Taiwan. Jpn. J. Ophthalmol. 2020, 64, 235–242. [Google Scholar] [CrossRef]
- Virgili, G.; Parravano, M.; Evans, J.R.; Gordon, I.; Lucenteforte, E. Anti-vascular endothelial growth factor for diabetic macular oedema: A network meta-analysis. Cochrane Database Syst. Rev. 2017, 6, CD007419. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Dorin, G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: A review. Curr. Diabetes Rev. 2012, 8, 274–284. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bottega, E.; Casciano, M.; Pilotto, E.; Convento, E.; Midena, E. Microperimetry and fundus autofluorescence in diabetic macular edema: Subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010, 30, 908–916. [Google Scholar] [CrossRef]
- Vujosevic, S.; Martini, F.; Longhin, E.; Convento, E.; Cavarzeran, F.; Midena, E. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: Morphologic and functional safety. Retina 2015, 35, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Sramek, C.; Palanker, D.; Spink, C.J.; Musch, D.C. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012, 32, 375–386. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014, 34, 2010–2020. [Google Scholar] [CrossRef]
- Mansouri, A.; Sampat, K.M.; Malik, K.J.; Steiner, J.N.; Glaser, B.M. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye 2014, 28, 1418–1424. [Google Scholar] [CrossRef]
- Lavinsky, D.; Cardillo, J.A.; Melo, L.A.S.; Dare, A.; Farah, M.E.; Belfort, R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4314–4323. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mitamura, Y.; Ogata, K.; Arai, M.; Takatsuna, Y.; Yamamoto, S. Functional and morphological changes of macula after subthreshold micropulse diode laser photocoagulation for diabetic macular oedema. Eye 2010, 24, 784–788. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Sandhu, R.; Tandon, A.; Sayed-Ahmed, K.; McHugh, D.A. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: A three-year follow up. Clin. Exp. Ophthalmol. 2007, 35, 640–644. [Google Scholar] [CrossRef]
- Chen, G.; Tzekov, R.; Li, W.; Jiang, F.; Mao, S.; Tong, Y. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: A meta-analysis of randomized controlled trials. Retina 2016, 36, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Gawęcki, M. Micropulse Laser Treatment of Retinal Diseases. J. Clin. Med. 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology 1986, 93, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A. Decreasing retinal photocoagulation damage: Principles and techniques. Semin. Ophthalmol. 1999, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Sabal, B.; Teper, S.; Wylęgała, E. Subthreshold micropulse laser for diabetic macular edema: A review. J. Clin. Med. 2022, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Abbassi, S.; Thinda, S.; Yoon, J.; Yiu, G.; Morse, L.S. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur. J. Ophthalmol. 2018, 28, 68–73. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Dehghani, A.; Pourmohammadi, R.; Asadpour, L.; Pourazizi, M. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J. Curr. Ophthalmol. 2019, 31, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.; Hagras, S.M.; AbdElhamid, A.; Torky, M.A.; Awad, E.A.; Abdelhameed, A.G. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1373–1380. [Google Scholar] [CrossRef]
- Abouhussein, M.A.; Gomaa, A.R. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int. Ophthalmol. 2020, 40, 1147–1154. [Google Scholar] [CrossRef]
- Kanar, H.S.; Arsan, A.; Altun, A.; Akı, S.F.; Hacısalihoglu, A. Can subthreshold micropulse yellow laser treatment change the anti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian J. Ophthalmol. 2020, 68, 145–151. [Google Scholar] [CrossRef]
- Furashova, O.; Strassburger, P.; Becker, K.A.; Engelmann, K. Efficacy of combining intravitreal injections of ranibizumab with micropulse diode laser versus intravitreal injections of ranibizumab alone in diabetic macular edema (ReCaLL): A single center, randomised, controlled, non-inferiority clinical trial. BMC Ophthalmol. 2020, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- El Matri, L.; Chebil, A.; El Matri, K.; Falfoul, Y.; Chebbi, Z. Subthreshold micropulse laser adjuvant to bevacizumab versus bevacizumab monotherapy in treating diabetic macular edema: One- year- follow-up. Ther. Adv. Ophthalmol 2021, 13, 25158414211040887. [Google Scholar] [CrossRef] [PubMed]
- Altınel, M.G.; Acikalin, B.; Alis, M.G.; Demir, G.; Mutibayraktaroglu, K.M.; Totuk, O.M.G.; Ardagil, A. Comparison of the efficacy and safety of anti-VEGF monotherapy versus anti-VEGF therapy combined with subthreshold micropulse laser therapy for diabetic macular edema. Lasers Med. Sci. 2021, 36, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Koushan, K.; Eshtiaghi, A.; Fung, P.; Berger, A.R.; Chow, D.R. Treatment of diabetic macular edema with aflibercept and micropulse laser (DAM study). Clin. Ophthalmol. 2022, 16, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Bıçak, F.; Kayıkçıoğlu, Ö.R.; Altınışık, M.; Doğruya, S.; Kurt, E. Efficacy of subthreshold micropulse laser combined with ranibizumab in the treatment of diabetic macular edema. Int. Ophthalmol. 2022, 42, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001, 20, 3875–3889. [Google Scholar] [CrossRef]
- Higgins, J.P.; Whitehead, A.; Turner, R.M.; Omar, R.Z.; Thompson, S.G. Meta-analysis of continuous outcome data from individual patients. Stat. Med. 2001, 20, 2219–2241. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- van Aert, R.C.M.; Wicherts, J.M.; van Assen, M.A.L.M. Publication bias examined in meta-analyses from psychology and medicine: A meta-meta-analysis. PLoS ONE 2019, 14, e0215052. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold micropulse laser modulates retinal neuroinflammatory biomarkers in diabetic macular edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef]
- Gawęcki, M. Subthreshold diode micropulse laser combined with intravitreal therapy for macular edema-a systematized review and critical approach. J. Clin. Med. 2021, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Friberg, T.R.; Karatza, E.C. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology 1997, 104, 2030–2038. [Google Scholar] [CrossRef]
- Chang, D.B.; Luttrull, J.K. Comparison of subthreshold 577 and 810 nm micropulse laser effects on heat-shock protein activation kinetics: Implications for treatment efficacy and safety. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
| Study | Trial Design | Population | Eyes (N) | Age (years) | Male (%) | Pre-BCVA (LogMAR) | Pre-CMT (μm) | HbA1c (%) | Anti-VEGF | SML Wavelength (nm) | Outcome | Follow-Up Duration (Months) | Intervention | Comparator |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisseiev (2018) [36] | Cohort study | Center-involved DME | 38(19/19) | 65.3/63.3 | 63.2/68.4 | 0.29/0.41 | 316.8/408.4 | NA | R | 577 | BCVA, CMT, IVI total number | 19.1/23.2 | IVI-R+SML | IVI-R |
| Akhlaghi (2019) [37] | RCT | Refractory DME | 42(21/21) | 60.86 * | 47.6 * | 0.81/0.70 | 513/494.38 | NA | B | 810 | BCVA, CMT | NA | IVI-B+SML | IVI-B |
| Khattab (2019) [38] | RCT | Center-involved DME | 54(27/27) | 59.4/55.7 | 40.7/59.3 | NA *** | 457.1/462 | NA | A | 577 | BCVA, CMT, CS | 18 | IVI-A+SML | IVI-A |
| Abouhussein (2020) [39] | RCT | Treatment-naïve center involved DME | 40(20/20) | 60.4/59.5 | 45/40 | 0.76/0.70 | 469.6/457.9 | 8.7/8.2 | A | 577 | BCVA, CMT, IVI total number | 12 | IVI-A+SML | IVI-A |
| Kanar (2020) [40] | RCT | Treatment-naïve center involved DME | 56(28/28) | 63.42/62.64 | 54/57 | 0.40/0.38 | 466.07/451.28 | 7.97/8.02 | A | 577 | BCVA, CMT, IVI total number, SFCT | 12 | IVI-A+SML | IVI-A |
| Matri (2021) [42] | Cohort study | Treatment-naïve center involved DME | 98(49/49) | 67.7/61.3 | 59.38/64.52 ** | 0.692/0.598 | 479.1/359.9 | 7.70/7.60 | B | 577 | BCVA, CMT, IVI total number | 12 | IVI-B+SML | IVI-B |
| Altınel (2021) [43] | Cohort study | Center-involved DME | 80(40/40) | 60.55/59.83 | 57.5/55 | 0.38/0.39 | 379.2/384.68 | 6.94/6.89 | B | 577 | BCVA, CMT, IVI total number | 11.48/11.1 | IVI-B+SML | IVI-B |
| Koushan (2022) [44] | RCT | Center-involved DME | 30(15/15) | 59.8/58.8 | 66.7/46.7 | 0.36/0.38 | 457.8/433.4 | NA | A | 532 | BCVA, CMT, IVI total number | 12 | IVI-A+SML | IVI-A |
| Bıçak (2022) [45] | Cohort study | Center-involved DME (CMT≤350 μm) | 97(52/45) | 62.4/61.6 | 53.8/44.4 | 0.43/0.41 | 426.6/406.0 | 6.91/6.85 | R | 577 | BCVA, CMT, IVI total number, MV | 9.25/9.29 | IVI-R+SML | IVI-R |
| Outcome | Follow-up period | Studies Number (N) | Patients Number (N) | Measurement (95% CIs) | Cochran Q p-Value | I2 (%) |
|---|---|---|---|---|---|---|
| logMAR BCVA | 3 months | 4 | 259 | random−effects; MD, −0.01 (−0.12 to 0.10) | 0.02 | 69% |
| 6 months | 4 | 206 | random−effects; MD, −0.02 (−0.10 to 0.06) | 0.01 | 72% | |
| 12 months | 6 | 342 | random−effects; MD, −0.05 (−0.10 to −0.01) | 0.28 | 20% | |
| Central Macular Thickness (CMT) | 3 months | 5 | 313 | random−effects; MD, −2.66 (−20.67 to 15.35) | 0.02 | 65% |
| 6 months | 5 | 260 | random−effects; MD, −3.69 (−22.59 to 15.22) | 0.04 | 60% | |
| 12 months | 7 | 396 | random−effects; MD, −18.27 (−27.36 to −9.18) | 0.20 | 29% | |
| Total Number of IVI | Overall | 5 | 302 | random−effects; MD, −2.22 (−3.13 to −1.31) | <0.01 | 84% |
| Outcome | Follow-Up Period/Subgroup | Studies Number (N) | Patients Number (N) | Measurement (95% CIs) | Cochran Q p-Value | I2 (%) |
|---|---|---|---|---|---|---|
| logMAR BCVA | 3 months–Overall | 4 | 259 | random−effects; MD, −0.01 (−0.12 to 0.10) | 0.02 | 69% |
| 3 months–810 nm | 1 | 42 | MD, −0.17 (−0.35 to 0.01) | - | - | |
| 3 months–577 nm | 3 | 217 | random−effects; MD, 0.03 (−0.07 to 0.13) | 0.06 | 63% | |
| 6 months–Overall | 4 | 206 | random−effects; MD, −0.02 (−0.10 to 0.06) | 0.01 | 72% | |
| 6 months–577 nm | 3 | 176 | random−effects; MD, 0.00 (−0.09 to 0.11) | 0.02 | 75% | |
| 6 months–532 nm | 1 | 30 | MD, −0.11 (−0.23 to 0.01) | - | - | |
| 12 months–Overall | 6 | 342 | random−effects; MD, −0.05 (−0.10 to −0.01) | 0.28 | 20% | |
| 12 months–577 nm | 5 | 312 | random−effects; MD, −0.05 (−0.10 to −0.01) | 0.28 | 20% | |
| 12 months–532 nm | 1 | 15 | MD, −0.10 (−0.23 to 0.03) | 0.93 | 0% | |
| Central Macular Thickness (CMT) | 3 months–Overall | 5 | 313 | random−effects; MD, −2.66 (−20.67 to 15.35) | 0.02 | 65% |
| 3 months–810 nm | 1 | 42 | MD, −94.28 (−168.80 to −19.76) | - | - | |
| 3 months–577 nm | 4 | 271 | random−effects; MD, 2.56 (−10.35 to 15.48) | 0.17 | 40% | |
| 6 months–Overall | 5 | 260 | random−effects; MD, −3.69 (−22.59 to 15.22) | 0.04 | 60% | |
| 6 months–577 nm | 4 | 230 | random−effects; MD, −3.60 (−25.76 to 18.56) | 0.02 | 69% | |
| 6 months–532 nm | 1 | 30 | MD, −7.30 (−48.06 to 33.46) | - | - | |
| 12 months–Overall | 7 | 396 | random−effects; MD, −18.27 (−27.36 to −9.18) | 0.20 | 29% | |
| 12 months–577 nm | 6 | 366 | random−effects; MD, −19.90 (−29.30 to −10.60) | 0.22 | 29% | |
| 12 months–532 nm | 1 | 30 | MD, 1.20 (−27.79 to 30.19) | - | - | |
| Total Number of IVI | Overall | 5 | 302 | random−effects; MD, −2.22 (−3.13 to −1.31) | <0.01 | 84% |
| 577 nm | 4 | 272 | random−effects; MD, −2.38 (−3.33 to −1.42) | <0.01 | 88% | |
| 532 nm | 1 | 30 | MD, −0.60 (−3.07 to 1.87) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-C.; Chen, P.-H.; Hsieh, Y.-H. Optimizing Diabetic Macular Edema Treatment: A Meta-Analysis of Subthreshold Micropulse Laser and Anti-Vascular Endothelial Growth Factor Combination Therapy. J. Clin. Med. 2024, 13, 4782. https://doi.org/10.3390/jcm13164782
Ma C-C, Chen P-H, Hsieh Y-H. Optimizing Diabetic Macular Edema Treatment: A Meta-Analysis of Subthreshold Micropulse Laser and Anti-Vascular Endothelial Growth Factor Combination Therapy. Journal of Clinical Medicine. 2024; 13(16):4782. https://doi.org/10.3390/jcm13164782
Chicago/Turabian StyleMa, Ching-Chih, Po-Huang Chen, and Yun-Hsiu Hsieh. 2024. "Optimizing Diabetic Macular Edema Treatment: A Meta-Analysis of Subthreshold Micropulse Laser and Anti-Vascular Endothelial Growth Factor Combination Therapy" Journal of Clinical Medicine 13, no. 16: 4782. https://doi.org/10.3390/jcm13164782






