Preoperative OCT Characteristics Contributing to Prediction of Postoperative Visual Acuity in Eyes with Macular Hole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Dividing Patients into Two Groups Based on the Best-Corrected Visual Acuity
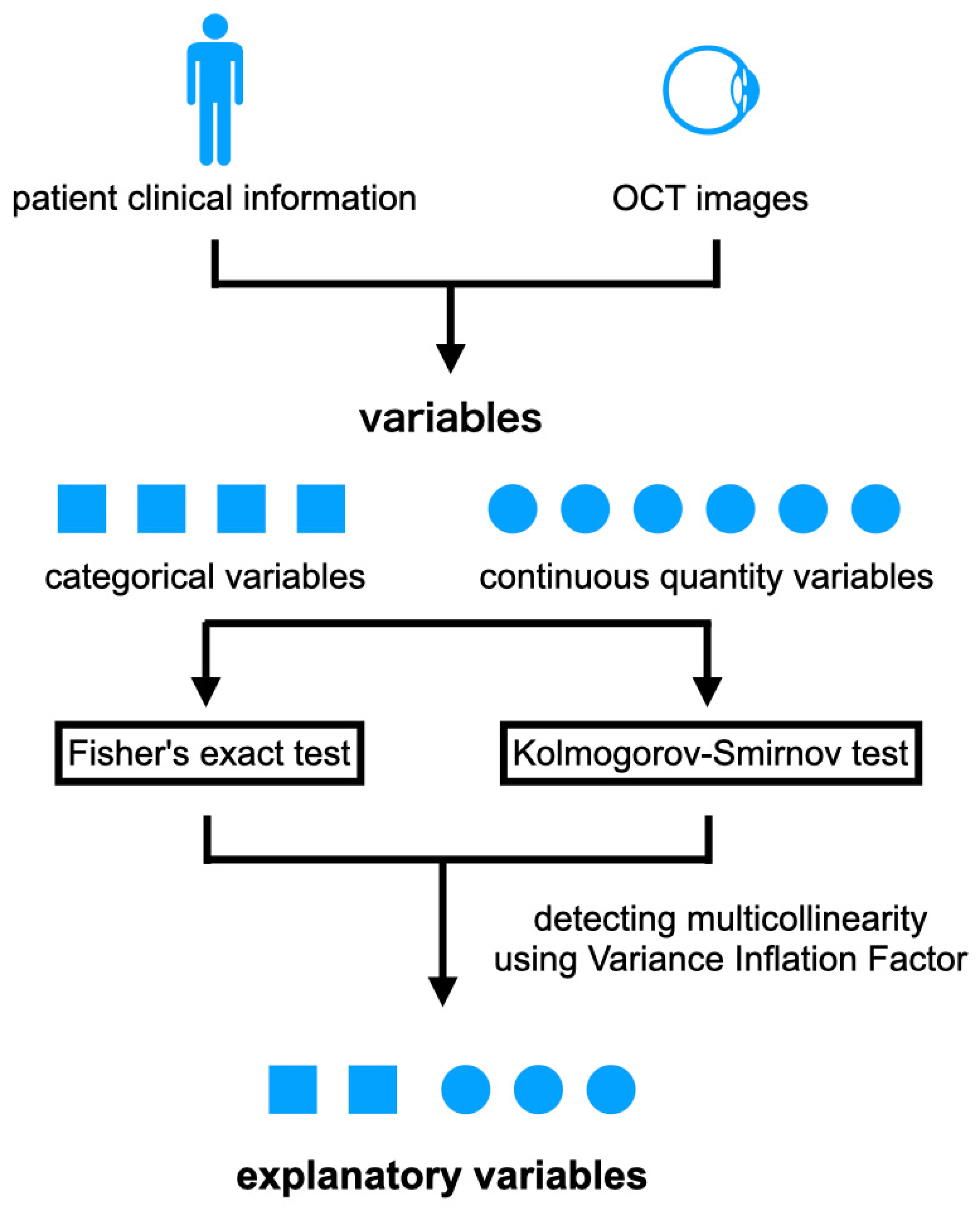
2.3. Preprocessing of OCT Images and Features
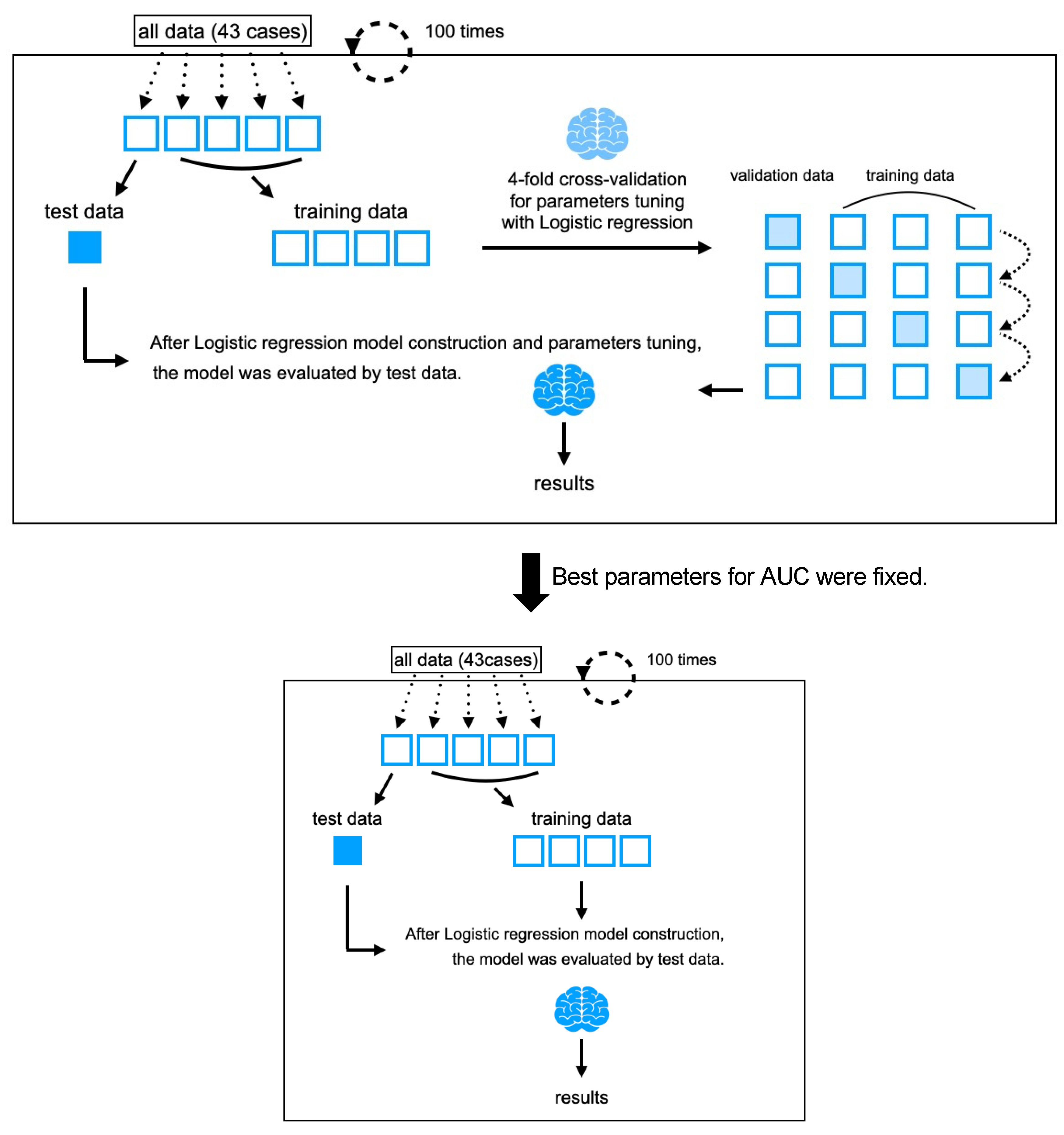
2.4. Machine Learning Algorithms Considering AI Alignment
2.5. Statistical Analyses
3. Results
3.1. Demographics of Group A and Group B
3.2. Explanatory Variables
3.3. Hyperparameters of Logistic Regression
3.4. Classification Performance
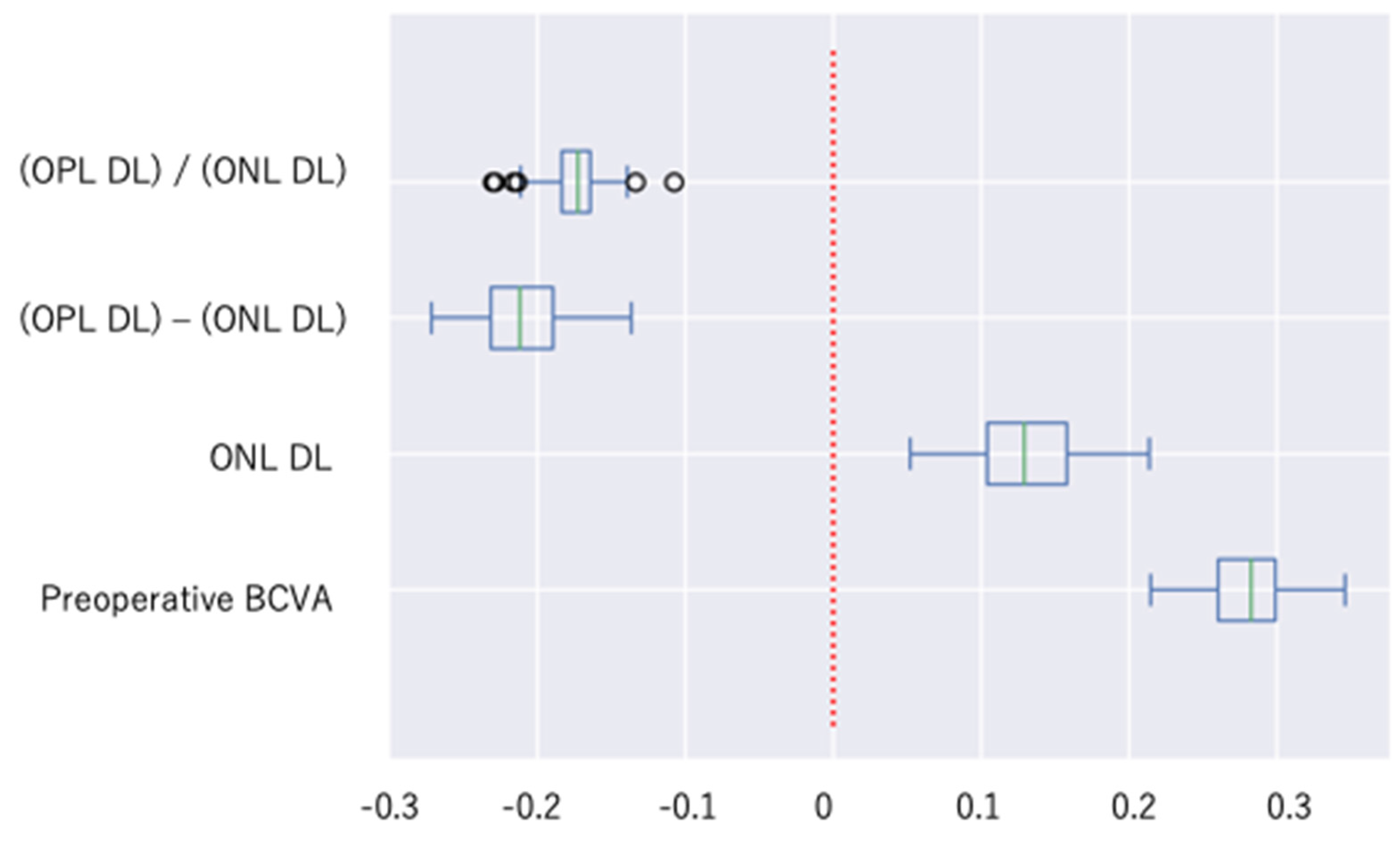
3.5. Specific Contributions of Explanatory Variables
3.6. Control Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, J.; Zarnegar, A.; Corradetti, G.; Singh, S.R.; Chhablani, J. Advances in optical coherence tomography imaging technology and techniques for choroidal and retinal disorders. J. Clin. Med. 2022, 11, 5139. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Teraoka Escaño, M.F.; Fujii, S.; Nakanishi, Y.; Tamura, Y.; Nagai, A.; Yamamoto, H.; Tsukahara, Y.; Negi, A. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am. J. Ophthalmol. 2004, 138, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, W.N.; Chen, K.J.; Wu, H.J.; Lee, C.L.; Chen, C.H.; Wu, K.-Y.; Wu, W.C. Correlation between the dynamic postoperative visual outcome and the restoration of foveal microstructures after macular hole surgery. Am. J. Ophthalmol. 2015, 160, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Oh, J.; Yang, S.M.; Ahn, S.E.; Kim, S.W.; Huh, K. Hyperreflective external limiting membranes after successful macular hole surgery. Retina 2012, 32, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A. Macula hole surgery: Simple or complex? Am. J. Ophthalmol. 2009, 147, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Houly, J.R.; Veloso, C.E.; Passos, E.; Nehemy, M.B. Quantitative analysis of external limiting membrane, ellipsoid zone and interdigitation zone defects in patients with macular holes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Aboudi, N.; Guetari, R.; Khlifa, N. Multi-objectives optimisation of features selection for the classification of thyroid nodules in ultrasound images. IET Image Process. 2020, 14, 1901–1908. [Google Scholar] [CrossRef]
- Khachnaoui, H.; Guetari, R.; Khlifa, N. A review on deep learning in thyroid ultrasound computer-assisted diagnosis systems. In Proceedings of the 2018 IEEE International Conference on Image Processing, Applications and Systems (IPAS), Sophia Antipolis, France, 12–14 December 2018; pp. 291–297. [Google Scholar]
- Matsui, Y.; Imamura, K.; Ooka, M.; Chujo, S.; Mase, Y.; Matsubara, H.; Kawanaka, H.; Kondo, M. Classification of good visual acuity over time in patients with branch retinal vein occlusion with macular edema using support vector machine. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Imamura, K.; Chujo, S.; Mase, Y.; Matsubara, H.; Sugimoto, M.; Kawanaka, H.; Kondo, M. Which Explanatory Variables Contribute to the Classification of Good Visual Acuity over Time in Patients with Branch Retinal Vein Occlusion with Macular Edema Using Machine Learning? J. Clin. Med. 2022, 11, 3903. [Google Scholar] [CrossRef]
- Zgolli, H.; k El Zarrug, H.H.; Meddeb, M.; Mabrouk, S.; Khlifa, N. Anatomical prognosis after idiopathic macular hole surgery: Machine learning based-predection. Libyan J. Med. 2022, 17, 2034334. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, Y.; Quan, W.; Zhang, B.; Wu, Y.; Wu, Q.; Liu, B.; Zheng, X.; Lin, Z.; Li, T.; et al. Machine learning-based prediction of anatomical outcome after idiopathic macular hole surgery. Ann. Transl. Med. 2021, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Lachance, A.; Godbout, M.; Antaki, F.; Hébert, M.; Bourgault, S.; Caissie, M.; Tourville, E.; Durand, A.; Dirani, A. Predicting visual improvement after macular hole surgery: A combined model using deep learning and clinical features. Transl. Vis. Sci. Technol. 2022, 11, 6. [Google Scholar] [CrossRef]
- Obata, S.; Ichiyama, Y.; Kakinoki, M.; Sawada, O.; Saishin, Y.; Ito, T.; Tomioka, M.; Ohji, M. Prediction of postoperative visual acuity after vitrectomy for macular hole using deep learning–based artificial intelligence. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 260, 1113–1123. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) the TRIPOD statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef]
- Miura, G.; Mizunoya, S.; Arai, M.; Hayashi, M.; Yamamoto, S. Early postoperative macular morphology and functional outcomes after successful macular hole surgery. Retina 2007, 27, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Unsal, E.; Cubuk, M.O.; Ciftci, F. Preoperative prognostic factors for macular hole surgery: Which is better? Oman J. Ophthalmol. 2019, 12, 20. [Google Scholar]
- The Official Website of the GNU Image Manipulation Program. Available online: https://www.gimp.org (accessed on 1 April 2021).
- Nick, T.G.; Campbell, K.M. Logistic regression. In Topics in Biostatistics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 273–301. [Google Scholar]
- Zeng, X.; Martinez, T.R. Distribution-balanced stratified cross-validation for accuracy estimation. J. Exp. Theor. Artif. Intell. 2000, 12, 1–12. [Google Scholar] [CrossRef]
- Chen, J.H.; Asch, S.M. Machine learning and prediction in medicine—Beyond the peak of inflated expectations. N. Engl. J. Med. 2017, 376, 2507. [Google Scholar] [CrossRef] [PubMed]
- The Official Website of Scikit-Learn. Available online: https://scikit-learn.org/stable/ (accessed on 1 April 2021).
- Choosing the Right Estimator. Available online: https://scikit-learn.org/1.3/tutorial/machine_learning_map/index.html (accessed on 1 April 2021).
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Omri, S.; Jonet, L.; Zhao, M.; Behar-Cohen, F.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Starr, M.R.; Salabati, M.; Mahmoudzadeh, R.; Patel, L.G.; Ammar, M.J.; Hsu, J.; Garg, S.; Ho, A.C.; Kuriyan, A.E. Fluctuations in central subfield thickness associated with worse visual outcomes in patients with diabetic macular edema in clinical trial setting. Am. J. Ophthalmol. 2021, 232, 90–97. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Oshima, Y.; Fujimoto, H.; Murakami, Y.; Sakaguchi, H.; Kusaka, S.; Tano, Y. Foveal microstructure and visual acuity after retinal detachment repair: Imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology 2009, 116, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Yamaguchi, Y.; Kishi, S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina Phila. Pa 2010, 30, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Jackson, T.L.; Chronopoulos, A.; Hattenbach, L.O.; Longo, A.; Bonfiglio, V.; Russo, A.; Avitabile, T.; Parisi, F.; Reibaldi, M.; et al. Factors predicting normal visual acuity following anatomically successful macular hole surgery. Acta Ophthalmol. 2021, 99, e324–e329. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Laidlaw, D.A.H.; Williamson, T.H.; Shah, S.P.; Wong, R.; Wren, S. Predicting visual success in macular hole surgery. Br. J. Ophthalmol. 2009, 93, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yu, Y.; You, Q.; Wang, Z.; Wang, J.; Liu, W. Hole diameter ratio for prediction of anatomical outcomes in stage III or IV idiopathic macular holes. BMC Ophthalmol. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.C.; AI-Zubaidy, M.; Lois, N.; Scott, N.; Steel, D.H.; Qu, J.; Zhao, M.; Sadda, S.; Manasa, S.; Alvin, K.K.H.; et al. The effect of macular hole duration on surgical outcomes: An individual participant data study of randomized controlled trials. Ophthalmology 2023, 130, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Staicu, C.; Pinero, D.P.; Montero, J.; Lugo, F.; Amat, P. Optical coherence tomography predictive factors for macular hole surgery outcome. Br. J. Ophthalmol. 2008, 92, 640–644. [Google Scholar] [CrossRef] [PubMed]




| Features | Definition | Variable Types 1 = Categorical 2 = Continuous |
|---|---|---|
| Sex | Man or woman | 1 |
| Age | N/A | 2 |
| Preoperative BCVA | N/A | 2 |
| Method | vitrectomy or phacovitrectomy | 1 |
| Affected eye | Right or left | 1 |
| Axial length | N/A | 2 |
| Stage | N/A | 1 |
| Disease duration | N/A | 2 |
| ILM | Peel or invert or not peel | 1 |
| VMT | Present or absent of VMT | 1 |
| PVD | PVD complete or not complete | 1 |
| BDM | basal diameter of MH | 2 |
| Hole-min | minimum linear diameter of MH | 2 |
| OPL-DL | DL for OPL | 2 |
| ELM-DL | DL for ELM | 2 |
| EZ-DL | DL for EZ | 2 |
| Green-sDL | shortest DL for green color area | 2 |
| Yellow-sDL (NL-DL) | shortest DL for yellow color area | 2 |
| Sky blue-sDL | shortest DL for sky blue color area | 2 |
| Blue-sDL | shortest DL for blue color area | 2 |
| Area IRF | Area of IRF | 2 |
| Area-green | Area from ILM to OPL not including boundary line | 2 |
| Area-yellow | Area from OPL to ELM not including boundary line, ONL | 2 |
| Area-sky_blue | Area from ELM to EZ not including boundary line | 2 |
| Area-blue | Area from EZ to RPE not including boundary line | 2 |
| (OPL-DL) − (ONL-DL) | (OPL-DL) minus (ONL-DL) | 2 |
| (ELM-DL) − (ONL-DL) | (ELM-DL) minus (ONL-DL) | 2 |
| (OPL-DL) − (Green-sDL) | (OPL-DL) minus (Green-sDL) | 2 |
| (ELM-DL) − (Sky blue-sDL) | (ELM-DL) minus (Sky blue-sDL) | 2 |
| (EZ-DL) − (Sky blue-sDL) | (EZ-DL) minus (Sky blue-sDL) | 2 |
| (EZ-DL) − (Blue-sDL) | (EZ-DL) minus (blue-sDL) | 2 |
| BDM − (Blue-sDL) | BDM minus (blue-sDL) | 2 |
| BDM − (hole-min) | BDM minus (hole-min) | 2 |
| (Green-sDL)/(OPL-DL) | (Green-sDL) divide by (OPL-DL) | 2 |
| (OPL-DL)/(ONL-DL) | (OPL-DL) divide by (ONL-DL) | 2 |
| (ELM-DL)/(ONL-DL) | (ELM-DL) divide by (ONL-DL) | 2 |
| (ELM-DL)/(Sky blue-sDL) | (ELM-DL) divide by (Sky blue-sDL) | 2 |
| (EZ-DL)/(Sky blue-sDL) | (EZ-DL) divide by (Sky blue-sDL) | 2 |
| (EZ-DL)/(Blue-sDL) | (EZ-DL) divide by (Blue-sDL) | 2 |
| BDM/(Blue-sDL) | BDM divided by (Blue-sDL) | 2 |
| BDM/(Hole-min) | BDM divide by (Hole-min) | 2 |
| Variables | Group A | Group B | p-Value |
|---|---|---|---|
| Sex Men, n (%) | 10 (30.3) | 4 (36.4) | 1.0 |
| Age years | 65.59 ± 6.51 | 67.18 ± 4.63 | 0.552 |
| Preoperative BCVA | 0.46 ± 0.24 | 0.81 ± 0.34 | * 0.007 |
| Methods PPV/phaco + PPV | 1/31 | 0/11 | 1.0 |
| Affected eye Right, n (%) | 18 (54.5) | 6 (36.0) | 1.0 |
| Axial length | 23.98 ± 1.38 | 24.05 ± 1.56 | 0.987 |
| Stage of MH (2, 3, 4) | 3, 24, 5 | 3, 7, 1 | 0.524 |
| Disease duration | 1.72 ± 2.05 | 3.18 ± 3.46 | 0.481 |
| ILM peeled, n (%) | 22 (84.6) | 4 (15.4) | 0.147 |
| VMT presence, n (%) | 20 (76.9) | 6 (23.1) | 0.728 |
| PVD exist, n (%) | 27 (73.0) | 10 (27.0) | 1.0 |
| BDM | 724.66 ± 189.13 | 958.45 ± 399.37 | 0.162 |
| Hole-min | 295.50 ± 107.49 | 423.09 ± 175.49 | 0.053 |
| OPL-DL | 465.09 ± 140.14 | 492.36 ± 170.95 | 0.784 |
| Yellow-sDL (ONL-DL) | 313.28 ± 115.73 | 434.09 ± 186.22 | 0.015 |
| ELM-DL | 320.63 ± 120.25 | 480.82 ± 221.77 | 0.017 |
| EZ-DL | 359.00 ± 172.47 | 517.09 ± 263.87 | 0.217 |
| Green-sDL | 462.91 ± 134.37 | 533.73 ± 148.32 | 0.180 |
| Sky blue-sDL | 297.13 ± 120.35 | 458.27 ± 220.81 | 0.053 |
| Blue-sDL | 372.25 ± 168.61 | 530.82 ± 256.59 | 0.136 |
| Area IRF | 1437.72 ± 910.70 | 2240.36 ± 1653.83 | 0.109 |
| Area-green | 7268.78 ± 1414.01 | 7122.45 ± 1195.36 | 0.721 |
| Area-yellow | 5263.59 ± 1220.27 | 5068.27 ± 1463.88 | 0.799 |
| Area-sky_blue | 1585.31 ± 327.78 | 1560.64 ± 215.41 | 0.721 |
| Area-blue | 1701.56 ± 417.81 | 1744.73 ± 472.78 | 0.552 |
| (OPL-DL) − (ONL-DL) | 151.81 ± 114.31 | 58.27 ± 46.37 | 0.020 |
| (ELM-DL) − (ONL-DL) | 7.34 ± 42.69 | 46.73 ± 76.45 | 0.336 |
| (OPL-DL) − (Green-sDL) | 2.19 ± 95.99 | −41.36 ± 55.85 | 0.362 |
| (ELM-DL) − (Sky blue-sDL) | 23.50 ± 30.58 | 22.55 ± 22.67 | 0.843 |
| (EZ-DL) − (Sky blue-sDL) | 61.88 ± 82.83 | 58.82 ± 51.32 | 0.516 |
| (EZ-DL) − (Blue-sDL) | −13.25 ± 25.11 | −13.73 ± 22.21 | 0.984 |
| BDM-(Blue-sDL) | 352.41 ± 127.58 | 427.64 ± 231.34 | 0.616 |
| BDM-(hole-min) | 429.16 ± 134.12 | 535.36 ± 250.01 | 0.616 |
| (Green-sDL)/(OPL-DL) | 1.01 ± 0.17 | 1.12 ± 0.17 | 0.362 |
| (OPL-DL)/(ONL-DL) | 1.63 ± 0.60 | 1.19 ± 0.19 | 0.020 |
| (ELM-DL)/(ONL-DL) | 1.03 ± 0.14 | 1.11 ± 0.16 | 0.336 |
| (ELM-DL)/(Sky blue-sDL) | 1.10 ± 0.14 | 1.05 ± 0.06 | 0.843 |
| (EZ-DL)/(Sky blue-sDL) | 1.20 ± 0.23 | 1.11 ± 0.09 | 0.516 |
| (EZ-DL)/(Blue-sDL) | 0.95 ± 0.08 | 0.96 ± 0.05 | 0.984 |
| BDM/(Blue-sL) | 2.19 ± 0.81 | 1.92 ± 0.41 | 0.784 |
| BDM/(Hole-min) | 2.62 ± 0.75 | 2.33 ± 0.43 | 0.267 |
| Variables | 1st R2 Score | 2nd R2 Score |
|---|---|---|
| Preoperative BCVA | 0.321 | 0.283 |
| ELM DL | 0.919 | NA |
| ONL DL | 0.918 | 0.521 |
| (OPL DL) − (ONL DL) | 0.722 | 0.660 |
| (OPL DL)/(ONL DL) | 0.767 | 0.755 |
| Algorithm | Mean | Standard Deviation | 95% Confidence Interval for Mean |
|---|---|---|---|
| Accuracy | 0.738 | 0.130 | 0.712~0.764 |
| Precision | 0.921 | 0.088 | 0.903~0.938 |
| Recall | 0.734 | 0.162 | 0.702~0.766 |
| F-measure | 0.804 | 0.116 | 0.781~0.827 |
| AUC | 0.843 | 0.117 | 0.820~0.866 |
| Variables | Mean | Standard Deviation | 95% Confidence Interval for Mean |
|---|---|---|---|
| Preoperative BCVA | 0.281 | 0.028 | 0.275~0.286 |
| ONL DL | 0.130 | 0.038 | 0.123~0.138 |
| (OPL DL)/(ONL DL) | −0.174 | 0.020 | −0.178~−0.170 |
| (OPL DL) − (ONL DL) | −0.212 | 0.029 | −0.217~−0.206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mase, Y.; Matsui, Y.; Imai, K.; Imamura, K.; Irie-Ota, A.; Chujo, S.; Matsubara, H.; Kawanaka, H.; Kondo, M. Preoperative OCT Characteristics Contributing to Prediction of Postoperative Visual Acuity in Eyes with Macular Hole. J. Clin. Med. 2024, 13, 4826. https://doi.org/10.3390/jcm13164826
Mase Y, Matsui Y, Imai K, Imamura K, Irie-Ota A, Chujo S, Matsubara H, Kawanaka H, Kondo M. Preoperative OCT Characteristics Contributing to Prediction of Postoperative Visual Acuity in Eyes with Macular Hole. Journal of Clinical Medicine. 2024; 13(16):4826. https://doi.org/10.3390/jcm13164826
Chicago/Turabian StyleMase, Yoko, Yoshitsugu Matsui, Koki Imai, Kazuya Imamura, Akiko Irie-Ota, Shinichiro Chujo, Hisashi Matsubara, Hiroharu Kawanaka, and Mineo Kondo. 2024. "Preoperative OCT Characteristics Contributing to Prediction of Postoperative Visual Acuity in Eyes with Macular Hole" Journal of Clinical Medicine 13, no. 16: 4826. https://doi.org/10.3390/jcm13164826






