Pathogenesis and Surgical Treatment of Dextro-Transposition of the Great Arteries (D-TGA): Part II
Abstract
:1. Introduction
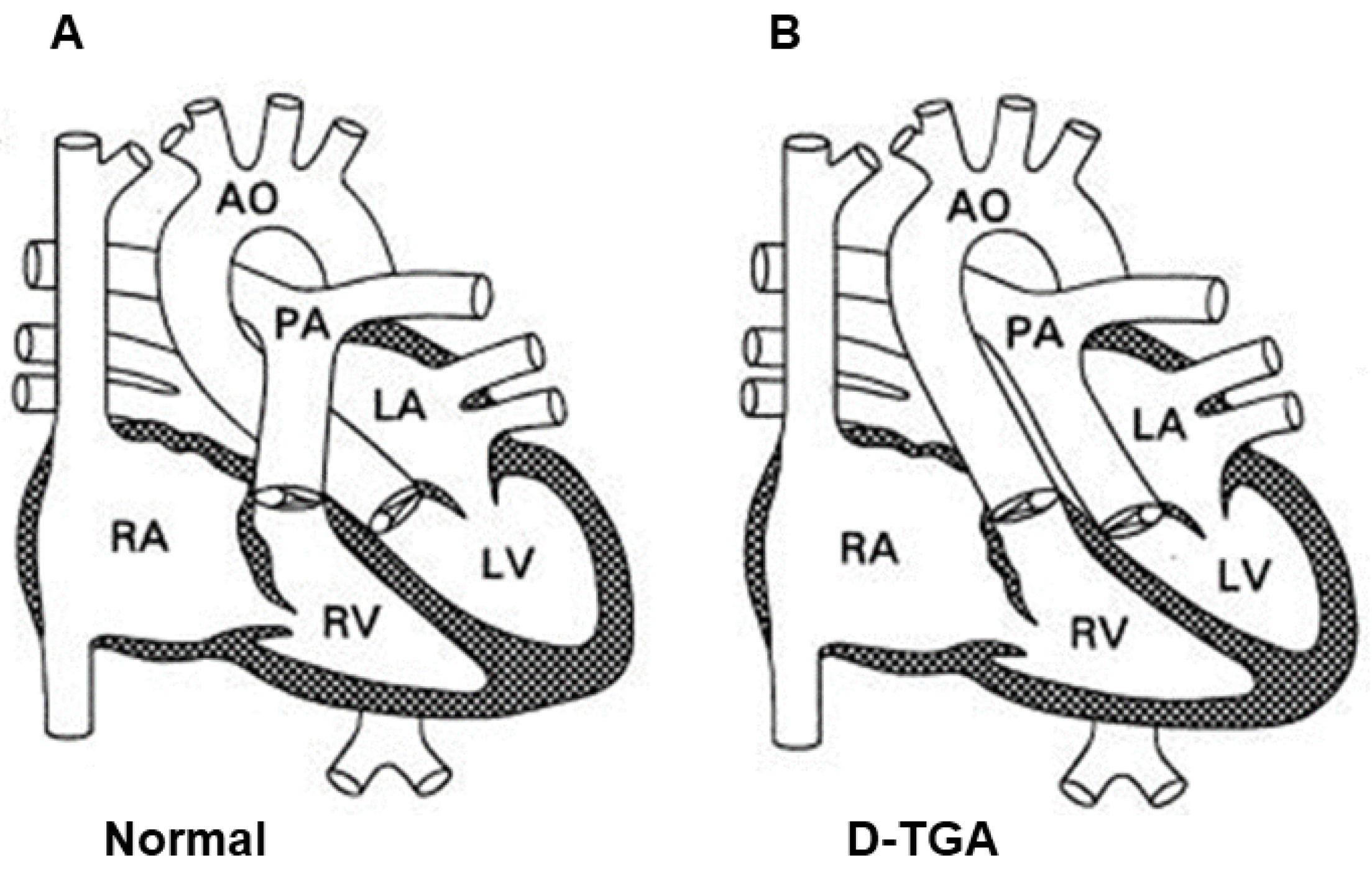
2. Definition of D-TGA and Its Historical Outline
3. Epidemiology
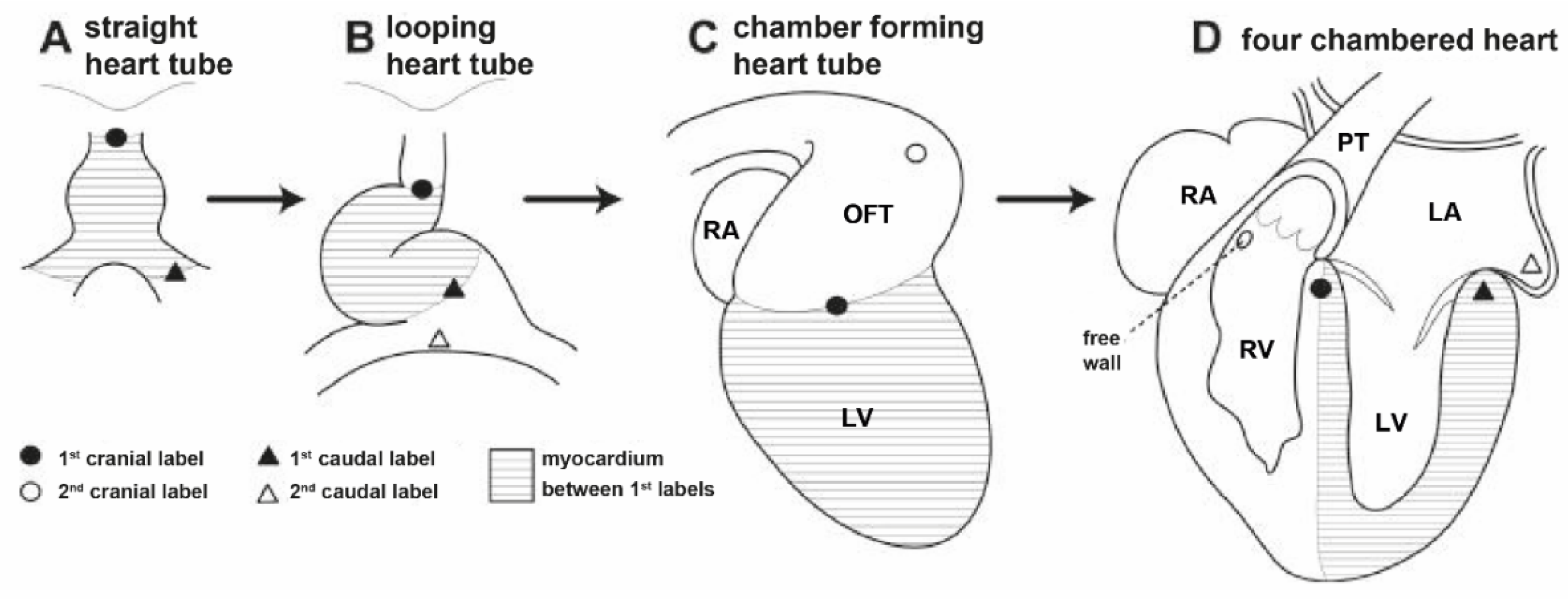
4. Etiology and Embryological Theories Explaining the Pathogenesis of D-TGA
5. The Influence of Various Risk Factors on the Etiology of D-TGA
6. Familial Recurrence of D-TGA
7. Cardiac Anomalies Associated with D-TGA
8. Classification of D-TGA Depending on the Presence of Concomitant Defects
9. Clinical Symptoms of D-TGA
10. Prenatal Detection of D-TGA
11. Diagnosis of D-TGA
11.1. Heart Auscultation
11.2. Electrocardiogram (ECG) in D-TGA
11.3. Chest Radiography (Chest X-ray)
11.4. Echocardiography of the Heart (ECHO)
11.5. Cardiac Catheterization and Angiography
11.6. Computed Tomography (CT)
11.7. Cardiac Magnetic Resonance (CMR)
12. Prognostic Significance of Markers of Systemic Right Ventricle (sRV) Insufficiency in Patients with D-TGA
13. Anatomy and Classification of Coronary Arteries in D-TGA
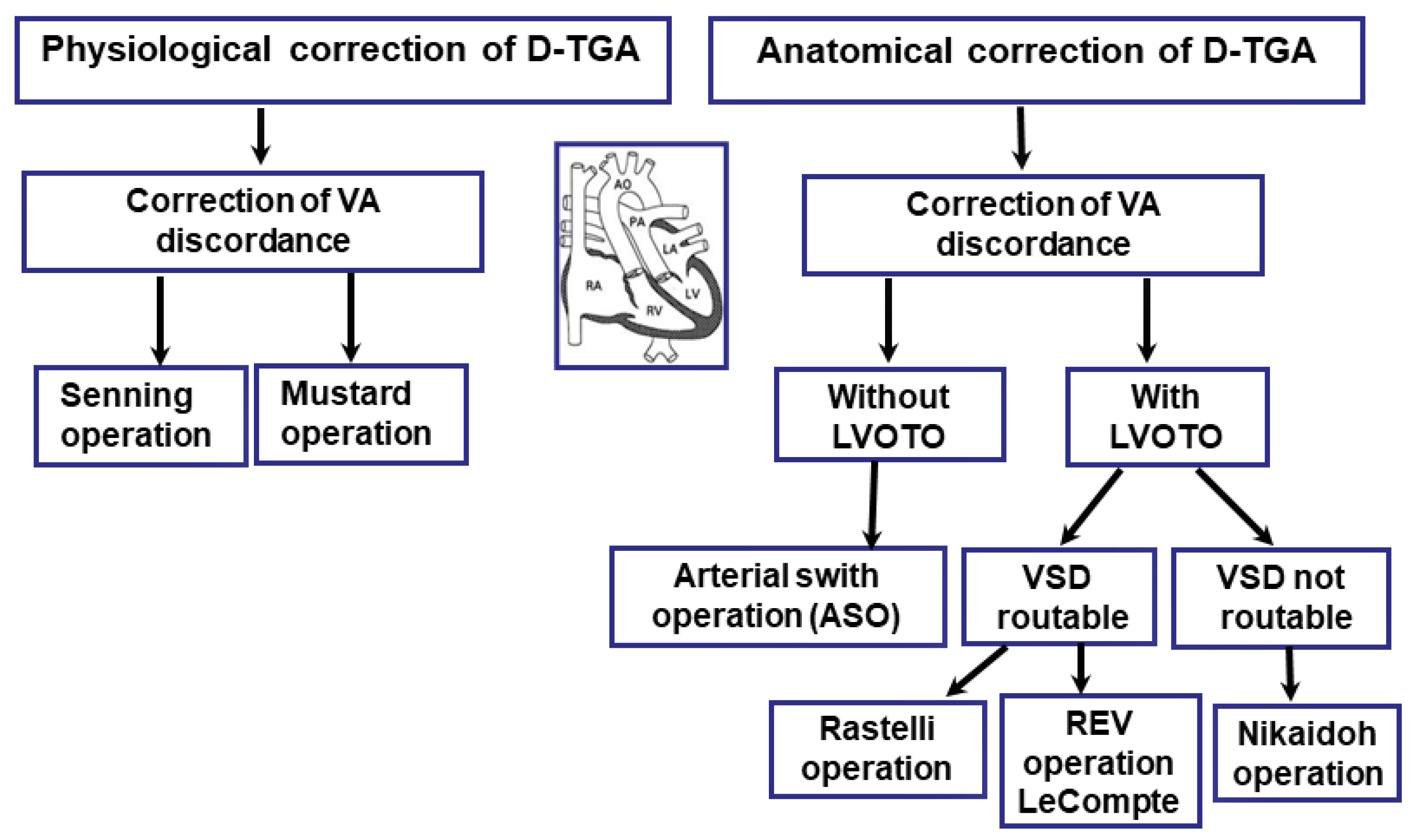
14. Surgical Treatment
Palliative Treatment
15. Anatomical Correction Using the Jatene Method (Arterial Switch Operation, ASO)
15.1. Indications for Surgery
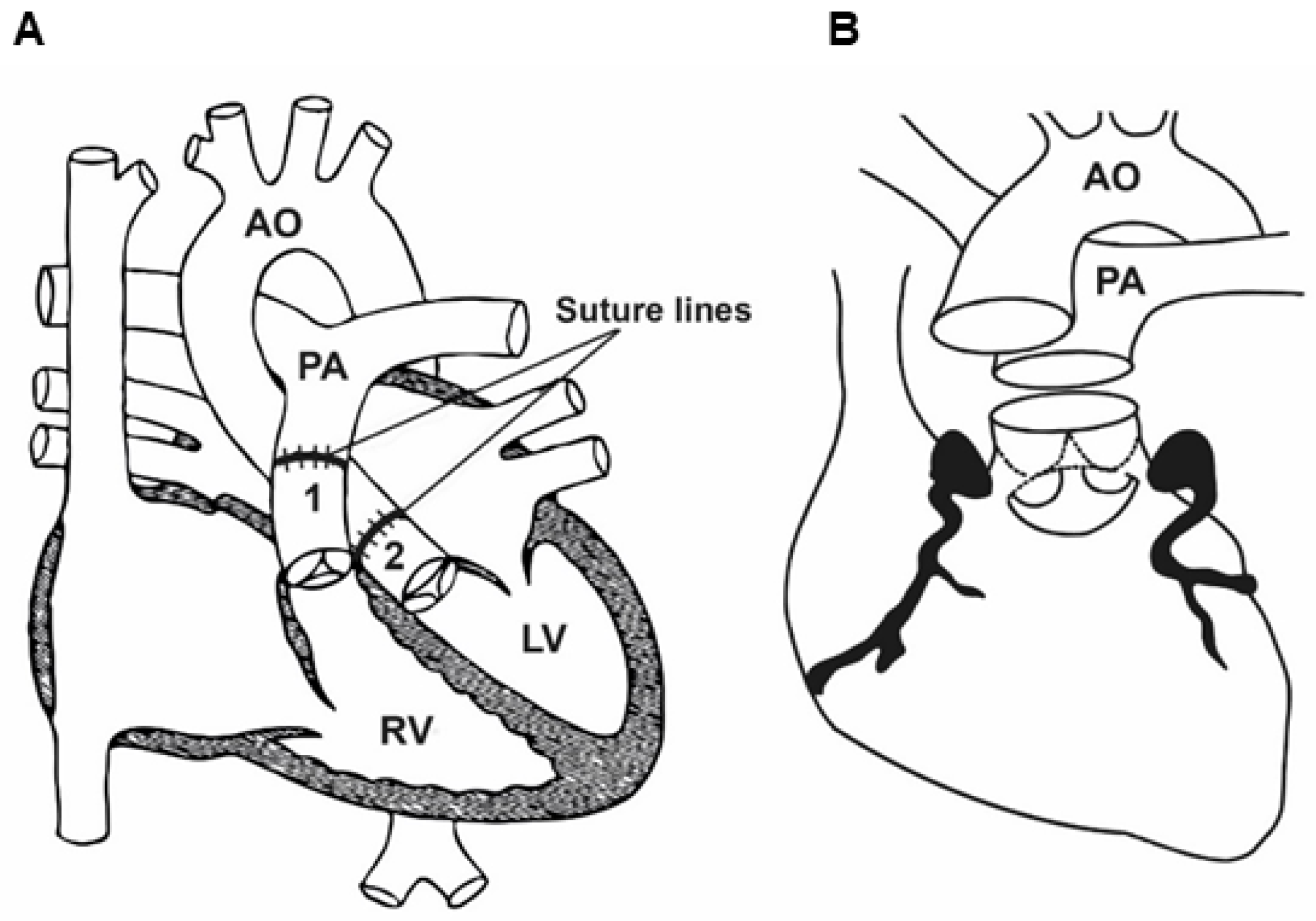
15.2. Surgical Technique
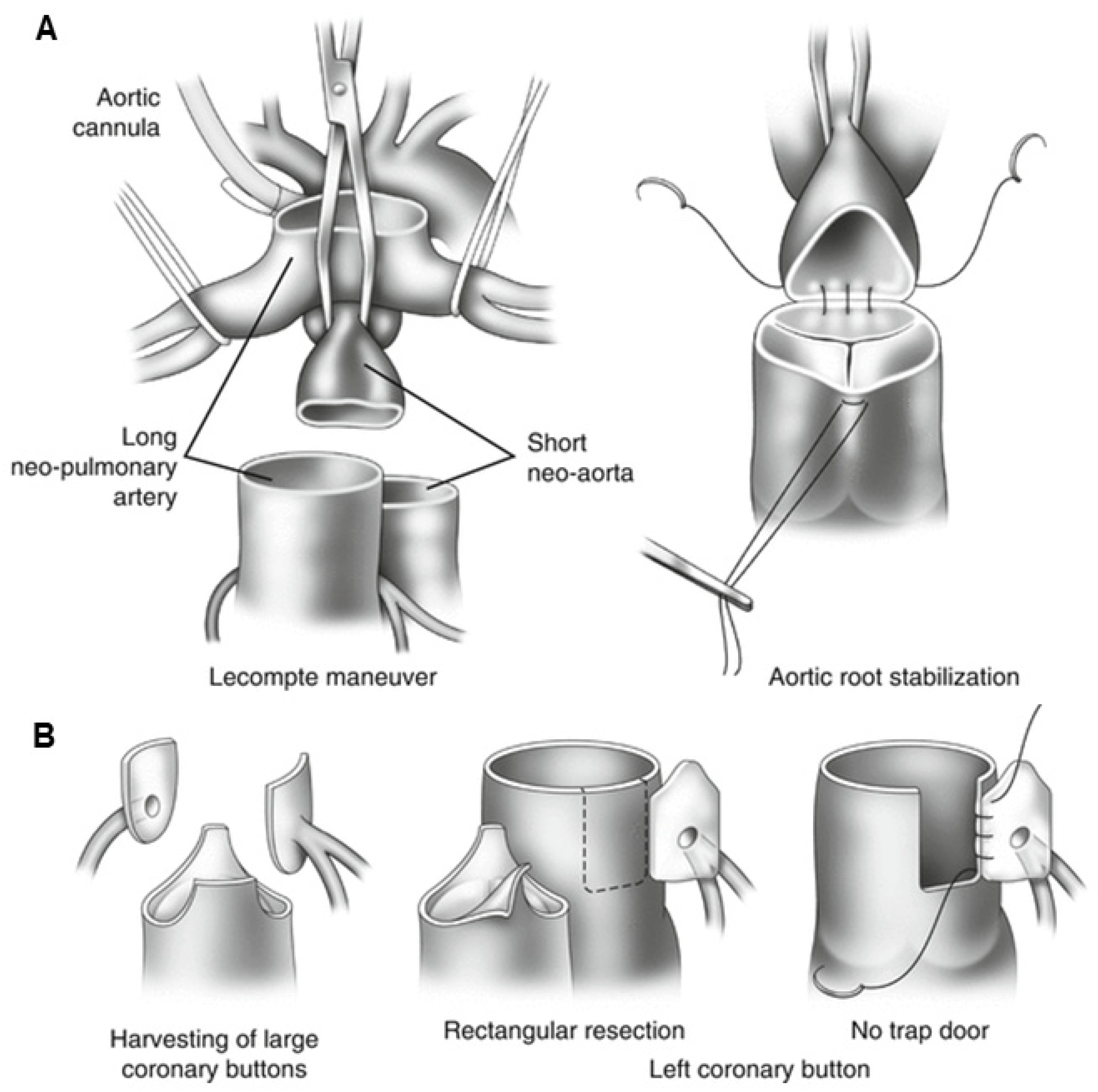
15.3. Post-Operative Prognosis of Patients Who Underwent ASO
16. Transposition of the Great Arteries (D-TGA) with Left Ventricular Outflow Tract Obstruction (LVOTO) and Ventricular Septal Defect (VSD)
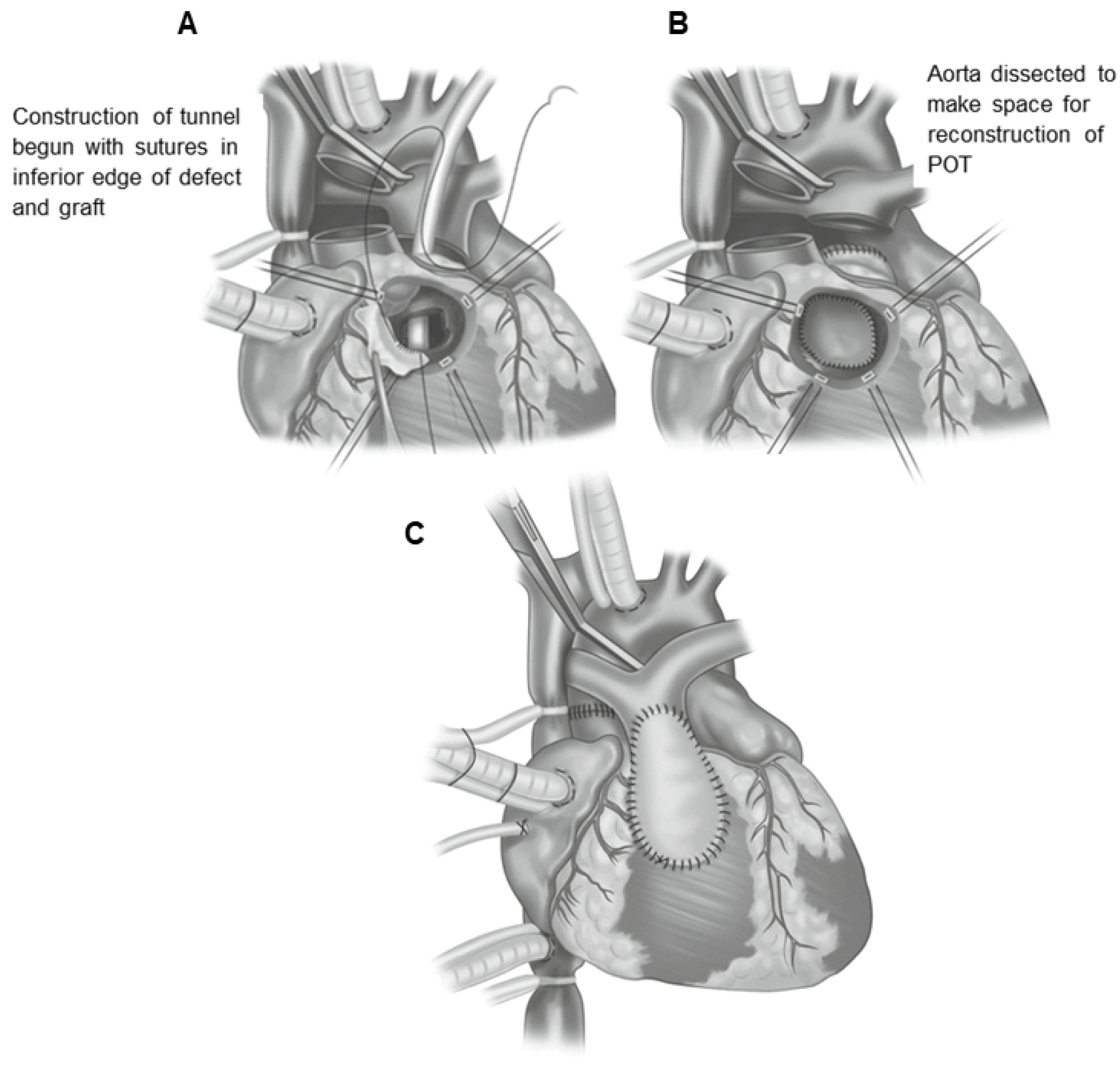
17. Rastelli Surgical Method
17.1. Indications for Surgery
17.2. Surgical Technique
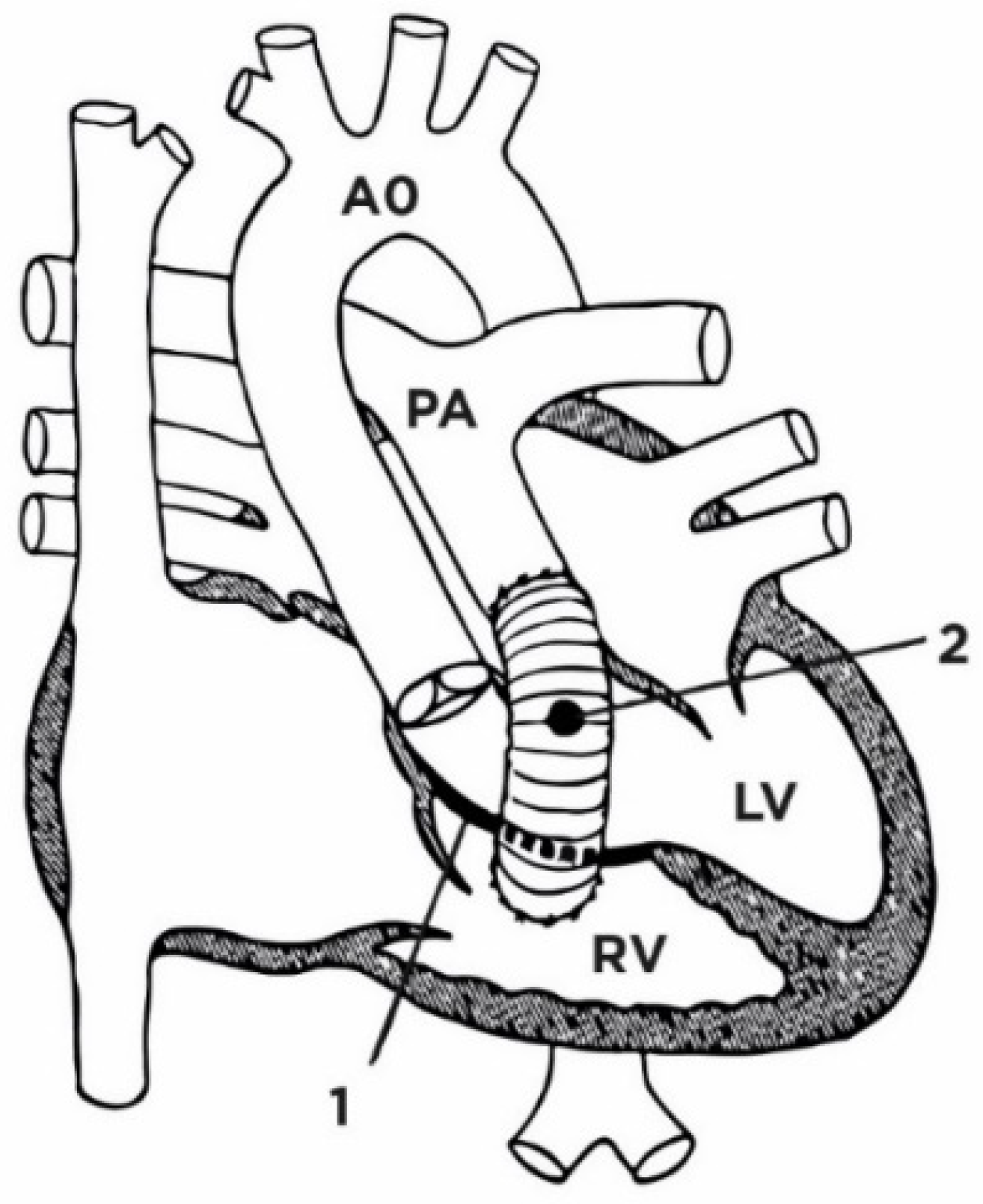
18. Réparation à l’Étage Ventriculaire (REV)
19. Nikaidoh Surgery
20. Physiological Correction
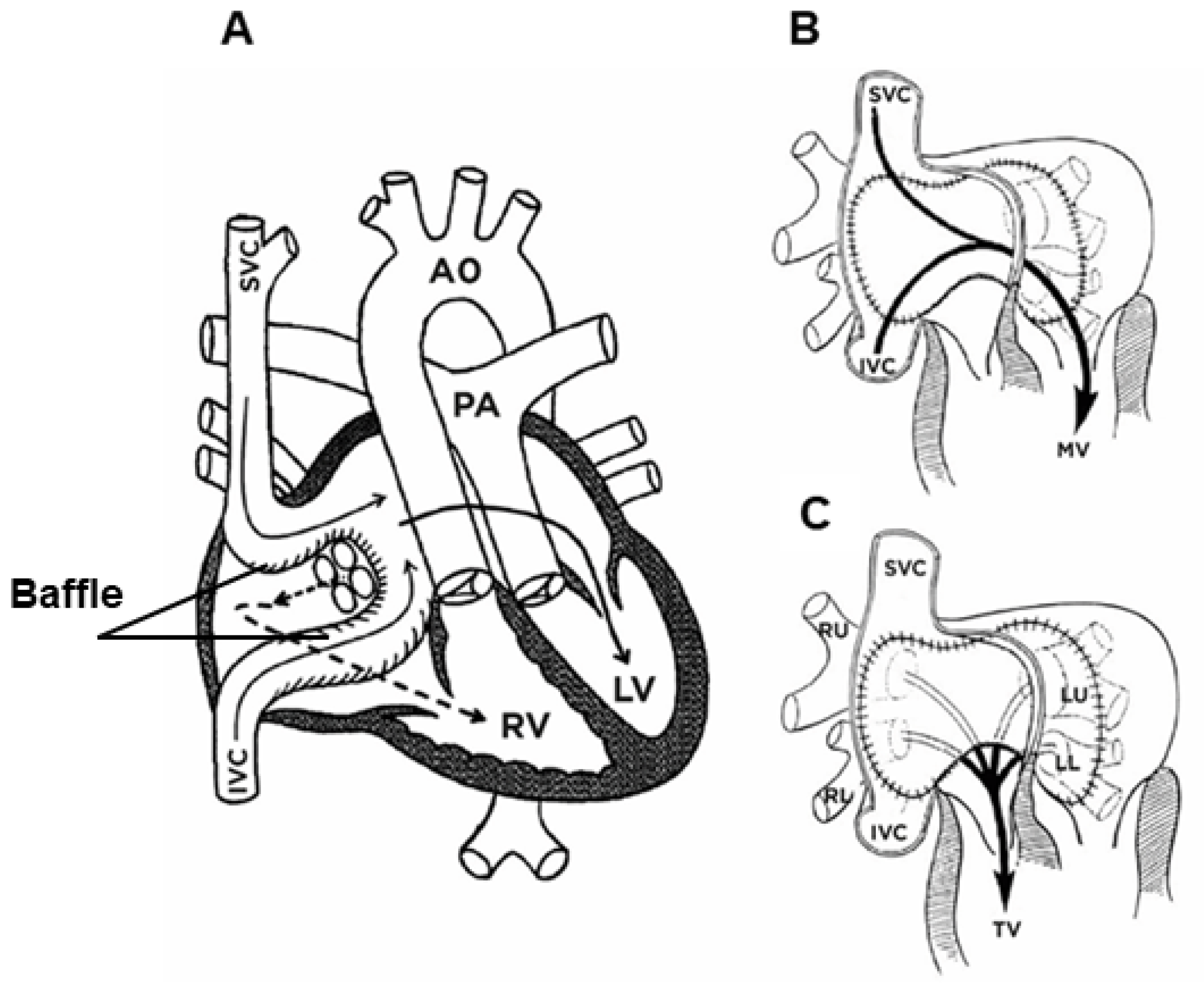
20.1. Physiological Correction with the Senning Procedure—Surgery Technique
20.2. Physiological Correction Using the Mustard Procedure—Surgery Technique
20.3. Postoperative Complications after the Mustard and Senning Operations
20.3.1. Dysfunction of the Right Ventricle (RV)
20.3.2. Tricuspid Valve Regurgitation (TR)
20.3.3. Arrhythmia, Conduction, and Stimulus Generation Disorders
21. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASD | atrial septal defect |
| ASO | arterial switch operation |
| AtrSR | atrial switch repair using the Senning or Mustard methods |
| AV | atrioventricular |
| AVSD | atrioventricular septal defects |
| BAS | balloon atrial septostomy |
| BNP | brain natriuretic peptide |
| cc-TGA | congenitally corrected transposition of the great arteries |
| CHD | congenital heart defect |
| CMR | cardiac magnetic resonance |
| CT | computed tomography |
| CTA | computed tomography angiography |
| Cx | circumflex artery |
| DA | ductus arteriosus |
| D-TGA | dextro-transposition of the great arteries |
| DORV | double-outlet right ventricle |
| ECG | electrocardiogram |
| ECHO | echocardiography |
| FO | foramen ovale |
| HF | heart failure |
| IART | intra-atrial reentrant tachycardia |
| IVC | inferior vena cava |
| LAD | anterior descending artery |
| LCA | left coronary artery trunk |
| LV | left ventricle |
| LVOT | left ventricle outflow tract |
| LVOTO | left ventricle outflow tract obstruction |
| miRNA | microRNA |
| MV | mitral valve |
| NT-pro BNP | N-terminal pro-B-type natriuretic peptide |
| PA | pulmonary artery |
| PGE1 | prostaglandin E1 |
| RA | right atrium |
| RCA | right coronary artery |
| REV | réparation a l’étage ventriculaire |
| RV | right ventricle |
| RVOT | right ventricle outflow tract |
| RVOTO | right ventricular outflow tract obstruction |
| sRV | systemic right ventricle |
| SVC | superior vena cava |
| TGA | transposition of the great arteries |
| TR | tricuspid valve regurgitation |
| TV | tricuspid valve |
| VA | ventriculoarterial |
| VSD | ventricular septal defect |
| X-ray | radiological examination |
References
- Samánek, M.; Slavík, Z.; Zbořilová, B.; Hroboňová, V.; Voříšková, M.; Škovránek, J. Prevalence, treatment, and outcome of heart disease in live-born children: A prospective analysis of 91,823 live-born children. Pediatric. Cardiol. 1989, 10, 205–211. [Google Scholar] [CrossRef]
- Rao, P.S. Diagnosis and management of cyanotic congenital heart disease: Part I. Indian J. Pediatr. 2009, 76, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, R. What determines whether the great arteries are normally or abnormally related? Am. J. Cardiol. 2016, 118, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.D.; Driscoll, D.J.; Shaddy, R.E.; Feltes, T.F. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult, 8th ed.; Williams and Wilkins: Baltimore, MD, USA, 2013; pp. 48–51. [Google Scholar]
- de la Cruz, M.V.; Sánchez Gómez, C.; Arteaga, M.M.; Argüello, C. Experimental study of the development of the truncus and the conus in the chick embryo. J. Anat. 1977, 123, 661–686. [Google Scholar] [PubMed]
- de la Cruz, M.V.; Arteaga, M.; Espino-Vela, J.; Quero-Jiménez, M.; Anderson, R.H.; Diaz, G.F. Complete transposition of the great arteries: Types and morphogenesis of ventriculoarterial discordance. Am. Heart J. 1981, 102, 271–281. [Google Scholar] [CrossRef]
- Goor, D.A.; Edwards, J.P. The spectrum of transposition of the great arteries: With special reference to developmental anatomy of the conus. Circulation 1973, 48, 406–415. [Google Scholar] [CrossRef]
- Anderson, R.H.; Wilkinson, J.L.; Arnold, R.; Becker, A.E.; Lubkiewicz, K. Morphogenesis of bulboventricular malformations: II. Observations on malformed hearts. Br. Heart J. 1974, 36, 948–970. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, R.; Van Praagh, S. Isolated ventricular inversion: A consideration of the morphogenesis, definition and diagnosis of nontransposed and transposed great arteries. Am. J. Cardiol. 1966, 17, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Versacci, P.; Pugnaloni, F.; Digilio, M.C.; Putotto, C.; Unolt, M.; Calcagni, G.; Baban, A.; Marino, B. Some Isolated Cardiac Malformations Can Be Related to Laterality Defects. J. Cardiovasc. Dev. Dis. 2018, 5, 24. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Li, W.; Priest, J.; Fu, Y.; Pang, K.J.; Ma, B.; Liu, X.; Han, B.; Hu, S.; et al. Exome-based case-control analysis highlights the pathogenic role of ciliary genes in transposition of the great arterie. Circ. Res. 2020, 126, 811–821. [Google Scholar] [CrossRef]
- Lei, L.; Lin, H.; Zhong, S.; Zhang, Z.; Chen, J.; Li, X.X.; Yu, X.; Liu, X.; Zhuang, J. Analysis of mutations in 7 candidate genes for dextro-Transposition of the great arteries in Chinese population. J. Thorac. Dis. 2014, 6, 491–496. [Google Scholar] [PubMed]
- Goldmuntz, E.; Bamford, R.; Karkera, J.D.; dela Cruz, J.; Roessler, E.; Muenke, M. CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am. J. Hum. Genet. 2002, 70, 776–780. [Google Scholar] [CrossRef] [PubMed]
- de Luca, A.; Sarkozy, A.; Consoli, F.; Ferese, R.; Guida, V.; Dentici, M.L.; Mingarelli, R.; Bellacchio, E.; Tuo, G.; Limongelli, G.; et al. Familial transposition of the great arteries caused by multiple mutations in laterality genes. Heart 2010, 96, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Unolt, M.; Putotto, C.; Silvestri, L.M.; Marino, D.; Scarabotti, A.; Massaccesi, V.; Caiaro, A.; Versacci, P.; Marino, B. Transposition of great arteries: New insights into the pathogenesis. Front. Pediatr. 2013, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, R.S.; Alharbi, S.H.; Tuwaijri, R.M.A.; Alzomaili, B.T.; Althubaiti, A.; Yelbuz, T.M. Transposition of the great arteries: A laterality defect in the group of heterotaxy syndromes or an outflow tract malformation? Ann. Pediatr. Cardiol. 2018, 11, 237–249. [Google Scholar] [PubMed]
- Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Grohmann, J.; Gummert, J.F.; Zubrzycka, M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. Int. J. Mol. Sci. 2024, 25, 7117. [Google Scholar] [CrossRef]
- Abu-Sulaiman, R.M.; Subaih, B. Congenital heart disease in infants of diabetic mothers: Echocardiographic study. Pediatr. Cardiol. 2004, 25, 137–140. [Google Scholar] [CrossRef]
- Loffredo, C.A.; Silbergeld, E.K.; Ferencz, C.; Zhang, J. Association of transposition of the great arteries in infants with maternalexposures to herbicides and rodenticides. Am. J. Epidemiol. 2001, 153, 529–536. [Google Scholar] [CrossRef]
- Okuda, H.; Nagao, T. Cardiovascular malformations induced by prenatal exposure to phenobarbital in rats. Congenit. Anom. 2006, 46, 97–104. [Google Scholar] [CrossRef]
- Ferencz, C.; Loffredo, C.A.; Corea-Villasenor, A.; Wilson, P.D. Genetic and environmental risk factors of major cardiovascular malformations: The Baltimore-Washington Infant Study, 1981–1989. In Perspectives in Pediatric Cardiology, 1st ed.; Ferencz, C., Loffredo, C.A., Corea-Villasenor, A., Wilson, P.D., Eds.; Futura Publishing Co. Inc.: Armonk, NY, USA, 1997; Volume 5, pp. 867–868. [Google Scholar]
- Marino, B.; Marcelletti, C. Complex congenital heart disease after in vitro fertilization. Am. J. Dis. Child. 1989, 143, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kurinczuk, J.J.; Milne, E.; de Klerk, N.; Bower, C. Assisted reproductive technology and birth defects: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 330–353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Y.; Huang, X.; Li, Y. Do the children born after assisted reproductive technology have an increased risk of birth defects? A systematic review and meta-analysis. J. Matern. Fetal Neonatal. Med. 2020, 33, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Galdini, A.; Fesslova, V.M.E.; Gaeta, G.; Candiani, M.; Pozzoni, M.; Chiarello, C.; Cavoretto, P.I. Prevalence of Congenital Heart Defects in Pregnancies Conceived by Assisted Reproductive Technology: A Cohort Study. J. Clin. Med. 2021, 10, 5363. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, R.; Geva, T.; Kreutzer, J. Ventricular septal defects: How shall we describe, name and classify them? J. Am. Coll. Cardiol. 1989, 14, 1298–1299. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.; Mills, L.; Fruitman, D.; Savard, W.; Colen, T.; Khoo, N.; Serrano-Lomelin, J.; Hornberger, L.K. Population trends in prenatal detection of transposition of great arteries: Impact of obstetric screening ultrasound guidelines. Ultrasound Obstet. Gynecol. 2018, 51, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Castela, E. Transposition of the great arteries. Orphanet. J. Rare Dis. 2008, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, F.; Pasqualin, G.; Ferrero, P.; Micheletti, A.; Negura, D.G.; D’Aiello, A.F.; Giamberti, A.; Chessa, M. Overview of Long-Term Outcome in Adults with Systemic Right Ventricle and Transposition of the Great Arteries: A Review. Diagnostics 2023, 13, 2205. [Google Scholar] [CrossRef]
- Warnes, C.A. Transposition of the Great Arteries. Circulation 2006, 114, 2699–2709. [Google Scholar] [CrossRef]
- Allen, H.D.; Driscoll, D.J.; Shaddy, R.E.; Feltes, T.F. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult, 7th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008; pp. 1039–1084. [Google Scholar]
- Villafañe, J.; Lantin-Hermoso, M.R.; Bhatt, A.B.; Tweddell, J.S.; Geva, T.; Nathan, M.; Elliott, M.J.; Vetter, V.L.; Paridon, S.M.; Kochilas, L.; et al. D-transposition of the great arteries: The current era of the arterial switch operation. J. Am. Coll. Cardiol. 2014, 64, 498–511. [Google Scholar] [CrossRef]
- Jatene, A.D.; Fontes, V.F.; Paulista, P.P.; De Souza, L.C.; Neger, F.; Galantier, M.; Souza, J.E. Successful anatomic correction of transposition of the great vessels. A preliminary report. Arq. Bras. Cardiol. 1975, 28, 461–464. [Google Scholar]
- Haas, F.; Wottke, M.; Poppert, H.; Meisner, H. Long-term survival and functional follow-up in patients after the arterial switch operation. Ann. Thorac. Surg. 1999, 68, 1692–1697. [Google Scholar] [CrossRef]
- Massin, M.M. Midterm results of the neonatal arterial switch operation. A review. J. Cardiothorac. Surg. 1999, 40, 517–522. [Google Scholar]
- Wernovsky, G.; Rome, J.J.; Tabbutt, S.; Rychik, J.; Cohen, M.S.; Paridon, S.M.; Weeb, G.; Dodds, K.M.; Gallagher, M.A.; Fleck, D.A.; et al. Guidelines for the Outpatient Management of Complex Congenital Heart Disease. Congenit. Heart Dis. 2006, 1, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Lalezari, S.; Bruggemans, E.F.; Blom, N.A.; Hazekamp, M.G. Thirty-year experience with the arterial switch operation. Ann. Thorac. Surg. 2011, 92, 973–979. [Google Scholar] [CrossRef]
- Moe, T.G.; Bardo, D.M.E. Long-term outcomes of the arterial switch operation for d-Transposition of the great arteries. Prog. Cardiovasc. Dis. 2018, 61, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, J.P.; Sheba, J. Management of the Adult with Arterial Switch. Methodist. Debakey Cardiovasc. J. 2019, 15, 133–137. [Google Scholar] [CrossRef]
- Canan, A.; Ashwath, R.; Prachi, P.; Agarwal, P.P.; François, C.; Rajiah, P. Multimodality Imaging of Transposition of the Great Arteries. Radiographics 2021, 41, 338–360. [Google Scholar] [CrossRef]
- Hutter, P.A.; Kreb, D.L.; Mantel, S.F.; Hitchcock, J.F.; Meijboom, E.J.; Bennink, G.B. Twenty-five years’ experience with the arterial switch operation. J. Thorac. Cardiovasc. Surg. 2002, 124, 790–797. [Google Scholar] [CrossRef]
- Tobler, D.; Williams, W.G.; Jegatheeswaran, A.; Van Arsdell, G.S.; McCrindle, B.W.; Greutmann, M.; Oechslin, E.N.; Silversides, C.K. Cardiac outcomes in young adult survivors of the arterial switch operation for transposition of the great arteries. J. Am. Coll. Cardiol. 2010, 56, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Baillie, M. The Morbid Anatomy of Some of the Most Important Parts of the Human Body; Johnson and Nicol: London, UK, 1797. [Google Scholar]
- Farre, J.R. Pathological Researches. Essay 1: On Malformation of the Human Heart; Longman, Hurst, Rees, Orme, Brown: London, UK, 1814; p. 28. [Google Scholar]
- Van Praagh, R. Transposition of the great arteries. II. Transposition clarified. Am. J. Cardiol. 1971, 28, 739–741. [Google Scholar] [CrossRef]
- Clark, E.B. Pathogenetic mechanism of congenital cardiovascular malformations revisited. Semin. Perinatol. 1996, 20, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Eidem, B.W.; Cetta, F.; Fogel, M.A.; Frommelt, P.C.; Ganame, J.; Han, B.K.; Kimball, T.R.; Johnson, R.K.; Mertens, L.; et al. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2016, 29, 571–621. [Google Scholar]
- Jaggers, J.J.; Cameron, D.E.; Herlong, J.R.; Ungerleider, R.M. Congenital Heart Surgery Nomenclature and Database Project: Transposition of the great arteries. Ann. Thorac. Surg. 2000, 69, S205–S235. [Google Scholar] [CrossRef]
- Amaral, F.; Valente, A.M.; Manso, P.H.; Gali, L.G.; Braggion-Santos, M.F.; Rocha, J.M.; de Andrade Vicente, W.V.; Schmidt, A. Congenitally Corrected Transposition of the Great Arteries in the Adult. Braz. J. Cardiovasc. Surg. 2022, 37, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Becker, A.E.; Freedom, R.M.; Macartney, F.J.; Quero-Jimenez, M.; Shinebourne, E.A.; Wilkinson, J.L.; Tynan, M. Sequential segmental analysis of congenital heart disease. Pediatr. Cardiol. 1984, 5, 281–287. [Google Scholar] [CrossRef]
- Anderson, R.H.; Ho, S.Y. Sequential segmental analysis—Description and cate-gorization for the millennium. Cardiol. Young 1997, 7, 98–116. [Google Scholar] [CrossRef]
- Paladini, D.; Rustico, M.; Todros, T.; Palmieri, S.; Gaglioti, P.; Benettoni, A.; Russo, M.G.; Chiappa, E.; D’Ottavio, G. Conotruncal anomalies in prenatal life. Ultrasound Obstet. Gynecol. 1996, 8, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Fyler, D.C. Nadas’ Pediatric Cardiology; Hanley and Belfus: Philadelphia, PA, USA, 1992. [Google Scholar]
- Thiene, G.; Frescura, C. Anatomical and pathophysiological classification of congenital heart disease. Cardiovasc. Pathol. 2010, 19, 259–274. [Google Scholar] [CrossRef]
- Frescura, C.; Thiene, G. The spectrum of congenital heart disease with transposition of the great arteries from the cardiac registry of the University of Padua. Front. Pediatr. 2016, 22, 84. [Google Scholar] [CrossRef]
- Bianca, S.; Ettore, G. Sex ratio imbalance in transposition of the great arteries and possible agricultural environmental risk factors. Images Paediatr. Cardiol. 2001, 8, 10–14. [Google Scholar]
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Haeffele, C.; Lui, G.K. dextro-transposition of the great arteries: Long-term sequelae of atrial and arterial switch. Cardiol. Clin. 2015, 33, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Güçer, S.; Ince, T.; Kale, G.; Akçören, Z.; Ozkutlu, S.; Talim, B.; Cağlar, M. Noncardiac malformations in congenital heart disease: A retrospective analysis of 305 pediatric autopsies. Turk. J. Pediatr. 2005, 47, 159–166. [Google Scholar] [PubMed]
- Liebman, J.; Cullum, L.; Belloc, N.B. Natural history of transposition of the great arteries. Anatomy and birth and death characteristics. Circulation 1969, 40, 237–262. [Google Scholar] [CrossRef]
- Aseervatham, R.; Pohlner, P.A. Clinical comparison of arterial and atrial repairs for transposition of the great arteries: Early and midterm survival and functional results. Aust. N. Z. J. Surg. 1998, 68, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Kutsche, L.M.; van Mierop, L.H. Anatomy and pathogenesis of aorticopulmonary septal defect. Am. J. Cardiol. 1987, 59, 443–447. [Google Scholar] [CrossRef]
- Van den Berg, G.; Moorman, A.F. Concepts of cardiac development in retrospect. Pediatr. Cardiol. 2009, 30, 580–587. [Google Scholar] [CrossRef]
- Van Praagh, R.; Perez-Trevino, C.; Lopez-Cuellar, M.; Baker, F.W.; Zuberbuhler, J.R.; Quero, M.; Perez, V.M.; Moreno, F.; Van Praagh, S. Transposition of the great arteries with posterior aorta, anterior pulmonary artery, subpulmonary conus and fibrous continuity between aortic and atrioventricular valves. Am. J. Cardiol. 1971, 28, 621–631. [Google Scholar] [CrossRef]
- Quero-Jiménez, M.; Perez Martinez, V. Uncommon conal pathology in complete dextroposition of the great arteries with ventricular septal defect. Chest 1974, 66, 411–417. [Google Scholar] [CrossRef]
- Takahashi, M.; Terasako, Y.; Yanagawa, N.; Kai, M.; Yamagishi, T.; Nakajima, Y. Myocardial progenitors in the pharyngeal regions migrate to distinct conotruncal regions. Dev. Dyn. 2012, 241, 284–293. [Google Scholar] [CrossRef]
- Kuehl, K.S.; Loffredo, C.A. Genetic and environmental influences on malformations of the cardiac outflow tract. Expert. Rev. Cardiovasc. Ther. 2005, 3, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Nakazawa, M.; Morishima, M.; Miyagawa-Tomita, S.; Momma, K. Morphological observations on the pathogenetic process of transposition of the great arteries induced by retinoic acid in mice. Circulation 1995, 91, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, M.; Kokubo, H.; Nakajima, Y.; Saga, Y. Ectopic retinoic acid signaling affects outflow tract cushion development through suppression of the myocardial Tbx2-Tgf 2 pathway. Development 2012, 139, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Pexieder, T.; Blanc, O.; Pelouch, V.; Ostàdalovà, I.; Milerovà, M.; Ostàdal, B. Late fetal development of retinoic acid-induced transposition of great arteries: Morphology, physiology, and biochemistry. In Developmental Mechanism of Heart Disease; Clark, E.B., Markwald, R.R., Takao, A., Eds.; Futura Publishing: Armonk, NY, USA, 1995; pp. 297–307. [Google Scholar]
- Cipollone, D.; Amati, F.; Carsetti, R.; Placidi, S.; Biancolella, M.; D’Amati, G.; Novelli, G.; Siracusa, G.; Marino, B. A multiple retinoic acid antagonist induces conotruncal anomalies, including transposition of the great arteries, in mice. Cardiovasc. Pathol. 2006, 15, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Muncke, N.; Jung, C.; Rudiger, H.; Ulmer, H.; Roeth, R.; Hubert, A.; Goldmuntz, E.; Driscoll, D.; Goodship, J.; Schon, K.; et al. Missense mutations and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries). Circulation 2003, 108, 2843–2850. [Google Scholar] [CrossRef]
- Mégarbané, A.; Salem, N.; Stephan, E.; Ashoush, R.; Lenoir, D.; Delague, V.; Kassab, R.; Loiselet, J.; Bouvagnet, P. X-linked transposition of the great arteries and incomplete penetrance among males with a nonsense mutation in ZIC3. Eur. J. Hum. Genet. 2000, 8, 704–708. [Google Scholar] [CrossRef]
- Roessler, E.; Ouspenskaia, M.V.; Karkera, J.D.; Vélez, J.I.; Kantipong, A.; Lacbawan, F.; Bowers, P.; Belmont, J.W.; Towbin, J.A.; Goldmuntz, E.; et al. Reduced NODAL signaling strength via mutation of several pathway members including FOXH1 is linked to human heart defects and holoprosencephaly. Am. J. Hum. Genet. 2008, 83, 18–29. [Google Scholar] [CrossRef]
- Mohapatra, B.; Casey, B.; Li, H.; Ho-Dawson, T.; Smith, L.; Fernbach, S.D.; Molinari, L.; Niesh, S.R.; Jefferies, J.L.; Craigen, W.J.; et al. Identification and functional characterization of NODAL rare variants in heterotaxy and isolated cardiovascular malformations. Hum. Mol. Genet. 2009, 18, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Reamon-Buettner, S.M.; Borlak, J. NKX2-5: An update on this hypermutable homeodomain protein and its role in human congenital heart disease (CHD). Hum. Mutat. 2010, 31, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, R.; Oneda, B.; Sheth, F.; Azzarello-Burri, S.; Baldinger, R.; Joset, P.; Latal, B.; Knirsch, W.; Desai, S.; Baumer, A.; et al. Dosage changes of MED13L further delineate its role in congenital heart defects and intellectual disability. Eur. J. Hum. Genet. 2013, 21, 1100–1104. [Google Scholar] [CrossRef]
- Škorić-Milosavljević, D.; Tadros, R.; Bosada, F.M.; Tessadori, F.; van Weerd, J.H.; Woudstra, O.I.; Tjong, F.V.Y.; Lahrouchi, N.; Bajolle, F.; Cordell, H.J.; et al. Common Genetic Variants Contribute to Risk of Transposition of the Great Arteries. Circ. Res. 2022, 130, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Melchionda, S.; Digilio, M.C.; Mingarelli, R.; Novelli, G.; Scambler, P.; Marino, B.; Dallapiccola, B. Transposition of the great arteries associated with deletion of chromosome 22q11. Am. J. Cardiol. 1995, 75, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y. Mechanism responsible for D-transposition of the great arteries: Is this part of the spectrum of right isomerism? Congenit. Anom. 2016, 56, 196–202. [Google Scholar] [CrossRef]
- Marino, B. Patterns of congenital heart disease and associated cardiac anomalies in children with Down syndrome. In Heart Disease in Persons with Down Syndrome; Marino, B., Pueschel, S.M., Eds.; Paul H Brookes Publishing: Baltimore, MD, USA, 1996; pp. 133–140. [Google Scholar]
- Alfarhan, A.; Alquayt, M.; Alshalhoub, M.; Alnahdi, M.A.; Masuadi, A.; Alhabshan, F. Risk factors for transposition of the great arteries in Saudi population. Saudi Med. J. 2020, 41, 1054–1062. [Google Scholar] [CrossRef]
- Marino, B.; Capolino, R.; Digilio, M.C.; Di Donato, R. Transposition of the great arteries in asplenia and polysplenia phenotypes. Am. J. Med. Genet. 2002, 110, 292–294. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, L.C.A.; Latney, B.C.; Paluru, P.C.; Goldmuntz, E. The Phenotypic Spectrum of ZIC3 Mutations Includes Isolated D-Transposition of the Great Arteries and Double Outlet Right Ventricle. Am. J. Med. Genet. A 2013, 161, 752–802. [Google Scholar] [CrossRef]
- Bajolle, F.; Zaffran, S.; Kelly, R.G.; Hadchouel, J.; Bonnet, D.; Brown, N.A.; Buckingham, M.E. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ. Res. 2006, 98, 421–428. [Google Scholar] [CrossRef]
- Gaio, U.; Schweickert, A.; Fischer, A.; Garratt, A.N.; Muller, T.; Ozcelik, C.; Lankes, W.; Strehle, M.; Britsch, S.; Blum, M.; et al. A role of the cryptic gene in the correct establishment of the left-right axis. Curr. Biol. 1999, 9, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.T.; Gritsman, K.; Ding, J.; Burdine, R.D.; Corrales, J.D.; Price, S.M.; Talbot, W.S.; Schier, A.F.; Shen, M.M. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 1999, 13, 2527–2537. [Google Scholar] [CrossRef]
- Becker, T.A.; Van Amber, R.; Moller, J.H.; Pierpont, M.E. Occurrence of cardiac malformations in relatives of children with transposition of the great arteries. Am. J. Med. Genet. 1996, 66, 28–32. [Google Scholar] [CrossRef]
- Peyvandi, S.; Ingall, E.; Woyciechowski, S.; Garbarini, J.; Mitchell, L.E.; Goldmuntz, E. Risk of congenital heart disease in relatives of probands with conotruncal cardiac defects: An evaluation of 1,620 families. Am. J. Med. Genet. A 2014, 164A, 1490–1495. [Google Scholar] [CrossRef]
- Digilio, M.C.; Casey, B.; Toscano, A.; Calabrò, R.; Pacileo, G.; Marasini, M.; Banaudi, E.; Giannotti, A.; Dallapiccola, B.; Marino, B. Complete transposition of the great arteries: Patterns of congenital heart disease in familial precurrence. Circulation 2001, 104, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Brennan, P.; Little, J.; Holloway, S.; Coffey, R.; Somerville, J.; Dennis, N.R.; Allan, L.; Arnold, R.; Deanfield, J.E.; et al. Recurrence risks in offspring of adults with major heart defects: Results from first cohort of British collaborative study. Lancet 1998, 351, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Fesslova, V.; Brankovic, J.; Lalatta, F.; Villa, L.; Meli, V.; Piazza, L.; Ricci, C. Recurrence of congenital heart disease in cases with familial risk screened prenatally by echocardiography. J. Pregnancy 2011, 2011, 368067. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.A.; Chokkalingam, A.; Sill, A.M.; Boughman, J.A.; Clark, E.B.; Scheel, J.; Brenner, J.I. Prevalence of congenital cardiovascular malformations among relatives of infants with hypoplastic left heart, coarctation of the aorta, and d-transposition of the great arteries. Am. J. Med. Genet. A 2004, 124A, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, W. A family study in transposition of the great vessels and in tricuspid atresia. Humangenetik 1968, 6, 148–157. [Google Scholar] [CrossRef]
- Nora, J.J.; Berg, K.; Nora, A.H. Cardiovascular Diseases: Genetics, Epidemiology and Prevention; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Digilio, M.C.; Marino, B.; Giannotti, A.; Dallapiccola, B. Familial recurrence of transposition of the great arteries and intact ventricular septum. Am. J. Med. Genet. 1997, 73, 93–94. [Google Scholar] [CrossRef]
- Marino, B.; Digilio, M.C.; Versacci, P.; Anaclerio, S.; Dallapiccola, B. Transposition of the great arteries. Understanding its pathogenesis. Ital. Heart J. 2002, 3, 154–160. [Google Scholar]
- Moene, R.J.; Oppenheimer-Dekker, A.; Wenink, A.C.; Bartelings, M.M.; Gittenberger-de Groot, A.C. Morphology of ventricular septal defect in complete transposition of the great arteries. Am. J. Cardiol. 1985, 55, 1566–1570. [Google Scholar] [CrossRef]
- Ibrahim, S.; Gaborit, B.; Lenoir, M.; Collod-Beroud, G.; Stefanovic, S. Maternal Pre-Existing Diabetes: A Non-Inherited Risk Factor for Congenital Cardiopathies. Int. J. Mol. Sci. 2023, 24, 16258. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, C.; Brenner, J.I.; Loffredo, C.; Kappetein, A.P.; Wilson, P.D. Transposition of great arteries: Etiologic distinctions of outflow tract defects in a case control study of risk factors. In Developmental Mechanism of Heart Disease; Clark, E.B., Markwald, R.R., Takao, A., Eds.; Futura Publishing: Armonk, NY, USA, 1995; pp. 639–653. [Google Scholar]
- Oster, M.E.; Aucott, S.W.; Glidewell, J.; Hackell, J.; Kochilas, L.; Martin, G.R.; Phillippi, J.; Pinto, N.M.; Saarinen, A.; Sontag, M.; et al. Lessons Learned From Newborn Screening for Critical Congenital Heart Defects. Pediatrics 2016, 137, e20154573. [Google Scholar] [CrossRef]
- Gardiner, H.M.; Kovacevic, A.; van der Heijden, L.B.; Pfeiffer, P.W.; Franklin, R.C.; Gibbs, J.L.; Averiss, I.E.; Larovere, J.M. Prenatal screening for major congenital heart disease: Assessing performance by combining national cardiac audit with maternity data. Heart 2014, 100, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Oboli, V.N.; Pizzolla, A.; Pattnaik, P. Diagnostic Dilemma: Transposition of the Great Arteries. Cureus 2023, 15, e38931. [Google Scholar] [CrossRef]
- Van Velzen, C.L.; Haak, M.C.; Reijnders, G.; Rijlaarsdam, M.E.; Bax, C.J.; Pajkrt, E.; Hruda, J.; Galindo-Garre, F.; Bilardo, C.M.; de Groot, C.J.M.; et al. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet. Gynecol. 2015, 45, 320–325. [Google Scholar] [CrossRef]
- Kliegman, R.; Stanton, B.; St Geme, J.W.; Schor, N.F.; Behrman, R.E.; Nelson, W.E. Nelson Textbook of Pediatrics; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Bravo-Valenzuela, N.J.; Peixoto, A.B.; Araujo, E., Jr. Prenatal diagnosis of transposition of the great arteries: An updated review. Ultrasonography 2020, 39, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Słodki, M. Dextro-transposition of great vessels: Difficult to detect prenatally, one of the most dangerous and one of the best prognosed. Transl. Pediat. 2022, 11, 783–788. [Google Scholar] [CrossRef] [PubMed]
- McGahan, J.P.; Moon-Grady, A.J.; Pahwa, A.; Towner, D.; Rhee-Morris, L.; Gerscovich, E.O.; Fogata, M. Potential pitfalls and methods of improving in utero diagnosis of transposition of the great arteries, including the baby bird’s beak image. J. Ultrasound. Med. 2007, 26, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.C.; Prefumo, F. Accuracy of ultrasonography at 11–14 weeks of gestation for detection of fetal structural anomalies: A systematic review. Obstet. Gynecol. 2013, 122, 1160–1167. [Google Scholar] [CrossRef]
- Schidlow, D.N.; Donofrio, M.T. Prenatal Maternal Hyperoxygenation Testing and Implications for Critical Care Delivery Planning among Fetuses with Congenital Heart Disease: Early Experience. Am. J. Perinatol. 2018, 35, 16–23. [Google Scholar]
- Jouannic, J.M.; Gavard, L.; Fermont, L.; Le Bidois, J.; Part, S.; Vouhe, P.R.; Dumez, Y.; Sidi, D.; Bonnet, D. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation 2004, 110, 1743–1746. [Google Scholar] [CrossRef]
- Pruetz, J.D.; Carroll, C.; Trento, L.U.; Chang, R.K.; Detterich, J.; Miller, D.A.; Sklansky, M. Outcomes of critical congenital heart disease requiring emergent neonatal cardiac intervention. Prenat. Diagn. 2014, 34, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Buca, D.; Winberg, P.; Rizzo, G.; Khalil, A.; Liberati, M.; Makatsariya, A.; Greco, F.; Nappi, L.; Acharya, G.; D’Antonio, F. Prenatal risk factors for urgent atrial septostomy at birth in fetuses with transposition of the great arteries: A systematic review and meta-analysis. J. Matern. Fetal Neonatal. Med. 2022, 35, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Sylwestrzak, O.; Słodki, M.; Respondek-Liberska, M. Maximal velocity of fetal pulmonary venous blood flow. Prenatal. Cardiol. 2019, 1, 17–19. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Bremer, Y.A.; Moskowitz, W.B. Diagnosis and management of restricted or closed foramen ovale in fetuses with congenital heart disease. Am. J. Cardiol. 2004, 94, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Talemal, L.; Donofrio, M.T. Hemodynamic consequences of a restrictive ductus arteriosus and foramen ovale in fetal transposition of the great arteries. J. Neonatal. Perinatal. Med. 2016, 9, 317–320. [Google Scholar] [CrossRef]
- Sanapo, L.; Pruetz, J.D.; Słodki, M.; Goens, M.B.; Moon-Grady, A.J.; Donofrio, M.T. Fetal echocardiography for planning perinatal and delivery room care of neonates with congenital heart disease. Echocardiography 2017, 34, 1804–1821. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.V.; Kamenir, S.A.; Sinclair, B.; van der Velde, M.E.; Smallhorn, J.F.; Hornberger, L.K. Prenatal features of ductus arteriosus constriction and restrictive foramen ovale in d-transposition of the great arteries. Circulation 1999, 99, 1209–1214. [Google Scholar] [CrossRef]
- Rasanen, J.; Wood, D.C.; Weiner, S.; Ludomirski, A.; Huchta, J.C. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation 1996, 94, 1068–1073. [Google Scholar] [CrossRef]
- Zhang, X.; Haneishi, H.; Liu, H. Impact of ductus arteriosus constriction and restrictive foramen ovale on global hemodynamics for term fetuses with d-TGA. Int. J. Numer. Method Biomed. Eng. 2021, 37, e3231. [Google Scholar] [CrossRef]
- Calderon, J.; Angeard, N.; Moutier, S.; Plumet, M.H.; Jambaque, I.; Bonnet, D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J. Pediatr. 2012, 161, 94–98. [Google Scholar] [CrossRef]
- Kutty, S.; Li, L.; Polak, A.; Gribben, P.; Danford, D.A. Echocardiographic knowledge-based reconstruction for quantification of the systemic right ventricle in young adults with repaired D-transposition of great arteries. Am. J. Cardiol. 2012, 109, 881–888. [Google Scholar] [CrossRef]
- Iriart, X.; Roubertie, F.; Jalal, Z.; Thambo, J.B. Quantification of Systemic Right Ventricle by Echocardiography. Arch. Cardiovasc. Dis. 2016, 109, 120–127. [Google Scholar] [CrossRef]
- Pasquini, L.; Sanders, S.P.; Parness, I.A.; Wernovsky, G.; Mayer, J.E., Jr.; Van der Velde, M.E.; Spevak, P.J.; Colan, S.D. Coronary echocardiography in 406 patients with d-loop transposition of the great arteries. J. Am. Coll. Cardiol. 1994, 24, 763–768. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.J.; El Said, H.G.; Feltes, T.F.; Watrin, C.H.; Hess, B.A.; Fraser, C.D., Jr. Preoperative identification of coronary arterial anatomy in complete transposition, and outcome after the arterial switch operation. Cardiol. Young 2002, 12, 240–247. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Baro’n-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Carazo, M.; Andrade, L.; Kim, Y.; Wilson, W.; Wu, F.M. Assessment and management of heart failure in the systemic right ventricle. Heart Fail. Rev. 2020, 25, 609–621. [Google Scholar] [CrossRef]
- DeVore, G.R.; Klas, B.; Cuneo, B.; Satou, G.; Sklansky, M. Review of speckle tracking analysis to measure the size, shape, and contractility of the fetal heart in fetuses with congenital heart defects. Echocardiography 2024, 41, e15870. [Google Scholar] [CrossRef]
- Meijboom, F.; Bouma, B. The use of contrast echo to detect shunts at atrial level in a Mustard or Senning repair for transposition of the great arteries: You can teach an old dog new tricks. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 536–537. [Google Scholar] [CrossRef]
- Celi, S.; Gasparotti, E.; Capellini, K.; Vignali, E.; Fanni, B.M.; Ali, L.; Cantinotti, M.; Murzi, M.; Bertiego, B.; Santoro, G.; et al. 3D printing in modern cardiology. Curr. Pharm. Des. 2021, 27, 1918–1930. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Krishnamoorthy, K.M.; Sivasubramonian, S. Balloon atrial septostomy at the bedside versus the catheterisation laboratory. Cardiol. Young 2019, 29, 454. [Google Scholar] [CrossRef] [PubMed]
- Meijer, F.M.M.; Kiès, P.; Verheijen, D.B.H.; Vliegen, H.W.; Jongbloed, M.R.M.; Hazekamp, M.G.; Lamb, H.J.; Egorova, A.D. Computed Tomography Derived Coronary Triangulated Orifice Area-Deduction of a New Parameter for Follow-up After Surgical Correction of Anomalous Aortic Origin of Coronary Arteries and Call for Validation. Front. Cardiovasc. Med. 2021, 8, 668503. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.; Hirtler, D.; Bürk, J.; Stiller, B.; Arnold, R.; Jung, B.; Langer, M.; Markl, M. Postoperative pulmonary and aortic 3D haemodynamics in patients after repair of transposition of the great arteries. Eur. Radiol. 2014, 24, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Quintana, E.; Marrero-Negrín, N.; Gopar-Gopar, S.; Rodríguez-González, F. Right ventricular function and N-terminal pro-brain natriuretic peptide levels in adult patients with simple dextro-transposition of the great arteries. Echocardiography 2017, 34, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, E.; Klisiewicz, A.; Rybicka, J.; Biernacka, E.K.; Hoffman, P. High sensitivity cardiac troponin T and systemic right ventricular function in adults with congenitally corrected transposition of the great arteries. Int. J. Cardiol. 2017, 241, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, E.; Klisiewicz, A.; Kowalski, M.; Rybicka, J.; Baranowski, R.; Biernacka, E.K.; Hoffman, P. High-Sensitive Cardiac Troponin T and Systemic Right Ventricular Area Predict Outcomes in Adults With Congenitally Corrected Transposition. Can. J. Cardiol. 2018, 34, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Geenen, L.W.; van Grootel, R.W.J.; Akman, K.; Baggen, V.J.M.; Menting, M.E.; Eindhoven, J.A.; Cuypers, J.A.E.; Boersma, E.; van den Bosch, A.E.; Roos-Hesselink, J.W. Exploring the Prognostic Value of Novel Markers in Adults with a Systemic Right Ventricle. J. Am. Heart Assoc. 2019, 8, e013745. [Google Scholar] [CrossRef] [PubMed]
- Popelova, J.R.; Tomkova, M.; Tomek, J. NT-proBNP predicts mortality in adults with transposition of the great arteries late after Mustard or Senning correction. Congenit. Heart Dis. 2017, 12, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Westhoff-Bleck, M.; Podewski, E.; Tutarel, O.; Wenzel, D.; Cappello, C.; Bertram, H.; Bauersahs, J.; Widder, J. Prognostic value of NT-proBNP in patients with systemic morphological right ventricles: A single-centre experience. Int. J. Cardiol. 2013, 169, 433–438. [Google Scholar] [CrossRef]
- de Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Tang, W.H. B-type natriuretic peptide: A critical review. Congest. Heart Fail. 2007, 13, 48–52. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Gallotta, M.; Quatrini, I.; Nuti, R. Natriuretic peptides (BNP and NT-proBNP): Measurement and relevance in heart failure. Vasc. Health Risk. Manag. 2010, 6, 411–418. [Google Scholar] [CrossRef]
- Kowalik, E.; Kwiatek-Wrzosek, A.; Klisiewicz, A.; Lutyńska, A.; Biernacka, E.K.; Kowalski, M.; Hoffman, P. Systemic right ventricle in elderly patients with congenitally corrected transposition of the great arteries: Clinical profile, cardiac biomarkers, and echocardiographic parameters. Anatol. J. Cardiol. 2020, 24, 92–96. [Google Scholar] [CrossRef]
- Lai, C.T.; Ng, E.K.; Chow, P.C.; Kwong, A.; Cheung, Y.F. Circulating microRNA expression profile and systemic right ventricular function in adults after atrial switch operation for complete transposition of the great arteries. BMC Cardiovasc. Disord. 2013, 13, 73. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Meese, E.; Abdul-Khaliq, H.; Raedle-Hurst, T. MicroRNA-183-3p Is a Predictor of Worsening Heart Failure in Adult Patients With Transposition of the Great Arteries and a Systemic Right Ventricle. Front. Cardiovasc. Med. 2021, 8, 730364. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Sauer, U.; Oppenheimer-Dekker, A.; Quaegebeur, J. Coronary arterial anatomy in transposition of the great arteries: A morphologic study. Pediatr. Cardiol. 1983, 4, 15–24. [Google Scholar]
- Wernovsky, G.; Sanders, S.P. Coronary artery anatomy and transposition of the great arteries. Coron. Artery Dis. 1993, 4, 148–157. [Google Scholar] [CrossRef]
- Katekaru-Tokeshi, D.I.; Jiménez-Santos, M.; Koppel, C.J.; Vliegen, H.W.; Díaz-Zamudio, M.; Castillo-Castellón, F.; Jongbloed, M.R.M.; Kimura-Hayama, E.J. Applicability of the Leiden Convention and the Lipton Classification in Patients with a Single Coronary Artery in the Setting of Congenital Heart Disease. Cardiovasc. Dev. Dis. 2021, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Koppel, C.J.; Vliegen, H.W.; Bökenkamp, R.; Harkel, D.J.T.; Kiès, P.; Egorova, A.D.; Jukema, J.W.; Hazekamp, M.G.; Schalij, M.J.; Gittenberger-de Groot, A.C.; et al. The Leiden Convention coronary coding system: Translation from the surgical to the universal view. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Koenraadt, W.M.C.; Bartelings, M.M.; Bökenkamp, R.; DeRuiter, M.C.; Hazekamp, M.G.; Bogers, A.; Quaegebeur, J.M.; Schalij, M.J.; Vliegen, H.W.; et al. Coding of coronary arterial origin and branching in congenital heart disease: The modified Leiden Convention. J. Thorac. Cardiovasc. Surg. 2018, 156, 2260–2269. [Google Scholar] [CrossRef]
- Quaegebeur, J.M. The Arterial Switch Operation. Rationale, Results and Perspectives; Leiden University: Rozengaard, The Netherlands, 1986. [Google Scholar]
- Al Anani, S.; Fughhi, I.; Taqatqa, A.; Elzein, C.; Ilbawi, M.N.; Polimenakos, A.C. Transposition of great arteries with complex coronary artery variants: Time-related events following arterial switch operation. Pediatr. Cardiol. 2017, 38, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.H.; Radley-Smith, R. Anatomy of the coronary arteries in transposition of the great arteries and methods for their transfer in anatomical correction. Thorax 1978, 33, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.K.; Hasselblad, V.; Li, J.S.; Kong, D.F.; Sanders, S.P. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: A meta-analysis. Circulation 2002, 106, 2575–2580. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.; Losay, J.; Touchot-Koné, A.; Serraf, A.; Belli, E.; Piot, J.D.; Lambert, V.; Capderou, A.; Planche, C. Coronary events after arterial switch operation for transposition of the great arteries. Circulation 2003, 108, 186–190. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Sauer, U.; Quaegebeur, J. Aortic intramural coronary artery in three hearts with transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 1986, 91, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Scheule, A.M.; Jonas, R.A. Management of transposition of the great arteries with single coronary artery. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2001, 4, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Massoudy, P.; Baltalarli, A.; de Leval, M.R.; Cook, A.; Neudorf, U.; Derrick, G.; McCarthy, K.P.; Anderson, R.H. Anatomic variability in coronary arterial distribution with regard to the arterial switch procedure. Circulation 2002, 106, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Lacour-Gayet, F.; Anderson, R.H. A uniform surgical technique for transfer of both simple and complex patterns of the coronary arteries during the arterial switch procedure. Cardiol. Young 2005, 15, 93–101. [Google Scholar] [CrossRef]
- Planche, C.; Lacour-Gayet, F.; Serraf, A. Arterial switch. Pediatr. Cardiol. 1998, 19, 297–307. [Google Scholar] [CrossRef]
- Khairy, P.; Clair, M.; Fernandes, S.M.; Blume, E.D.; Powell, A.J.; Newburger, J.W.; Landzberg, M.J.; Mayer Jr, J. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013, 127, 331–339. [Google Scholar] [CrossRef]
- Raisky, O.; Bergoend, E.; Agnoletti, G.; Ou, P.; Bonnet, D.; Sidi, D.; Vouhé, P.R. Late coronary artery lesions after neonatal arterial switch operation: Results of surgical coronary revascularization. Eur. J. Cardiothorac. Surg. 2007, 31, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.P.; Wolfe, L.T.; Millington, K.A.; Myers, J.L.; Clark, J.B. Occult coronary ostial obstruction late after arterial switch operation. J. Card. Surg. 2013, 28, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Thrupp, S.F.; Gentles, T.L.; Kerr, A.R.; Finucane, K. Arterial switch operation: Early and late outcome for intramural coronary arteries. Ann. Thorac. Surg. 2012, 94, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Mair, D.D.; Ritter, D.G. Factors influencing intercirculatory mixing in patients with complete transposition of the great arteries. Am. J. Cardiol. 1972, 30, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Butts, R.J.; Ellis, A.R.; Bradley, S.M.; Hulsey, T.C.; Atz, A.M. Effect of prostaglandin duration on outcomes in transposition of the great arteries with intact ventricular septum. Congenit. Heart Dis. 2012, 7, 387–391. [Google Scholar] [CrossRef]
- Rashkind, W.J.; Miller, W.W. Creation of an atrial septal defect without thoracotomy: A palliative approach to complete transposition of the great arteries. JAMA 1966, 196, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.W.; Ruckman, R.N.; Galioto, F.M., Jr.; Shapiro, S.R.; Potter, B.M.; Scott, L.P. Echocardiographically assisted balloon atrial septostomy. Pediatrics 1982, 70, 403–408. [Google Scholar] [CrossRef] [PubMed]
- McQuillen, P.S.; Hamrick, S.E.; Perez, M.J.; Barkovich, A.J.; Glidden, D.V.; Karl, T.R.; Teitel, D.; Miller, S.P. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation 2006, 113, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Lindsay, M.; Zhang, Y.; Lardaro, T.; Osen, H.; Chang, D.C.; Brenner, J.I.; Abdullah, F. Analysis of 8681 neonates with transposition of the great arteries: Outcomes with and without Rashkind balloon atrial septostomy. Cardiol. Young 2010, 20, 373–380. [Google Scholar] [CrossRef]
- Polito, A.; Ricci, Z.; Fragasso, T.; Cogo, P.E. Balloon atrial septostomy and pre-operative brain injury in neonates with transposition of the great arteries: A systematic review and a meta-analysis. Cardiol. Young 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Petit, C.J.; Rome, J.J.; Wernovsky, G.; Mason, S.E.; Shera, D.M.; Nicolson, S.C.; Montenegro, L.M.; Tabbutt, S.; Zimmerman, R.A.; Licht, D.J. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation 2009, 119, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Applegate, S.E.; Lim, D.S. Incidence of stroke in patients with d-transposition of the great arteries that undergo balloon atrial septostomy in the University Healthsystem Consortium Clinical Data Base/Resource Manager. Cathet. Cardivasc. Interv. 2010, 76, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Thanopoulos, B.D.; Georgakopoulos, D.; Tsaousis, G.S.; Simeunovic, S. Percutaneous balloon dilatation of the atrial septum: Immediate and midterm results. Heart 1996, 76, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.D., Jr. The neonatal arterial switch operation: Technical pearls. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2017, 20, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Korun, O.; Dedemoglu, M.; Çiçek, M.; Biçer, M.; Altin, H.F.; Yurdakök, O.; Şaşmazel, A.; Aydemir, N.A. Clinical outcomes of primary arterial switch operation in treatment of Taussig-Bing anomaly. Turk. Gogus. Kalp. Dama 2017, 25, 374–380. [Google Scholar] [CrossRef]
- Jatene, A.D.; Fontes, V.F.; Paulista, P.P.; Souza, L.C.; Neger, F.; Galantier, M.; Souza, J.E. Anatomic correction of transposition of the great vessels. J. Thorac. Cardiovasc. Surg. 1976, 72, 364–370. [Google Scholar] [CrossRef]
- Hornung, T.S.; Derrick, G.P.; Deanfield, J.E.; Redington, A.N. Transposition complexes in the adult: A changing perspective. Cardiol. Clin. 2002, 20, 405–420. [Google Scholar] [CrossRef]
- Yacoub, M.H.; Bernhard, A.; Lange, P. Clinical and hemodynamic results of the two-stage anatomic correction of simple transposition of the great arteries. Circulation 1980, 62, 190. [Google Scholar]
- Bove, E.L. Current technique of the arterial switch procedure for transposition of the great arteries. J. Card. Surg. 1989, 4, 193–199. [Google Scholar] [CrossRef]
- Sarris, G.E.; Balmer, C.; Bonou, P.; Comas, J.V.; da Cruz, E.; Di Chiara, L.; Di Donato, R.M.; Fragata, J.; Jokinen, T.E.; Kirvassilis, G.; et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum. Cardiol. Young 2017, 27, 530–569. [Google Scholar] [CrossRef] [PubMed]
- Brawn, W.J.; Mee, R.B.B. Early results for anatomic correction of transposition of the great arteries and for double-outlet right ventricle with subpulmonary ventricular septal defect. J. Thorac. Cardiovasc. Surg. 1988, 95, 230–238. [Google Scholar] [CrossRef]
- Dedemoğlu, M.; Coşkun, G.; Özdemir, F.; Yurdakök, O.; Korun, O.; Çiçek, M.; Biçer, M.; Coşkun, F.I.; Aydemir, N.A.; Şaşmazel, A. Modified Closed Coronary Transfer is a Good Alternative to the Trap-Door Method During Arterial Switch Operation: A Retrospective Propensity-Matched Comparison. Braz. J. Cardiovasc. Surg. 2020, 35, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Bove, E.L. The arterial switch procedure: Closed coronary artery transfer. Oper. Tech. Thorac. Cardiovasc. Surg. 2009, 14, 309–316. [Google Scholar] [CrossRef]
- Formigari, R.; Toscano, A.; Giardini, A.; Gargiulo, G.; Di Donato, R.; Picchio, F.M.; Pasquini, L. Prevalence and predictors of neoaortic regurgitation after arterial switch operation for transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2003, 126, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Lacour-Gayet, F. Chapter 14—Arterial Switch in TGA-IVS: Coronary Transfer. In Surgery of Conotruncal Anomalies; Lacour-Gayet, F., Bove, E.L., Hraška, V., Morell, V.O., Spray, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 247–268. [Google Scholar]
- Kim, H.; Sung, S.C.; Kim, S.H.; Chang, Y.H.; Ahn, H.Y.; Lee, H.D. Arterial switch operation in patients with intramural coronary artery: Early and mid-term results. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 115–122. [Google Scholar] [CrossRef]
- Sithamparanathan, S.; Padley, S.P.G.; Rubens, M.B.; Gatzoulis, M.A.; Ho, S.Y.; Nicol, E.D. Great vessel and coronary artery anatomy in transposition and other coronary anomalies; a universal descriptive and alphanumerical sequential classification. JACC Cardiovasc. Imaging 2013, 6, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Rudra, H.S.; Mavroudis, C.; Backer, C.L.; Kaushal, S.; Russell, H.; Stewart, R.D.; Webb, C.; Sullivan, C. The arterial switch operation: 25-year experience with 258 patients. Ann. Thorac. Surg. 2011, 92, 1742–1746. [Google Scholar] [CrossRef]
- Blume, E.D.; Altmann, K.; Mayer, J.E.; Colan, S.D.; Gauvreau, K.; Geva, T. Evolution of risk factors influencing early mortality of the arterial switch operation. J. Am. Coll. Cardiol. 1999, 33, 1702–1709. [Google Scholar] [CrossRef]
- Jatene, M.B.; Miana, L.A. Intramural coronary artery course in Jatene operation for transposition of great arteries: Still a challenge. Braz. J. Cardiovasc. Surg. 2016, 31, III. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budtsa, W.; Chessa, M.; Dillera, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Fricke, T.A.; d’Udekem, Y.; Richardson, M.; Thuys, C.; Dronavalli, M.; Ramsay, J.M.; Wheaton, G.; Grigg, L.E.; Brizard, C.P.; Konstantinov, I.E. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann. Thorac. Surg. 2012, 94, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ou, P.; Khraiche, D.; Celermajer, D.S.; Agnoletti, G.; Le Quan Sang, K.H.; Thalabard, J.C.; Quintin, M.; Raisky, O.; Vouhe, P.; Sidi, D.; et al. Mechanisms of coronary complications after the arterial switch for transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2013, 145, 1263–1269. [Google Scholar] [CrossRef]
- Rastelli, G.C. A new approach to “anatomic” repair of transposition of the great arteries. Mayo Clin. Proc. 1969, 44, 1–12. [Google Scholar]
- Rastelli, G.C.; McGoon, D.C.; Wallace, R.B. Anatomic correction of transposition of the great arteries with ventricular septal defect and subpulmonary stenosis. J. Thorac. Cardiovasc. Surg. 1969, 58, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.C.; Wallace, R.B.; Ongley, P.A. Complete repair of transposition of the great arteries with pulmonary stenosis. A review and report of a case corrected by using a new surgical technique. Circulation 1969, 39, 83–95. [Google Scholar] [CrossRef]
- Kreutzer, C. Chapter 17—TGA-VSD and LVOTO: Rastelli Procedure. In Surgery of Conotruncal Anomalies; Lacour-Gayet, F., Bove, E.L., Hraška, V., Morell, V.O., Spray, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 303–316. [Google Scholar]
- Lecompte, Y.; Neveux, J.Y.; Leca, F.; Zannini, L.; Tu, T.V.; Duboys, Y.; Jarreau, M.M. Reconstruction of the pulmonary outflow tract without prosthetic conduit. J. Thorac. Cardiovasc. Surg. 1982, 84, 727–733. [Google Scholar] [CrossRef]
- Lecompte, Y.; Vouhé, P. Réparation à l’Etage Ventriculaire (REV procedure): Not a Rastelli procedure without conduit. Oper. Tech. Thorac. Cardiovasc. Surg. 2003, 8, 150–159. [Google Scholar] [CrossRef]
- Vouhé, P.R.; Raisky, O. Chapter 18—TGA-VSD-LVOT Obstruction: REV (Réparation à l’Etage Ventriculaire) Procedure. In Surgery of Conotruncal Anomalies; Lacour-Gayet, F., Bove, E.L., Hraška, V., Morell, V.O., Spray, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 317–332. [Google Scholar]
- Di Carlo, D.; Tomasco, B.; Cohen, L.; Vouhe, P.; Lecompte, Y. Long-term results of the REV (reparation à l’etage ventriculaire) operation. J. Thorac. Cardiovasc. Surg. 2011, 142, 336–343. [Google Scholar] [CrossRef]
- Bex, J.P.; Lecompte, Y.; Baillot, F.; Hazan, E. Anatomical correction of transposition of the great arteries. Ann. Thorac. Surg. 1980, 29, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Nikaidoh, H. Aortic translocation and biventricular outflow tract reconstruction. A new surgical repair for transposition of the great arteries associated with ventricular septal defect and pulmonary stenosis. J. Thorac. Cardiovasc. Surg. 1984, 88, 365–372. [Google Scholar] [CrossRef]
- Morell, V.O.; Jacobs, J.P.; Quintessenza, J.A. Aortic translocation in the management of transposition of the great arteries with ventricular septal defect and pulmonary stenosis: Results and follow-up. Ann. Thorac. Surg. 2005, 79, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernandez, V.; Marx, G.R.; Bacha, E.A.; del Nido, P.J. Aortic root translocation plus arterial switch for transposition of the great arteries with left ventricular outflow tract obstruction: Intermediate-term results. J. Am. Coll. Cardiol. 2007, 49, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, M.G.; Nevvazhay, T.; Sojak, V. Nikaidoh vs. réparation à l’etage ventriculaire vs. Rastelli. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2018, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Raju, V.; Myers, P.O.; Quinonez, L.G.; Emani, S.M.; Mayer, J.E., Jr.; Pigula, F.A.; del Nido, P.J.; Baird, C.W. Aortic root translocation (Nikaidoh procedure): Intermediate follow-up and impact of conduit type. J. Thorac. Cardiovasc. Surg. 2015, 149, 1349–1355. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Supreet, M.; Shilpa, M.; Nelson, A.; Agarwal, V. Options for coronary translocation and other considerations in aortic root translocation (Bex Nikaidoh procedure). Ann. Pediatr. Cardiol. 2019, 12, 228–232. [Google Scholar] [CrossRef]
- Morell, W.O. Chapter 20—The Aortic Translocation (Nikaidoh) Procedure. In Surgery of Conotruncal Anomalies; Lacour-Gayet, F., Bove, E.L., Hraška, V., Morell, V.O., Spray, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 345–355. [Google Scholar]
- Dearani, J.A.; Danielson, G.K.; Puga, F.J.; Mair, D.D.; Schleck, C.D. Late results of the Rastelli operation for transposition of the great arteries. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2001, 4, 3–15. [Google Scholar] [CrossRef]
- Agarwal, V.; Vaidyanathan, S. Aortic root translocation: The Bex-Nikaidoh procedure. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Shuntoh, K.; Matsushita, T.; Fujiwara, K.; Shinkawa, T.; Miyazaki, T.; Kitamura, N. Half-turned truncal switch operation for complete transposition of the great arteries with ventricular septal defect and pulmonary stenosis. J. Thorac. Cardiovasc. Surg. 2003, 125, 966–968. [Google Scholar] [CrossRef]
- Hu, S.S.; Li, S.J.; Wang, X.; Wang, L.Q.; Xiong, H.; Li, L.H.; Yan, F.; Wang, X. Pulmonary and aortic root translocation in the management of transposition of the great arteries with ventricular septal defect and left ventricular outflow tract obstruction. J. Thorac. Cardiovasc. Surg. 2007, 133, 1090–1092. [Google Scholar] [CrossRef]
- Hu, S.S.; Liu, Z.G.; Li, S.J.; Shen, X.D.; Wang, X.; Liu, J.P.; Yan, F.X.; Wang, L.Q.; Li, Y.Q. Strategy for biventricular outflow tract reconstruction: Rastelli, REV, or Nikaidoh procedure? J. Thorac. Cardiovasc. Surg. 2008, 135, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Angeli, E.; Raisky, O.; Bonnet, D.; Sidi, D.; Vouhé, P.R. Late reoperations after neonatal arterial switch operation for transposition of the great arteries. Eur. J. Cardiothorac. Surg. 2008, 34, 32–36. [Google Scholar] [CrossRef]
- Kreutzer, C.; De Vive, J.; Kreutzer, J.; Gauvreau, K.; Freed, M.; Mayer, J.; Jonas, R.; Del Nido, P. Twenty-five year experience with Rastelli repair for transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2000, 120, 211–223. [Google Scholar] [CrossRef]
- Yeh, T., Jr.; Ramaciotti, C.; Leonard, S.R.; Roy, L.; Nikaidoh, H.J. The aortic translocation (Nikaidoh) procedure: Midterm results superior to the Rastelli procedure. J. Thorac. Cardiovasc. Surg. 2007, 133, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Blalock, A.; Hanlon, C.R. The surgical treatment of complete transposition of the aorta and the pulmonary artery. Surg. Gynecol. Obstet. 1950, 90, 1–15. [Google Scholar] [PubMed]
- Lillehei, C.W.; Varco, R.L. Certain physiologic, pathologic, and surgical features of complete transposition of the great vessels. Surgery 1953, 34, 376–400. [Google Scholar]
- Baffes, T.G. A new method for surgical correction of transposition of the aorta and pulmonary artery. Sur. Gynecol. Obstet. 1956, 102, 227–233. [Google Scholar]
- Müller, J.; Hess, J.; Hörer, J.; Hager, A. Persistent superior exercise performance and quality of life long-term after arterial switch operation compared to that after atrial redirection. Int. J. Cardiol. 2013, 166, 381–384. [Google Scholar] [CrossRef]
- Albert, H.M. Surgical correction of transposition of the great vessels. Sur. Forum 1954, 5, 74–77. [Google Scholar] [CrossRef]
- Mustard, W.T. Successful Two-Stage Correction of Transposition of the Great Vessels. Surgery 1964, 55, 469–472. [Google Scholar]
- Rooshesselink, J. Decline in Ventricular Function and Clinical Condition after Mustard Repair for Transposition of the Great Arteries (a Prospective Study of 22–29 Years). Eur. Heart J. 2004, 25, 1264–1270. [Google Scholar] [CrossRef]
- Deanfield, J.; Camm, J.; Macartney, F.; Cartwright, T.; Douglas, J.; Drew, J.; de Leval, M.; Stark, J. Arrhythmia and late mortality after Mustard and Senning operation for transposition of the great arteries: An eight-year prospective study. J. Thorac. Cardiovasc. Surg. 1988, 96, 569–576. [Google Scholar] [CrossRef]
- Bolger, A.P.; Sharma, R.; Li, W.; Leenarts, M.; Kalra, P.R.; Kemp, M.; Coats, A.J.S.; Anker, S.D.; Gatzoulis, M.A. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 2002, 106, 92–99. [Google Scholar] [CrossRef]
- Wong, K.Y.; Venables, A.W.; Kelly, M.J.; Kalff, V. Longitudinal studies of ventricular function after the Mustard operation of transposition of the great arteries: A long term follow up. Br. Heart J. 1988, 60, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Landzberg, M.J.; Lambert, J.; O’Donnell, C.P. Longterm outcomes after the atrial switch for surgical correction of transposition: A meta-analysis comparing the Mustard and Senning procedures. Cardiol. Young 2004, 14, 284–292. [Google Scholar] [CrossRef]
- Khairy, P.; Dore, A.; Talajic, M.; Dubuc, M.; Poirier, N.; Roy, D.; Mercier, L.A. Arrhythmias in adult congenital heart disease. Expert. Rev. Cardiovasc. Ther. 2006, 4, 83–95. [Google Scholar] [CrossRef]
- Broberg, C.S.; van Dissel, A.; Minnier, J.; Aboulhosn, J.; Kauling, R.M.; Ginde, S.; Krieger, E.V.; Rodriguez, F., 3rd; Gupta, T.; Shah, S.; et al. Long-term outcomes after atrial switch operation for transposition of the great arteries. J. Am. Coll. Cardiol. 2022, 80, 951–963. [Google Scholar] [CrossRef]
- Dos, L.; Teruel, L.; Ferreira, I.J.; Rodriguez-Larrea, J.; Miro, L.; Girona, J.; Albert, D.C.; Goncalves, A.; Murtra, M.; Casaldaliga, J. Late outcome of Senning and Mustrad procedures for correction of transposition of the great arteries. Heart Br. Card. Soc. 2005, 91, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Gewillig, M.; Sluysmans, T.; Verhaaren, H.; Viart, P.; Massin, M.; Suys, B.; Budts, W.; Pasquet, A.; De Wolf, D.; et al. Long term outcome up to 30 years after the Mustard or Senning operation: A nationwide multicentre study in Belgium. Heart 2004, 90, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Tynan, M.; Anderson, R.H. Congenital heart disease. In Diseases of the Heart; Julian, D.G., Camm, A.J., Fox, K.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1996; pp. 681–755. [Google Scholar]
- Andrade, L.; Carazo, M.; Wu, F.; Kim, Y.; Wilson, W. Mechanisms for heart failure in systemic right ventricle. Heart Fail. Rev. 2020, 25, 599–607. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Ferreira, J.P.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- Ephrem, G.; McCollum, J.C.; Green-Hess, D.; Guglin, M.E.; Sawada, S.G.; Rao, R.A. Subjective and Objective Impact of Angiotensin Receptor-Neprilysin Inhibitors on Systemic Right Ventricle Patients. Heart Lung Circ. 2022, 31, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Love, B.A.; Mehta, D.; Fuster, V.F. Evaluation and management of the adult patient with transposition of the great arteries following atrial-level (Senning or Mustard) repair. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Evans, A.T.; Maw, A.M.; Pashun, R.A.; Patel, A.; Kim, L.; Feldman, D.; Minutello, R.; Wong, S.C.; Stribling, J.C.; et al. Predictors of Late Mortality in D-Transposition of the Great Arteries After Atrial Switch Repair: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012932. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, T.; Kiès, P.; Maan, A.; Man, S.C.; Bootsma, M.; Vliegen, H.; Egorova, A.; Mertens, B.; Holman, E.; Schalij, M.; et al. Association between reduced heart rate variability components and supraventricular tachyarrhythmias in patients with a systemic right ventricle. Auton. Neurosci. Basic Clin. 2020, 227, 102696. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.; Giardi, D.; Cheema, K.P.; Espinosa, S.; Umadat, G.; Hodge, D.O.; Madhavan, M.; Asirvatham, S.; Phillips, S.D.; McLeod, C.J. Atrial arrhythmia predicts late events and mortality in patients with D-transposition of the great arteries and atrial switch repair. IJC Congenit. Heart Dis. 2024, 15, 100491. [Google Scholar] [CrossRef]
- Khairy, P.; Harris, L.; Landzberg, M.J.; Fernandes, S.M.; Barlow, A.; Mercier, L.A.; Viswanathan, S.; Chetaille, P.; Gordon, E.; Dore, A.; et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: A multicenter study. Circ. Arrhythm. Electrophysiol. 2008, 1, 250–257. [Google Scholar] [CrossRef]
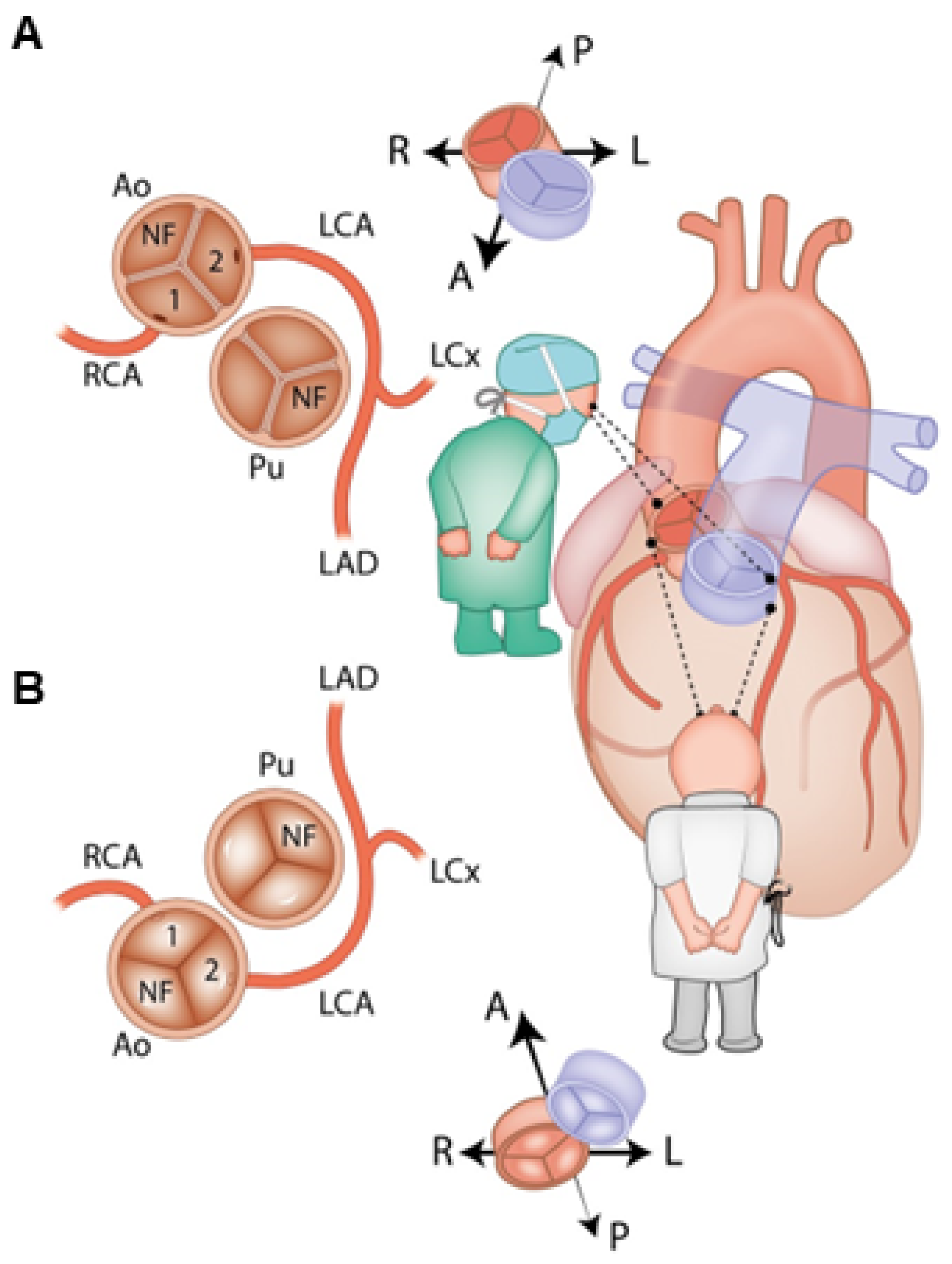



| Type A | 1LCx-2R (70–74%) | Type D | 1RL-2Cx (2–6%) |
| Type B | 1L-2CxR (10–12%) | Type E | 2LCxR (5–6%) |
| Type C | 1R-2LCx (1–2%) | Type F | 1LCxR (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Morshuis, M.; Gummert, J.F.; Zubrzycka, M. Pathogenesis and Surgical Treatment of Dextro-Transposition of the Great Arteries (D-TGA): Part II. J. Clin. Med. 2024, 13, 4823. https://doi.org/10.3390/jcm13164823
Zubrzycki M, Schramm R, Costard-Jäckle A, Morshuis M, Gummert JF, Zubrzycka M. Pathogenesis and Surgical Treatment of Dextro-Transposition of the Great Arteries (D-TGA): Part II. Journal of Clinical Medicine. 2024; 13(16):4823. https://doi.org/10.3390/jcm13164823
Chicago/Turabian StyleZubrzycki, Marek, Rene Schramm, Angelika Costard-Jäckle, Michiel Morshuis, Jan F. Gummert, and Maria Zubrzycka. 2024. "Pathogenesis and Surgical Treatment of Dextro-Transposition of the Great Arteries (D-TGA): Part II" Journal of Clinical Medicine 13, no. 16: 4823. https://doi.org/10.3390/jcm13164823





