Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Echocardiography Measurements
2.3. Statistical Analysis
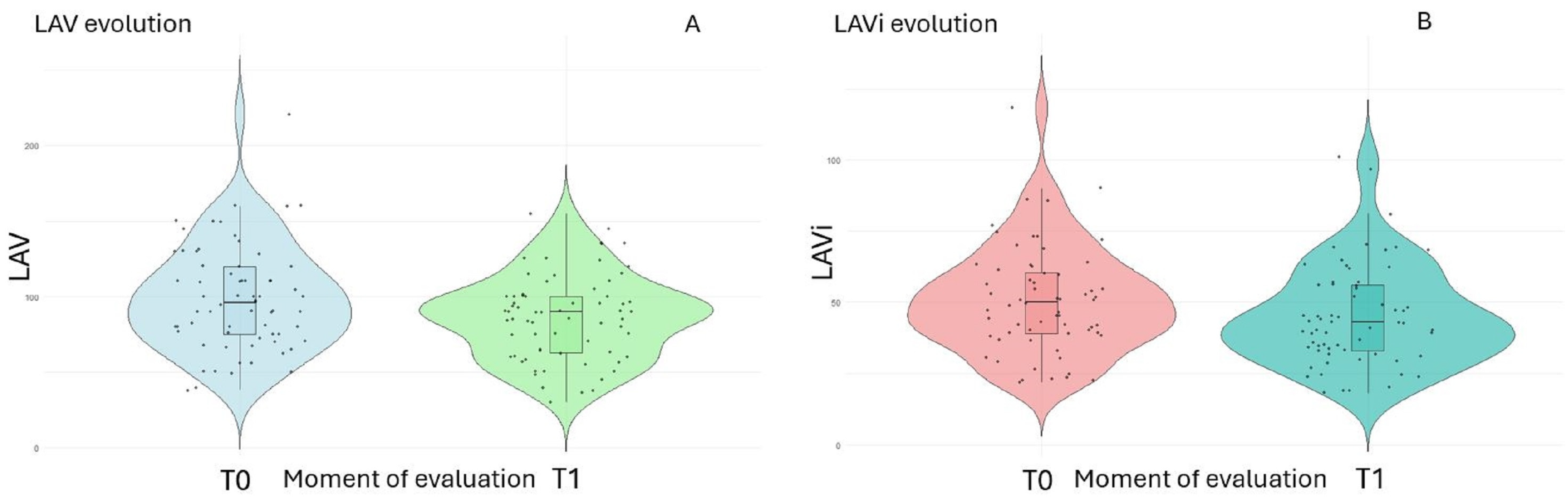
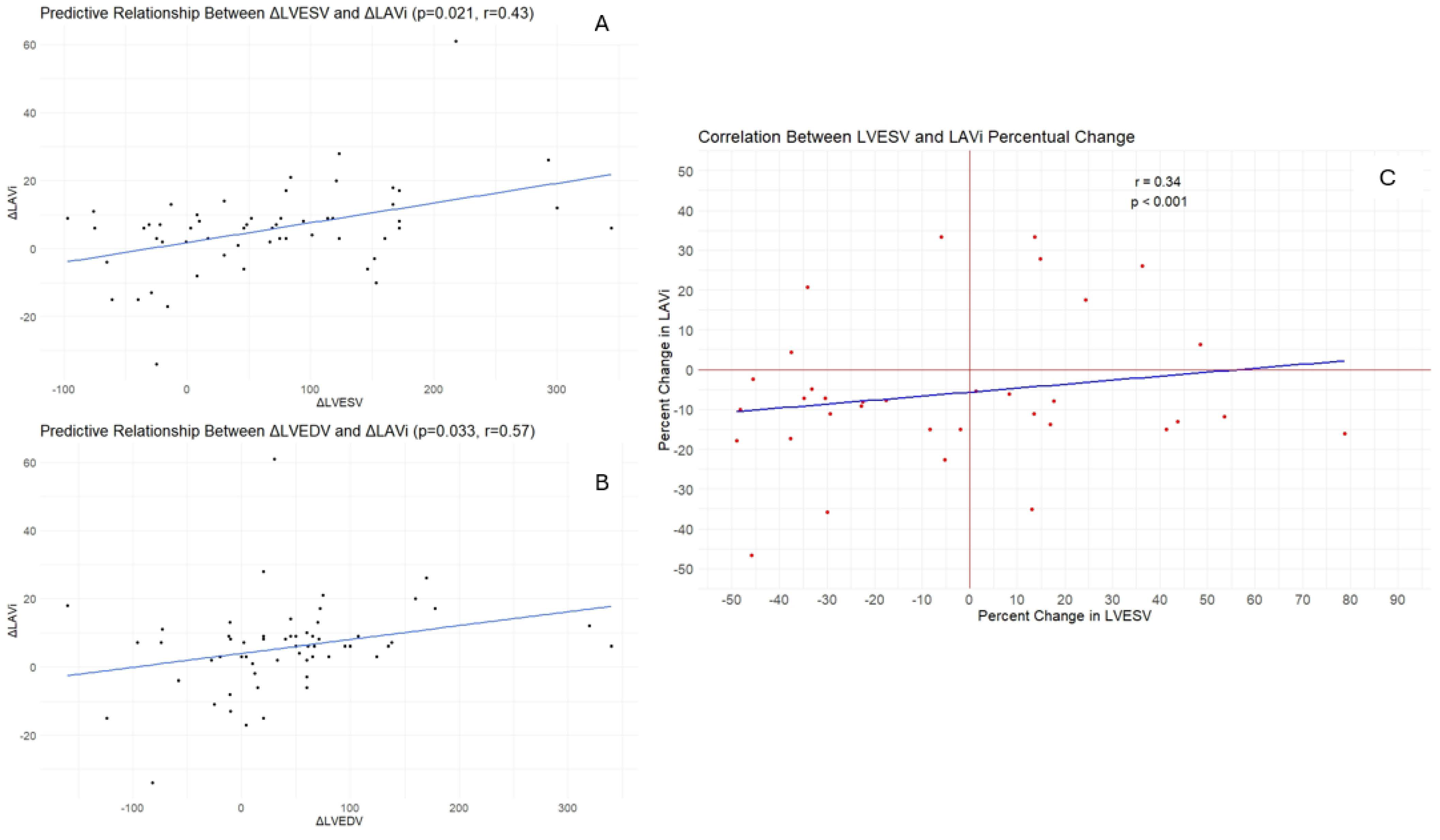
3. Results
4. Discussions
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglas, P.S. The left atrium: A biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J. Am. Coll. Cardiol. 2003, 42, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Lam, C.S. Function over form? Assessing the left atrium in heart failure. Eur. Heart J. 2015, 36, 711–714. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Lefy atrial volume as an index of left atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef]
- Feneon, D.; Behaghel, A.; Bernard, A.; Fournet, M.; Mabo, P.; Daubert, J.C.; Leclercq, C.; Donal, E. Left atrial function, a new predictor of response to cardiac resynchronization therapy? Heart Rhythm 2015, 12, 1800–1806. [Google Scholar] [CrossRef]
- Badran, H.A.; Abdelhamid, M.; Ibrahim, M.T.; Abdelmoteleb, A.M.; Zarif, J.K. Left atrium in cardiac resynchronization therapy: Active participant or innocent bystander. J. Saudi Heart Assoc. 2017, 29, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Bajraktari, G.; Lindqvist, P.; Henein, M.Y. Improved Left Atrial Function in CRT Responders: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 298. [Google Scholar] [CrossRef]
- Gasparini, M.; Birnie, D.; Lemke, B.; Aonuma, K.; Lee, K.L.; Gorcsan, J., 3rd; Landolina, M.; Klepfer, R.; Meloni, S.; Cicconelli, M.; et al. Adaptive Cardiac Resynchronization Therapy Reduces Atrial Fibrillation Incidence in Heart Failure Patients with Prolonged AV Conduction: The Adaptive CRT Randomized Trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007260. [Google Scholar] [CrossRef] [PubMed]
- Cozma, D.; Vacarescu, C.; Petrescu, L.; Mornos, C.; Goanta, E.; Feier, H.; Luca, C.T.; Gusetu, G.; Vatasescu, R. CRT Pacing: Midterm Follow-Up in LV Only Pacing without RV Lead in Patients with Normal AV Conduction. J. Clin. Med. 2018, 7, 531. [Google Scholar] [CrossRef]
- Goanță, E.V.; Luca, C.T.; Vacarescu, C.; Crișan, S.; Petrescu, L.; Vatasescu, R.; Lazăr, M.A.; Gurgu, A.; Turi, V.R.; Cozma, D. Nonischemic Super-Responders in Fusion CRT Pacing with Normal Atrioventricular Conduction. Diagnostics 2022, 23, 122032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burri, H.; Prinzen, F.W.; Gasparini, M.; Leclercq, C. Left univentricular pacing for cardiac resynchronization therapy. Europace 2017, 19, 912–919, Erratum in Europace 2017, 19, 1415. https://doi.org/10.1093/europace/euw441; Erratum in Europace 2017, 19, 920. https://doi.org/10.1093/europace/eux204. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Auricchio, A.; Martens, P.; Witte, K.; Cowie, M.R.; Delgado, V.; Dickstein, K.; Linde, C.; Vernooy, K.; Leyva, F.; et al. Optimized implementation of cardiac resynchronization therapy: A call for action for referral and optimization of care: A joint position statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 2349–2369. [Google Scholar] [CrossRef] [PubMed]
- Vacarescu, C.; Cozma, D.; Petrescu, L.; Dragan, S.; Mornos, C.; Crisan, S.; Feier, H.; Lazar, M.A.; Cozlac, R.A.; Luca, C.T. Exercise test is essential in LV-only fusion CRT pacing without right ventricle lead. Clin. Interv. Aging 2019, 14, 969–975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632, Erratum in Eur. Heart J. 2022, 43, 2022. https://doi.org/10.1093/eurheartj/ehac051. [Google Scholar] [CrossRef] [PubMed]
- Auger, D.; Ducharme, A.; Harel, F.; Thibault, B.; O’Meara, E. Patient assessment for cardiac resynchronization therapy: Past, present and future of imaging techniques. Can. J. Cardiol. 2010, 26, 27–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ratajczak, P.; Sławińska, A.; Martynowska-Rymer, I.; Strześniewski, P.; Rusak, G. Anatomical Evaluation of the Pulmonary Veins and the Left Atrium Using Computed Tomography Before Catheter Ablation: Reproducibility of Measurements. Pol. J. Radiol. 2016, 81, 228–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pastore, G.; Zanon, F.; Baracca, E.; Aggio, S.; Corbucci, G.; Boaretto, G.; Roncon, L.; Noventa, F.; Barold, S.S. The risk of atrial fibrillation during right ventricular pacing. Europace 2016, 18, 353–358. [Google Scholar] [CrossRef]
- Xie, J.M.; Fang, F.; Zhang, Q.; Chan, J.Y.; Yip, G.W.; Sanderson, J.E.; Lam, Y.Y.; Yan, B.P.; Yu, C.M. Left atrial remodeling and reduced atrial pump function after chronic right ventricular apical pacing in patients with preserved ejection fraction. Int. J. Cardiol. 2012, 157, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, H.; Li, X.; Yao, Y.; Liu, Z.; Fan, X. Left bundle branch area pacing versus right ventricular pacing in patients with persistent atrial fibrillation requiring ventricular pacing. Pacing Clin. Electrophysiol. 2021, 44, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Chao, C.J. Adverse effects of right ventricular pacing on cardiac function: Prevalence, prevention and treatment with physiologic pacing. Trends Cardiovasc. Med. 2023, 33, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Sharma, P.S.; Patel, N.R.; Dommaraju, S.; Zalavadia, D.V.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. New-Onset Atrial Fibrillation in Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing. Circ. Arrhythmia Electrophysiol. 2022, 15, 264–266. [Google Scholar] [CrossRef]
- Radu, A.D.; Zlibut, A.; Scarlatescu, A.; Cojocaru, C.; Bogdan, S.; Scafa-Udriște, A.; Dorobantu, M. Cardiac Resynchronization Therapy and Left Atrial Remodeling: A Novel Insight? Biomedicines 2023, 11, 1156. [Google Scholar] [CrossRef]
- Singh, J.P.; Cha, Y.M.; Lunati, M.; Chung, E.S.; Li, S.; Smeets, P.; O’Donnell, D. Real-world behavior of CRT pacing using the AdaptivCRT algorithm on patient outcomes: Effect on mortality and atrial fibrillation incidence. J. Cardiovasc. Electrophysiol. 2020, 31, 825–833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Y.; Hua, W.; Shen, F.; Zou, J.; Tang, B.; Chen, K.; Liang, Y.; He, L.; Zhou, X.; Zhang, X.; et al. Left ventricular-only fusion pacing versus cardiac resynchronization therapy in heart failure patients: A randomized controlled trial. Clin. Cardiol. 2021, 44, 1225–1232. [Google Scholar] [CrossRef]
- Thibault, B.; Ducharme, A.; Harel, F.; White, M.; O’Meara, E.; Guertin, M.C.; Lavoie, J.; Frasure-Smith, N.; Dubuc, M.; Guerra, P.; et al. Left ventricular versus simultaneous biventricular pacing in patients with heart failure and a QRS complex ≥120 milliseconds. Circulation 2011, 124, 2874–2881. [Google Scholar] [CrossRef]
- Boriani, G.; Kranig, W.; Donal, E.; Calo, L.; Casella, M.; Delarche, N.; Lozano, I.F.; Ansalone, G.; Biffi, M.; Boulogne, E.; et al. A randomized double-blind comparison of biventricular versus left ventricular stimulation for cardiac resynchronization therapy: The Biventricular versus Left Univentricular Pacing with ICD Back-up in Heart Failure Patients (B-LEFT HF) trial. Am. Heart J. 2010, 159, 1052–1058.e1. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.M.; Ellenbogen, K.A.; Gold, M.R.; Lemke, B.; Lozano, I.F.; Mittal, S.; Spinale, F.G.; VAN Eyk, J.E.; Waggoner, A.D.; Meyer, T.E. Smart Delay determined AV optimization: A comparison of AV delay methods used in cardiac resynchronization therapy (SMART-AV): Rationale and design. Pacing Clin. Electrophysiol. 2010, 33, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Waddingham, P.H.; Lambiase, P.; Muthumala, A.; Rowland, E.; Chow, A.W. Fusion Pacing with Biventricular, Left Ventricular-only and Multipoint Pacing in Cardiac Resynchronisation Therapy: Latest Evidence and Strategies for Use. Arrhythm. Electrophysiol. Rev. 2021, 10, 91–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Zhang, Y.; Sun, Y.; Chen, M.; Wang, Z.; Ma, C. Success rates, challenges and troubleshooting of left bundle branch area pacing as a cardiac resynchronization therapy for treating patients with heart failure. Front. Cardiovasc. Med. 2023, 9, 1062372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.T.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacingand resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European HeartRhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force oncardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar]
- Tabbah, R.; Francis, J.; Skouri, H.; Khoury, M.; Abi-Saleh, B. Ejection fraction improvement in left ventricular-only pacing vs. BiVentricular pacing in patients with heart failure. Heart Vessel. Transplant. 2024, 8, 191–199. [Google Scholar] [CrossRef]
- Sweeney, M.O.; Bank, A.J.; Nsah, E.; Koullick, M.; Zeng, Q.C.; Hettrick, D.; Sheldon, T.; Lamas, G.A. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N. Engl. J. Med. 2007, 357, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Veasey, R.A.; Arya, A.; Silberbauer, J.; Sharma, V.; Lloyd, G.W.; Patel, N.R.; Sulke, A.N. The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: The long-MinVPACE study. Europace 2011, 13, 815–820. [Google Scholar] [CrossRef]
- Eriksson, P.; Wilhelmsen, L.; Rosengren, A. Bundle-branch block in middle-aged men: Risk of complications and death over 28 years: The Primary Prevention Study in Göteborg, Sweden. Eur. Heart J. 2005, 26, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Mosoiu, D.; Rogozea, L.; Landon, A.; Bisoc, A.; Tint, D. Palliative Care in Heart Failure: A Public Health Emergency. Am. J. Ther. 2020, 27, e204–e223. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Tilz, R.; Boveda, S.; Dobreanu, D.; Sciaraffia, E.; Mansourati, J.; Papiashvili, G.; Dagres, N. Implantable cardioverter defibrillator use for primary prevention in ischaemic and non-ischaemic heart disease-indications in the post-DANISH trial era: Results of the European Heart Rhythm Association survey. Europace 2017, 19, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Lupu, S.; Mitre, A.; Sus, I.; Rudzik, R.; Beke, I.; Dobreanu, D. Changes in left atrial size and function early after cardiac resynchronization therapy as assessed by conventional two-dimensional echocardiography. Med. Ultrason. 2018, 20, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cozma, D.; Kalifa, J.; Pescariu, S.; Lighezan, D.; Stiubei, M.; Luca, C.T.; Deharo, J.C.; Djiane, P.; Dragulescu, S.I. Can simple Doppler measurements estimate interatrial conduction time? Pacing Clin. Electrophysiol. 2003, 26, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Bajraktari, G.; Henein, M.Y. Left atrial volume index predicts response to cardiac resynchronisation therapy: A systematic review and meta-analysis. Arch. Med. Sci. 2020, 18, 930–938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouwmeester, S.; Mast, T.P.; Keulards, D.C.J.; de Lepper, A.G.W.; Herold, I.H.F.; Dekker, L.R.; Prinzen, F.W.; Houthuizen, P. Left atrial reverse remodeling predicts long-term survival after cardiac resynchronization therapy. J. Echocardiogr. 2022, 20, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dokuni, K.; Matsumoto, K.; Tatsumi, K.; Suto, M.; Tanaka, H.; Fukuzawa, K.; Hirata, K.I. Cardiac resynchronization therapy improves left atrial reservoir function through resynchronization of the left atrium in patients with heart failure with reduced ejection fraction. Int. J. Cardiovasc. Imaging 2020, 36, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, T.; Ammirati, G.; Pergola, V.; Imparato, L.; Carella, C.; Koci, E.; Chiappetti, R.; Abbate, F.G.; La Fazia, V.M.; Viggiano, A.; et al. Contrast-induced nephropathy after cardiac resynchronization therapy implant impairs the recovery of ejection fraction in responders. ESC Heart Fail. 2019, 6, 1266–1273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All Patients (n = 73) | ||
|---|---|---|
| Mean age (years), mean ± SD | 63.7 ± 9.3 | |
| Male, n (%) | 38 (52%) | |
| NYHA functional class (n, %) | II | 33 (45%) |
| III | 40 (55%) | |
| Electrocardiogram | PR interval (ms), mean ± SD | 181.3 ± 21.9 |
| QRS complex (ms), mean ± SD | 159.8 ± 18.2 | |
| Hypertension, n (%) | 39 (53%) | |
| Diabetes mellitus, n (%) | 30 (41%) | |
| Chronic kidney disease, n (%) * | 26 (37%) | |
| Baseline therapy | Beta blockers, n (%) | 64 (88%) |
| Ivabradine, n (%) | 39 (53%) | |
| ACEI/ARB, n (%) | 51 (70%) | |
| Diuretics, n (%) | 69 (95%) | |
| Antialdosteronics, n (%) | 62 (85%) | |
| Sacubitril, n (%) | 20 (27%) | |
| SGLT2 inhibitors, n (%) | 14 (19%) | |
| Basic Echocardiographic Parameters | All Patients (n = 73) | All Patients (n= 73) | |
|---|---|---|---|
| Mean ± SD | Range | Asynchronism Parameters | |
| IVS (cm) | 1.1 ± 0.2 | 0.8–1.8 | Septal flash (n, %) |
| LVEDD (cm) | 6.4 ± 0.9 | 4.7–8.9 | 68 (93%) |
| LVEDV (mL) | 234.3 ± 84.5 | 110–520 | Atrioventricular asynchronism, (n, %) |
| LVESV (mL) | 176.9 ± 84.1 | 80–446 | 57 (78%) |
| LVEF (%) | 27.9 ± 5.1 | 15–35 | |
| LAV (mL) | 98.6 ± 34.2 | 56–187 | Intraventricular asynchronism, (n, %) |
| LAVi (mL/m2) | 51.1 ± 18.6 | 118–22 | 63 (86%) |
| sPAP (mmHg) | 41.5 ± 16 | 20–80 | Interventricular asynchronism, (n, %) |
| E/A ratio | 1.3 ± 0.8 | 0.45–4.3 | 58 (79%) |
| Valvulopaties | mild | moderate | severe |
| Mitral regurgitation, n (%) | 11 (15%) | 34 (47%) | 28 (38%) |
| Tricuspid regurgitation, n (%) | 33 (45%) | 29 (40%) | 11 (15%) |
| Aortic stenosis, n (%) | 1 (1%) | 1 (1%) | 0 |
| Aortic regurgitation, n (%) | 12 (16%) | 4 (5%) | 2 (3%) |
| Before Fusion CRT-P | Follow-Up 6.4 Years ± 27 Months | p Value | |
|---|---|---|---|
| LVEF %, mean ± SD | 27.9 ± 5.1 | 40.4 ± 8.5 | <0.0001 |
| LVEDV (mL), mean ± SD | 234.3 ± 84.5 | 190.1 ± 84.4 | 0.0019 |
| LVESV (mL), mean ± SD | 176.9 ± 84.1 | 123.9 ± 68.8 | 0.0001 |
| LAV (mL), mean ± SD | 98.6 ± 34.2 | 85.9 ± 27.9 | 0.0151 |
| LAVi (mL/m2), mean ± SD | 51.1 ± 18.6 | 45.1 ± 17.6 | 0.0472 |
| sPAP (mmHg), mean ± SD | 41.5 ± 16 | 34.1 ± 11.2 | 0.0015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Văcărescu, C.; Cozma, D.; Crișan, S.; Gaiță, D.; Anutoni, D.-D.; Margan, M.-M.; Faur-Grigori, A.-A.; Roteliuc, R.; Luca, S.-A.; Lazăr, M.-A.; et al. Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence. J. Clin. Med. 2024, 13, 4814. https://doi.org/10.3390/jcm13164814
Văcărescu C, Cozma D, Crișan S, Gaiță D, Anutoni D-D, Margan M-M, Faur-Grigori A-A, Roteliuc R, Luca S-A, Lazăr M-A, et al. Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence. Journal of Clinical Medicine. 2024; 13(16):4814. https://doi.org/10.3390/jcm13164814
Chicago/Turabian StyleVăcărescu, Cristina, Dragoș Cozma, Simina Crișan, Dan Gaiță, Debora-Delia Anutoni, Mădălin-Marius Margan, Adelina-Andreea Faur-Grigori, Romina Roteliuc, Silvia-Ana Luca, Mihai-Andrei Lazăr, and et al. 2024. "Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence" Journal of Clinical Medicine 13, no. 16: 4814. https://doi.org/10.3390/jcm13164814
APA StyleVăcărescu, C., Cozma, D., Crișan, S., Gaiță, D., Anutoni, D.-D., Margan, M.-M., Faur-Grigori, A.-A., Roteliuc, R., Luca, S.-A., Lazăr, M.-A., Pătru, O., Cirin, L., Baneu, P., & Luca, C.-T. (2024). Left Atrium Reverse Remodeling in Fusion CRT Pacing: Implications in Cardiac Resynchronization Response and Atrial Fibrillation Incidence. Journal of Clinical Medicine, 13(16), 4814. https://doi.org/10.3390/jcm13164814






