Mortality Outcomes and Contributing Risk Factors in Patients with Hospital-Associated Disability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Automated Screening System for Hospital-Associated Disability
2.3. Diagnostic Criteria for Hospital-Associated Disability
2.4. Quantitative Assessment of Functional Decline
2.5. Measurement of Body Composition Using Bioelectrical Impedance Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Risk Factors Associated with 3-Month Mortality
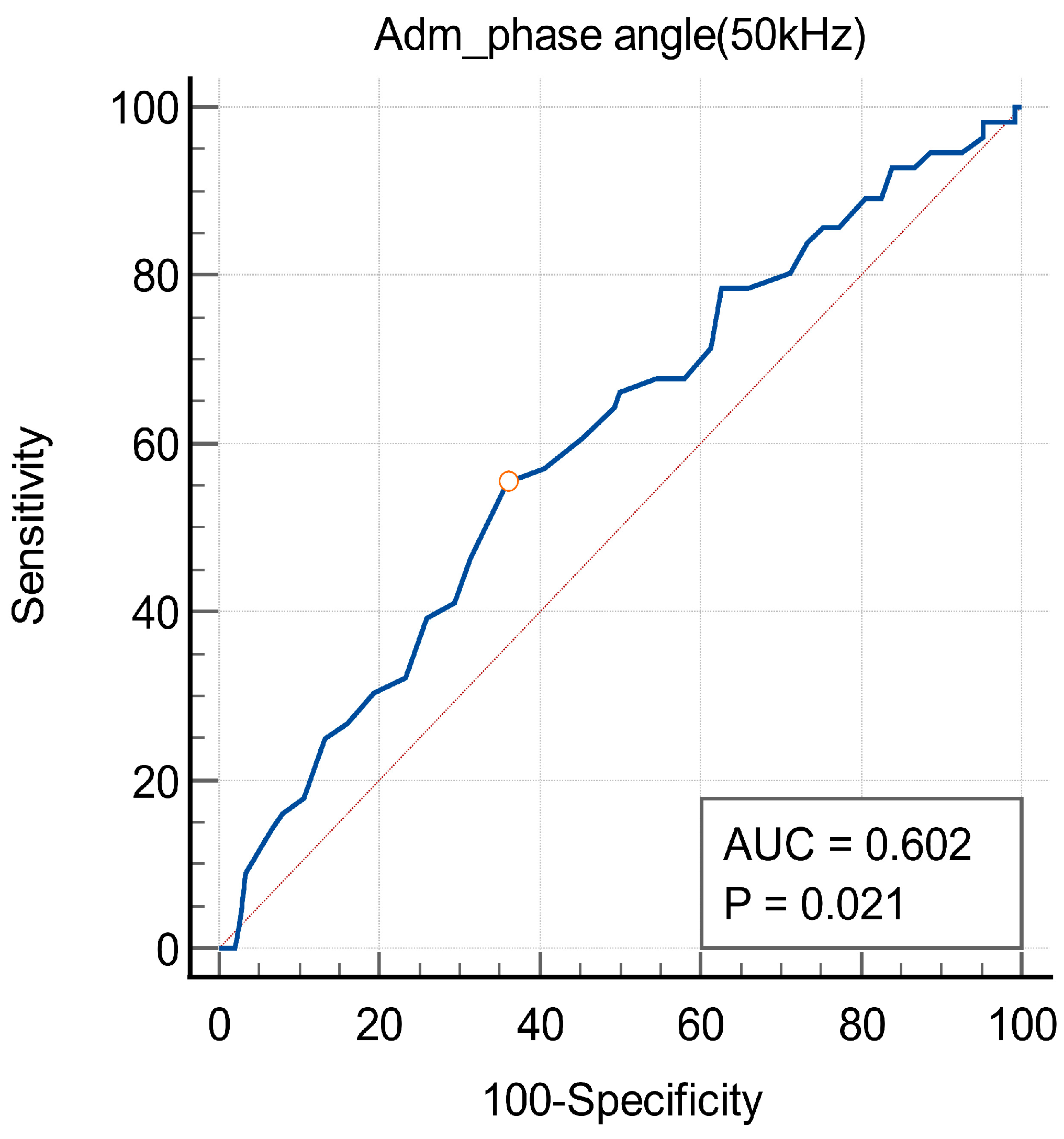
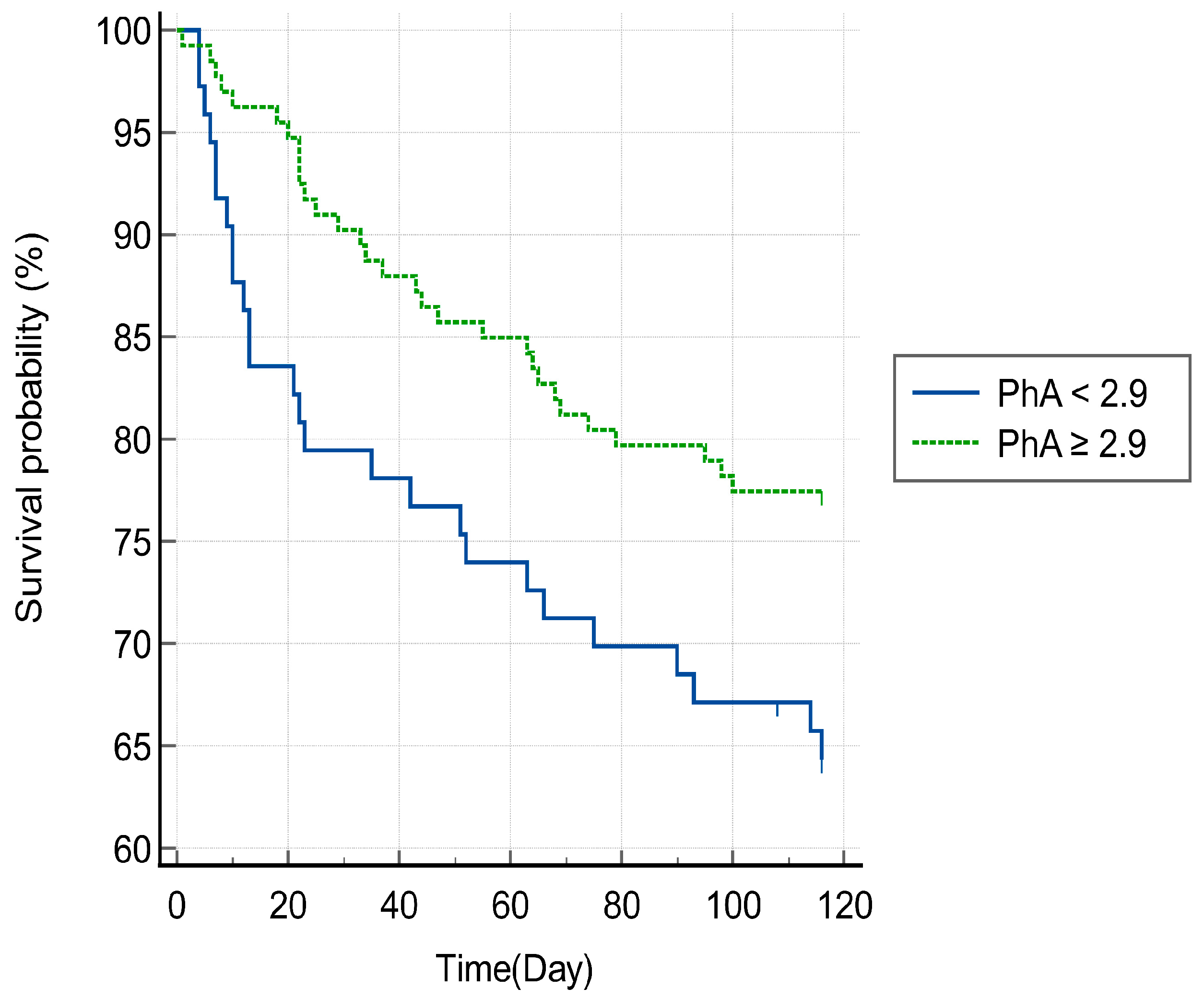
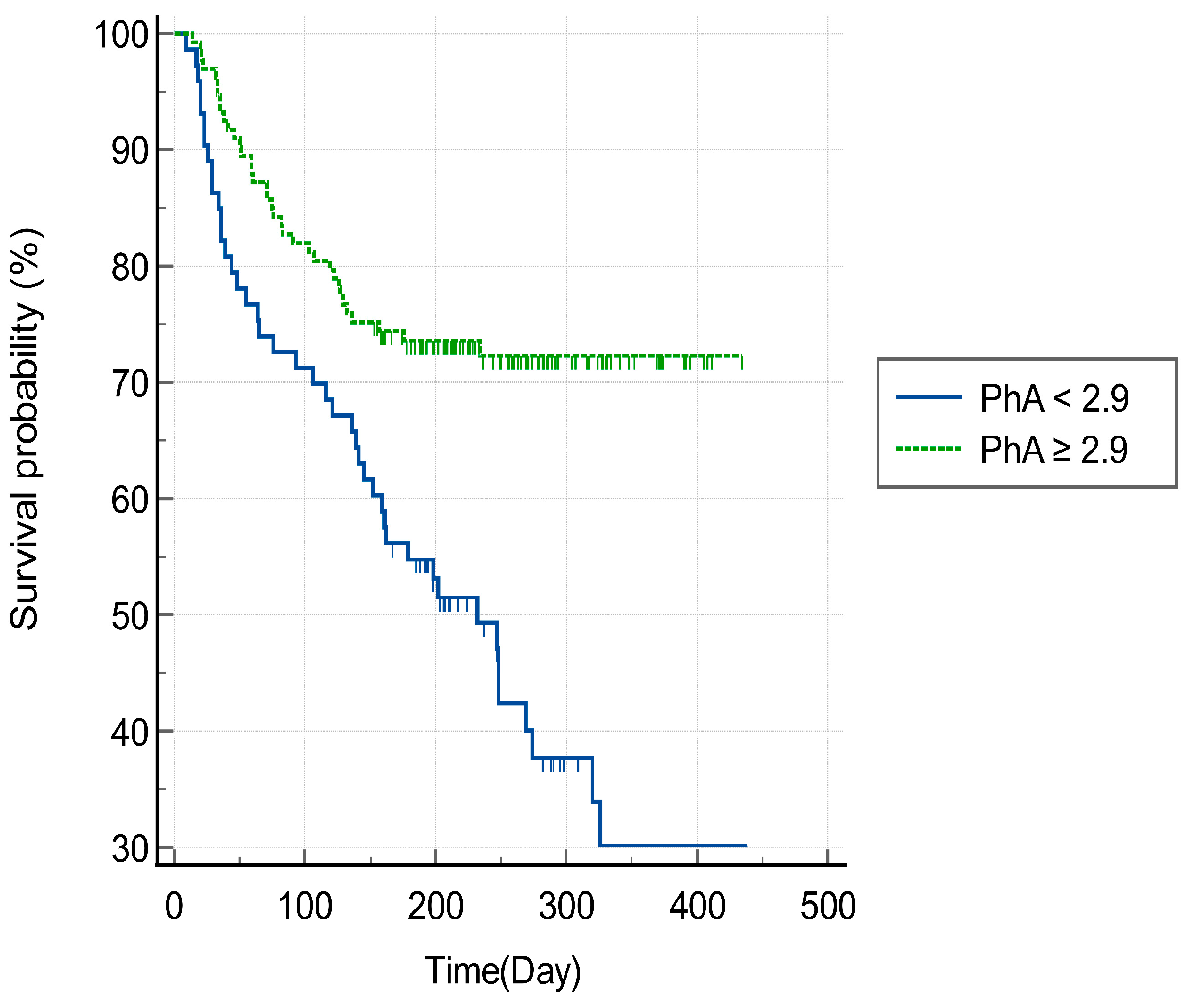
3.3. Phase Angle and Mortality of Patients with HAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Kresevic, D.; Burant, C.J.; Landefeld, C.S. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-Associated Disability “She Was Probably Able to Ambulate, but I’m Not Sure”. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Loyd, C.; Markland, A.D.; Zhang, Y.; Fowler, M.; Harper, S.; Wright, N.C.; Carter, C.S.; Buford, T.W.; Smith, C.H.; Kennedy, R.; et al. Prevalence of Hospital-Associated Disability in Older Adults: A Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 455–461. [Google Scholar] [CrossRef]
- Gillick, M.R.; Serrell, N.A.; Gillick, L.S. Adverse consequences of hospitalization in the elderly. Soc. Sci. Med. 1982, 16, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.A.; Franke, T.; Inouye, S.K.; Landefeld, C.S.; Morgan, T.M.; Rudberg, M.A.; Sebens, H. Functional outcomes of acute medical illness and hospitalization in older persons. Arch. Intern. Med. 1996, 156, 645–652. [Google Scholar] [CrossRef]
- Sager, M.A.; Rudberg, M.A.; Jalaluddin, M.; Franke, T.; Inouye, S.K.; Landefeld, C.S.; Siebens, H.; Winograd, C.H. Hospital admission risk profile (HARP): Identifying older patients at risk for functional decline following acute medical illness and hospitalization. J. Am. Geriatr. Soc. 1996, 44, 251–257. [Google Scholar] [CrossRef]
- Gallego-González, E.; Mayordomo-Cava, J.; Vidán, M.T.; Valadés-Malagón, M.I.; Serra-Rexach, J.A.; Ortiz-Alonso, J. Functional trajectories associated with acute illness and hospitalization in oldest old patients: Impact on mortality. Front. Physiol. 2022, 13, 937115. [Google Scholar] [CrossRef] [PubMed]
- Zisberg, A.; Shadmi, E.; Gur-Yaish, N.; Tonkikh, O.; Sinoff, G. Hospital-associated functional decline: The role of hospitalization processes beyond individual risk factors. J. Am. Geriatr. Soc. 2015, 63, 55–62. [Google Scholar] [CrossRef]
- Inouye, S.K.; Peduzzi, P.N.; Robison, J.T.; Hughes, J.S.; Horwitz, R.I.; Concato, J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 1998, 279, 1187–1193. [Google Scholar] [CrossRef]
- Gill, T.M.; Allore, H.G.; Holford, T.R.; Guo, Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004, 292, 2115–2124. [Google Scholar] [CrossRef]
- Chodos, A.H.; Kushel, M.B.; Greysen, S.R.; Guzman, D.; Kessell, E.R.; Sakar, U.; Goldman, L.E.; Critchfield, J.M.; Pierluissi, E. Hospitalization-Associated Disability in Adults Admitted to a Safety-Net Hospital. J. Gen. Intern. Med. 2015, 30, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Onder, G.; Cavalieri, M.; Guerra, G.; Sioulis, F.; Maraldi, C.; Zuliani, G.; Fellin, R.; Italian Group of Pharmacoepidemiology in the Elderly Study (GIFA). Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J. Gen. Intern. Med. 2007, 22, 668–674. [Google Scholar] [CrossRef]
- McCusker, J.; Kakuma, R.; Abrahamowicz, M. Predictors of functional decline in hospitalized elderly patients: A systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M569–M577. [Google Scholar] [CrossRef] [PubMed]
- Admi, H.; Shadmi, E.; Baruch, H.; Zisberg, A. From research to reality: Minimizing the effects of hospitalization on older adults. Rambam Maimonides Med. J. 2015, 6, e0017. [Google Scholar] [CrossRef]
- Boyd, C.M.; Landefeld, C.S.; Counsell, S.R.; Palmer, R.M.; Fortinsky, R.H.; Kresevic, D.; Burant, C.; Covinsky, K.E. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J. Am. Geriatr. Soc. 2008, 56, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.M.; Pierluissi, E.; Boscardin, W.J.; Kirby, K.A.; Walter, L.C.; Chren, M.M.; Palmer, R.M.; Counsell, S.R.; Landefeld, C.S. A clinical index to stratify hospitalized older adults according to risk for new-onset disability. J. Am. Geriatr. Soc. 2011, 59, 1206–1216. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in stage IIIB and IV non-small cell lung cancer. BMC Cancer 2009, 9, 37. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez, E.; Guevara, P.; Núñez-Valencia, C.; Oñate-Ocaña, L.F.; Flores, D.; Arrieta, O. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: A prospective study. Nutr. Cancer 2012, 64, 526–534. [Google Scholar] [CrossRef]
- Wirth, R.; Volkert, D.; Rösler, A.; Sieber, C.C.; Bauer, J.M. Bioelectric impedance phase angle is associated with hospital mortality of geriatric patients. Arch. Gerontol. Geriatr. 2010, 51, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R.S.; Gonzalez, M.C.; Freire, S.M.; Lourenço, R.A. Low phase angle in critically ill older patients is associated with late mortality: A prospective study. Nutrition 2023, 105, 111852. [Google Scholar] [CrossRef]
- Thibault, R.; Makhlouf, A.M.; Mulliez, A.; Cristina Gonzalez, M.; Kekstas, G.; Kozjek, N.R.; Preiser, J.C.; Rozalen, I.C.; Dadet, S.; Krznaric, Z.; et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: The international prospective observational study Phase Angle Project. Intensive Care Med. 2016, 42, 1445–1453. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service, Republic of Korea. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020049000100&brdScnBltNo=4&brdBltNo=67#none (accessed on 14 July 2020).
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Kleyweg, R.P.; van der Meché, F.G.; Schmitz, P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991, 14, 1103–1109. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, G.M. Comparison of the Berg Balance Scale and Fullerton Advanced Balance Scale to predict falls in community-dwelling adults. J. Phys. Ther. Sci. 2017, 29, 232–234. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Santos, A.R.; Amaral, T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty-a systematic review. BMC Geriatr. 2017, 17, 238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jan, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J. Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 311–317. [Google Scholar] [CrossRef]
- Narain, P.; Rubenstein, L.Z.; Wieland, G.D.; Rosbrook, B.; Strome, L.S.; Pieetruszka, F.; Morley, J.E. Predictors of immediate and 6-month outcomes in hospitalized elderly patients: The importance of functional status. J. Am. Geriatr. Soc. 1988, 36, 775–783. [Google Scholar] [CrossRef]
- Walter, L.C.; Brand, R.J.; Counsell, S.R.; Palmer, R.M.; Landefeld, C.S.; Fortinsky, R.H.; Covinsky, K.E. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 2001, 285, 2987–2994. [Google Scholar] [CrossRef]
- Gbeasor-Komlanvi, F.A.; Tchankoni, M.K.; Bakoubayi, A.W.; Lokossou, M.Y.; Sadio, A.; Zida-Compaore, W.I.C.; Djibril, M.; Belo, M.; Agbonon, A.; Ekouevi, D.K. Predictors of three-month mortality among hospitalized older adults in Togo. BMC Geriatr. 2020, 51, 507. [Google Scholar] [CrossRef]
- Baztán, J.J.; Gálvez, C.P.; Socorro, A. Recovery of functional impairment after acute illness and mortality: One-year follow-up study. Gerontology 2009, 55, 269–274. [Google Scholar] [CrossRef]
- Statistics Korea. 2022 Cause of Death Statistics Results. Available online: https://kostat.go.kr/board.es?mid=a10301060200&bid=218&act=view&list_no=427216 (accessed on 21 September 2023).
- Kim, K.W.; Jang, S. Characteristics and mortality risk factors in geriatric hospital patients visiting one region-wide emergency department. J. Korean Acad. Community Health Nurs. 2016, 27, 327–336. [Google Scholar] [CrossRef][Green Version]
- Brandt, C.; Pedersen, B.K. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. BioMed Res. Int. 2010, 2010, 520258. [Google Scholar] [CrossRef]
- Lightfoot, A.; McArdle, A.; Griffiths, R.D. Muscle in defense. Crit. Care Med. 2009, 37, S384–S390. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef]
- Looijaard, W.G.; Stapel, S.N.; Dekker, I.M.; Rusticus, H.; Remmelzwaal, S.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Identifying critically ill patients with low muscle mass: Agreement between bioelectrical impedance analysis and computed tomography. Clin. Nutr. 2020, 39, 1809–1817. [Google Scholar] [CrossRef]
- Toptas, M.; Yalcin, M.; Akkoc, İ.; Demir, E.; Metin, C.; Savas, Y.; Kalyoncuoglu, M.; Can, M.M. The relation between sarcopenia and mortality in patients at intensive care unit. BioMed Res. Int. 2018, 1, 5263208. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Shibuya, K.; Rao, C.; Mathers, C.D.; Hansell, A.L.; Held, L.S.; Schmid, V.; Buist, S. Chronic obstructive pulmonary disease: Current burden and future projections. Eur. Respir. J. 2006, 27, 397–412. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Scichilone, N.; Natoli, G.; Nobili, A.; Corazza, G.R.; Mannucci, P.M.; Pericone, F.; Corrao, S.; REPOSI Investigators. Pattern of comorbidities and 1-year mortality in elderly patients with COPD hospitalized in internal medicine wards: Data from the RePoSI Registry. Intern. Emerg. Med. 2021, 16, 389–400. [Google Scholar] [CrossRef]
- Hasegawa, W.; Yamauchi, Y.; Yasunaga, H.; Sunohara, M.; Jo, T.; Matsui, H.; Fushimi, K.; Takami, K.; Nagase, T. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax 2012, 67, 957–963. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.J.; Yang, J.H.; Kim, C.M.; Choi, W.S. The Association between Phase Angle of Bioelectrical Impedance Analysis and Survival Time in Advanced Cancer Patients: Preliminary Study. Korean J. Fam. Med. 2014, 35, 251–256. [Google Scholar] [CrossRef]
- Colín-Ramírez, E.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Durán, M.; Rodríguez, A.E.; Keirns-Davis, C. Bioelectrical impedance phase angle as a prognostic marker in chronic heart failure. Nutrition 2012, 28, 901–905. [Google Scholar] [CrossRef]
- Ko, S.J.; Cho, J.; Choi, S.M.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Lee, J. Phase angle and frailty are important prognostic factors in critically ill medical patients: A prospective cohort study. J. Nutr. Health Aging 2021, 25, 218–223. [Google Scholar] [CrossRef]
- Stapel, S.N.; Looijaard, W.G.; Dekker, I.M.; Girbes, A.R.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Working Group on Functional Outcome Measures for Clinical Trials. Functional outcomes for clinical trials in frail older persons: Time to be moving. J. Gerontol. Biol. Sci. Med. Sci. 2008, 63, 160. [Google Scholar] [CrossRef]

| Variable | Total Population, n = 206 (100%) | 3 Months Survivors, n = 150 (72.8%) | 3 Months Non-Survivors, n = 56 (27.2%) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) | 73.1 (12.5) | 73.2 (12.6) | 72.8 (12.4) | 0.85 |
| Sex (Male, %) | 115 (55.8) | 73 (48.7) | 42 (75.0) | <0.001 |
| BMI, mean (SD) | 21.4 (3.9) | 21.2 (3.9) | 21.8 (4.1) | 0.40 |
| Former/current smoker (%) | 65 (31.6) | 42 (28.0) | 23 (41.1) | 0.07 |
| Comorbidities | ||||
| Hypertension (%) | 108 (52.4) | 80 (53.3) | 28 (50.0) | 0.67 |
| Diabetes mellitus (%) | 93 (45.2) | 71 (47.3) | 22 (39.3) | 0.30 |
| Chronic kidney disease (%) | 43 (20.9) | 31 (20.7) | 12 (21.4) | 0.90 |
| Coronary artery disease (%) | 32 (15.5) | 24 (16.0) | 8 (14.3) | 0.76 |
| Heart failure (%) | 20 (9.7) | 15 (10.0) | 5 (8.9) | 0.82 |
| Cerebrovascular disease (%) | 33 (16.0) | 23 (15.3) | 10 (17.9) | 0.66 |
| Asthma (%) | 9 (4.4) | 7 (4.7) | 2 (3.6) | 1.00 |
| COPD (%) | 12 (5.8) | 9 (6.0) | 3 (5.4) | 1.00 |
| Cancer (%) | 73 (35.4) | 44 (29.3) | 29 (51.8) | <0.01 |
| History of ICU admission (%) | 44 (21.4) | 37 (24.7) | 7 (12.5) | 0.06 |
| Total ICU days, mean (SD) (a) | 11.6 (9.3) | 11.8 (9.7) | 10.4 (7.0) | 0.72 |
| Physical function | ||||
| MMT, mean (SD) | 39.2 (7.6) | 39.5 (6.6) | 38.2 (9.6) | 0.36 |
| BBS, mean (SD) | 7.2 (9.9) | 7.9 (10.4) | 5.6 (8.5) | 0.14 |
| Grip (b) (Low, %) | 200 (97.1) | 145 (96.7) | 55 (96.7) | 1.00 |
| Activities of daily living | ||||
| MBI, mean (SD) | 26.7 (24.3) | 28.6 (23.8) | 21.5 (25.1) | 0.07 |
| Body composition | ||||
| SMI (c) (Low, %) | 199 (79.3) | 159 (77.2) | 40 (71.4) | 0.23 |
| Phase angle, mean (SD) | 3.4 (1.2) | 3.5 (1.2) | 3.1 (1.1) | <0.05 |
| PBF (%), mean (SD) | 28.1 (11.2) | 29.1 (10.9) | 25.4 (11.4) | <0.05 |
| Univariate | Multivariate | |||||
| Variables | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Sex (male) | 3.16 | 1.60–6.27 | <0.01 | 3.23 | 1.58–6.61 | <0.01 |
| Cancer | 2.59 | 1.38–4.86 | <0.01 | 2.18 | 1.13–4.20 | <0.05 |
| Phase angle (50 kHz) | 0.74 | 0.54–0.99 | <0.05 | 0.69 | 0.50–0.95 | <0.05 |
| PBF (%) | 0.97 | 0.94–0.99 | <0.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.-J.; Lee, S.-H.; Min, H.-J.; Kim, H.-J.; Kong, H.-H. Mortality Outcomes and Contributing Risk Factors in Patients with Hospital-Associated Disability. J. Clin. Med. 2024, 13, 4798. https://doi.org/10.3390/jcm13164798
Jo S-J, Lee S-H, Min H-J, Kim H-J, Kong H-H. Mortality Outcomes and Contributing Risk Factors in Patients with Hospital-Associated Disability. Journal of Clinical Medicine. 2024; 13(16):4798. https://doi.org/10.3390/jcm13164798
Chicago/Turabian StyleJo, Soo-Jeong, So-Hee Lee, Hyo-Jin Min, Hee-Ji Kim, and Hyun-Ho Kong. 2024. "Mortality Outcomes and Contributing Risk Factors in Patients with Hospital-Associated Disability" Journal of Clinical Medicine 13, no. 16: 4798. https://doi.org/10.3390/jcm13164798
APA StyleJo, S.-J., Lee, S.-H., Min, H.-J., Kim, H.-J., & Kong, H.-H. (2024). Mortality Outcomes and Contributing Risk Factors in Patients with Hospital-Associated Disability. Journal of Clinical Medicine, 13(16), 4798. https://doi.org/10.3390/jcm13164798






