Demineralized Dentin Matrix Incorporated with rhBMP-2 Composite Graft for Treating Medication-Related Osteonecrosis of the Jaw
Abstract
:1. Introduction
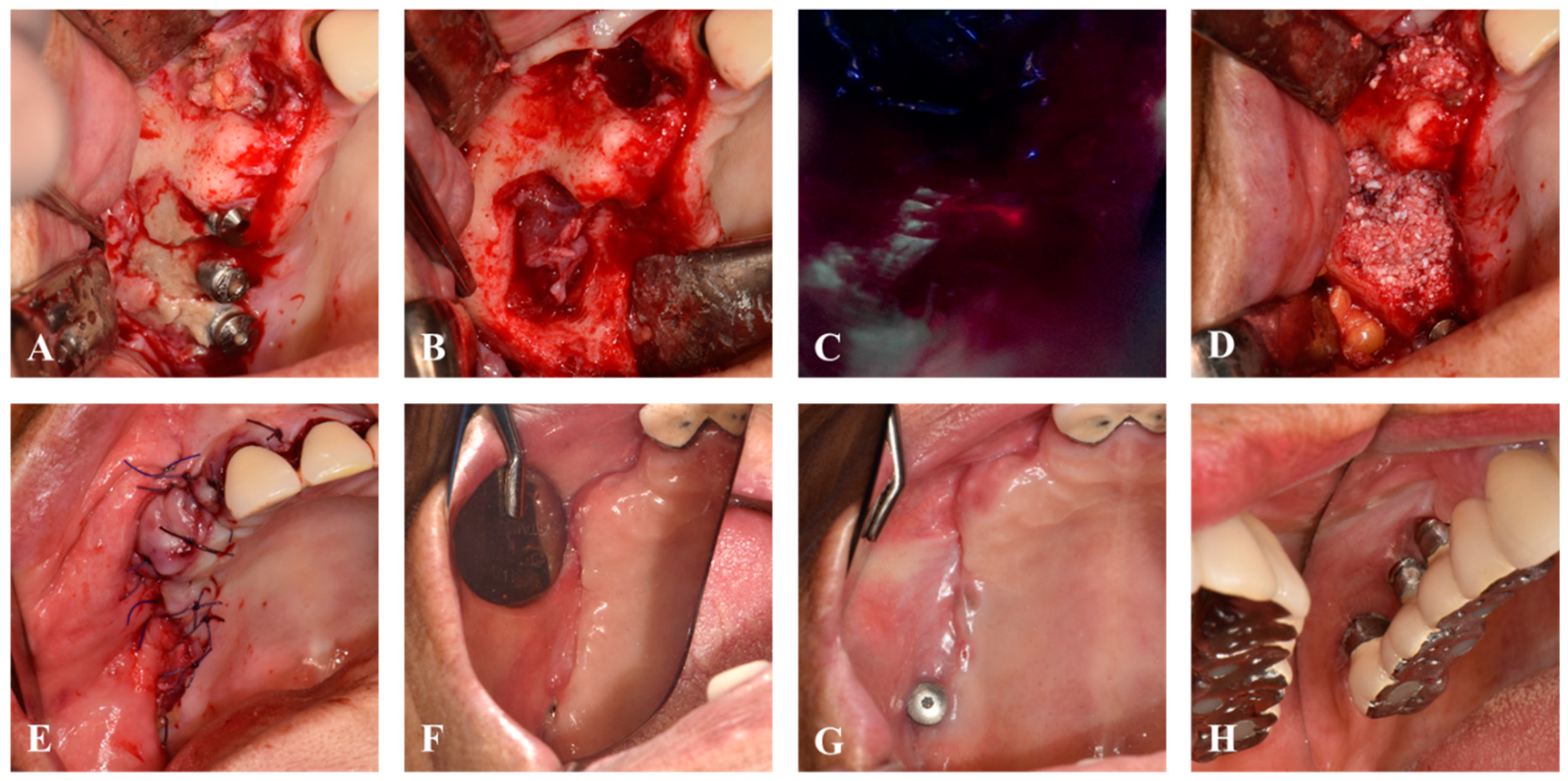
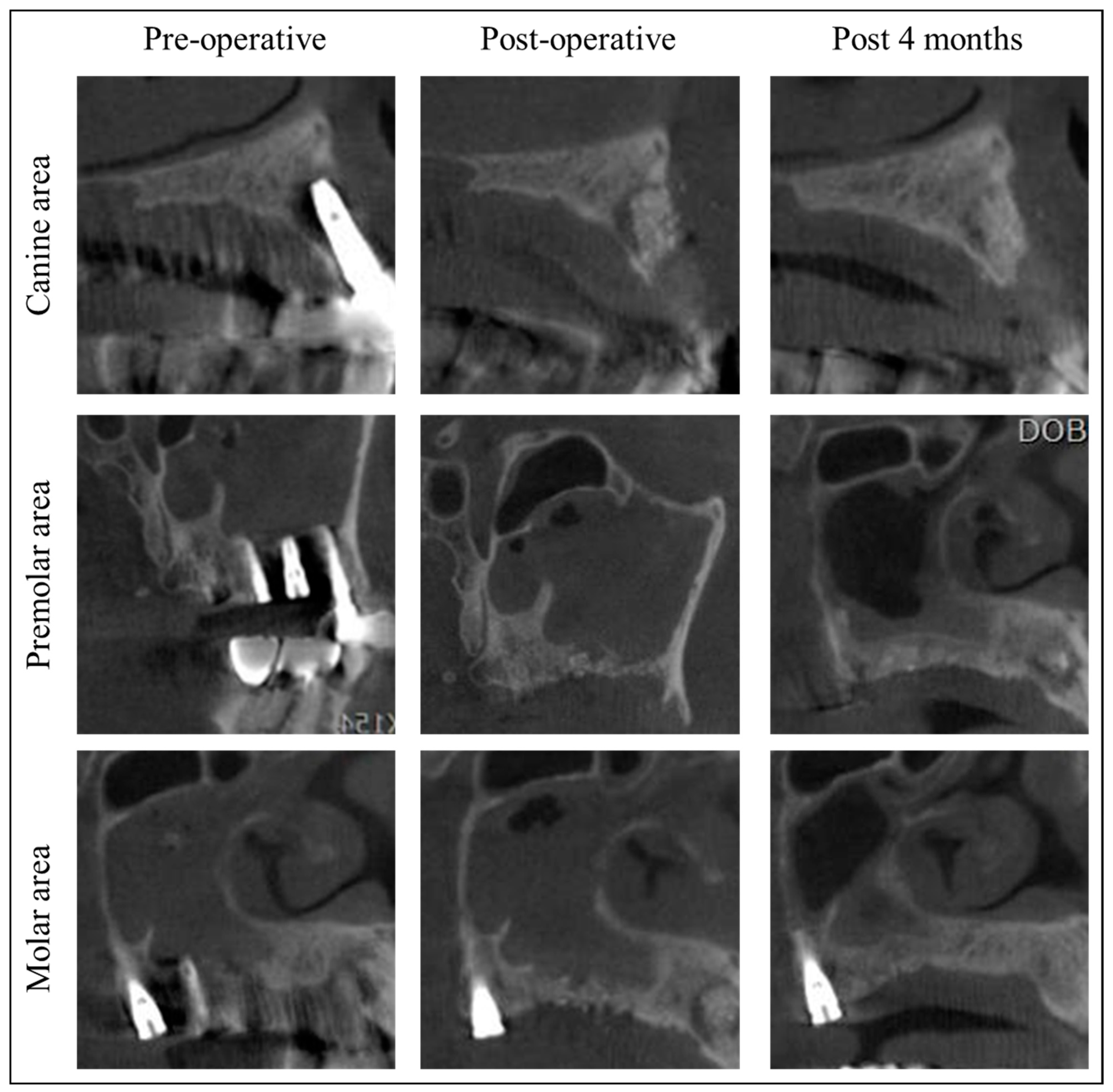
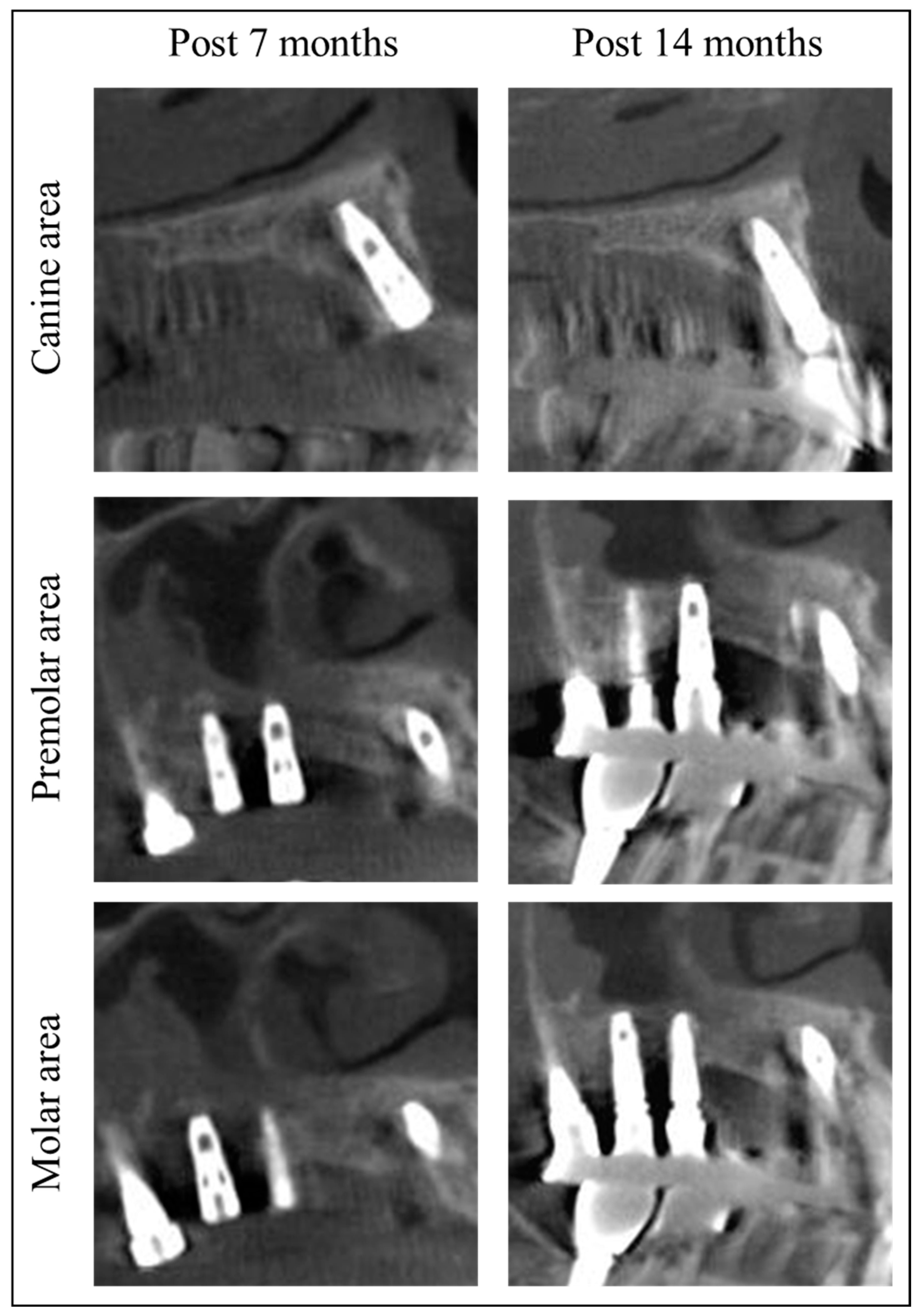
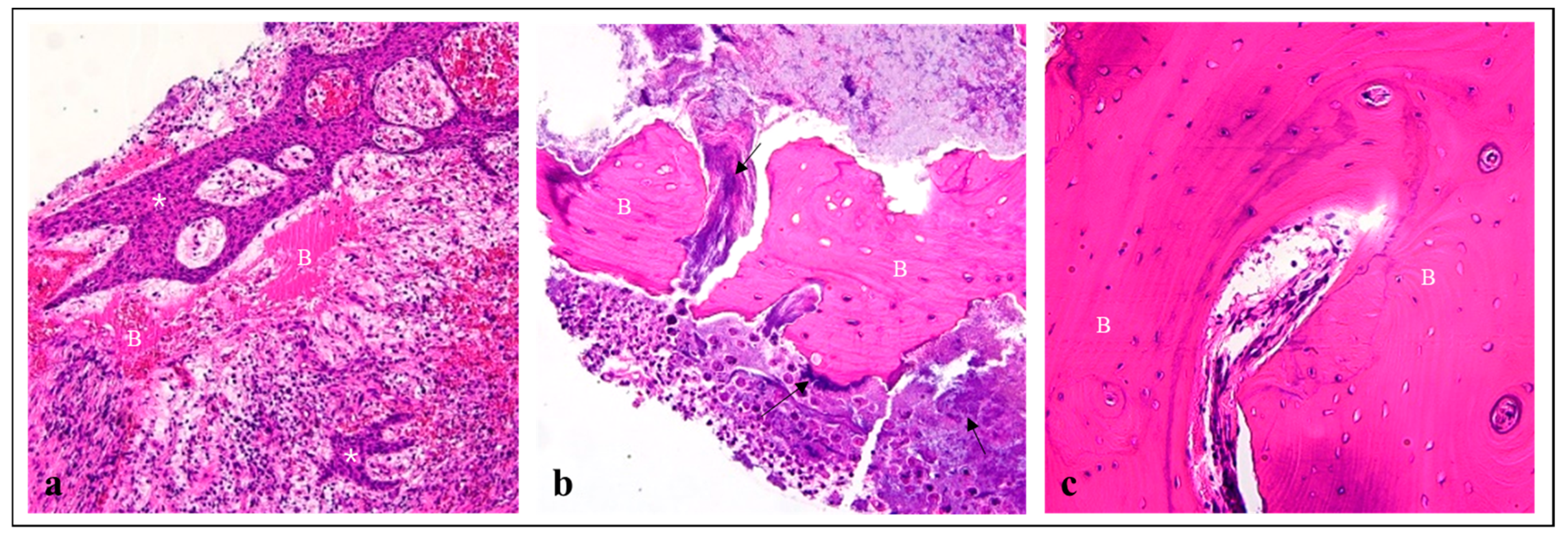
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-related osteonecrosis of the jaw (mronj): A review of pathophysiology, risk factors, preventive measures and treatment strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Bassan Marinho Maciel, G.; Marinho Maciel, R.; Linhares Ferrazzo, K.; Cademartori Danesi, C. Etiopathogenesis of medication-related osteonecrosis of the jaws: A review. J. Mol. Med. 2024, 102, 353–364. [Google Scholar] [CrossRef]
- Cerrato, A.; Zanette, G.; Boccuto, M.; Angelini, A.; Valente, M.; Bacci, C. Actinomyces and mronj: A retrospective study and a literature review. J. Stomatol. Oral. Maxillofac. Surg. 2021, 122, 499–504. [Google Scholar] [CrossRef]
- Zirk, M.; Wenzel, C.; Buller, J.; Zöller, J.E.; Zinser, M.; Peters, F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin. Oral. Investig. 2019, 23, 2143–2151. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jung, Y.S.; Park, W.; Choi, Y.J.; Kim, J.Y. Can medication-related osteonecrosis of the jaw be attributed to specific microorganisms through oral microbiota analyses? A preliminary study. BMC Oral. Health 2024, 24, 160. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American association of oral and maxillofacial surgeons’ position paper on medication-related osteonecrosis of the jaws—2022 update. J. Oral. Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.R. Management of antiresorptive osteonecrosis of the jaws with primary surgical resection. J. Oral. Maxillofac. Surg. 2014, 72, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B.; Kang, A.; Lee, C.; Dyalram, D.; Shih, P.; Twaddell, W.S.; Lubek, J.E. Risk factors for infection recurrence after surgical resection of advanced stage osteonecrosis of the mandible. J. Oral. Maxillofac. Surg. 2024, 82, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, M.; Thomas, R.; Huysmans, M.; De Soet, J. Red autofluorescence of dental plaque bacteria. Caries Res. 2006, 40, 542–545. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, E.S.; Kim, B.I. Can red fluorescence be useful in diagnostic decision making of residual dentin caries? Photodiagnosis Photodyn. Ther. 2019, 26, 43–44. [Google Scholar] [CrossRef]
- Kühnisch, J.; Heinrich-Weltzien, R. Quantitative light-induced fluorescence (qlf)—A literature review. Int. J. Comput. Dent. 2004, 7, 325–338. [Google Scholar]
- Kim, Y.; Jung, H.I.; Kim, Y.K.; Ku, J.K. Histologic analysis of osteonecrosis of the jaw according to the different aspects on quantitative light-induced fluorescence images. Photodiagnosis Photodyn. Ther. 2021, 34, 102212. [Google Scholar] [CrossRef]
- Kim, Y.; Ku, J.K. Quantitative light-induced fluorescence-guided surgery for medication-related osteomyelitis of the jaw. Photodiagnosis Photodyn. Ther. 2024, 45, 103867. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; de Josselin de Jong, E.; Kim, E.; Kim, B.I. Real-time optical detection of endodontic infection using bacterial autofluorescence. J. Dent. 2023, 136, 104600. [Google Scholar] [CrossRef]
- Murata, M. Bone engineering using human demineralized dentin matrix and recombinant human bmp-2. J. Hard Tissue Biol. 2005, 14, 80–81. [Google Scholar] [CrossRef]
- Avery, S.J.; Sadaghiani, L.; Sloan, A.J.; Waddington, R.J. Analysing the bioactive makeup of demineralised dentine matrix on bone marrow mesenchymal stem cells for enhanced bone repair. Eur. Cell Mater. 2017, 34, 1–14. [Google Scholar] [CrossRef]
- Murata, M. Collagen biology for bone regenerative surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 321–325. [Google Scholar] [CrossRef]
- Um, I.-W.; Ku, J.-K.; Kim, Y.-M.; Yun, P.-Y.; Chang, N.-H.; Kim, Y.-K.; Choi, Y. Allogeneic demineralized dentin matrix graft for guided bone regeneration in dental implants. Appl. Sci. 2020, 10, 4661. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, S.-G.; Byeon, J.-H.; Lee, H.-J.; Um, I.-U.; Lim, S.-C.; Kim, S.-Y. Development of a novel bone grafting material using autogenous teeth. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2010, 109, 496–503. [Google Scholar] [CrossRef]
- Um, I.-W.; Jun, S.-H.; Yun, P.-Y.; Kim, Y.-K. Histological comparison of autogenous and allogenic demineralized dentin matrix loaded with recombinant human bone morphogenetic protein-2 for alveolar bone repair: A preliminary report. J. Hard Tissue Biol. 2017, 26, 417–424. [Google Scholar] [CrossRef]
- Mustakim, K.R.; Eo, M.Y.; Lee, J.Y.; Seo, M.H.; Kim, S.M. Clinical significance of drug cessation on medication-related osteonecrosis of the jaw in patients with osteoporosis. J. Korean Assoc. Oral. Maxillofac. Surg. 2023, 49, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, Cd012432. [Google Scholar]
- Min, S.H.; Kang, N.E.; Song, S.I.; Lee, J.K. Regenerative effect of recombinant human bone morphogenetic protein-2/absorbable collagen sponge (rhbmp-2/acs) after sequestrectomy of medication-related osteonecrosis of the jaw (mronj). J. Korean Assoc. Oral. Maxillofac. Surg. 2020, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Friess, W.; Uludag, H.; Foskett, S.; Biron, R.; Sargeant, C. Characterization of absorbable collagen sponges as rhbmp-2 carriers. Int. J. Pharm. 1999, 187, 91–99. [Google Scholar] [CrossRef] [PubMed]
- On, S.W.; Park, S.Y.; Yi, S.M.; Park, I.Y.; Byun, S.H.; Yang, B.E. Current status of recombinant human bone morphogenetic protein-2 (rhbmp-2) in maxillofacial surgery: Should it be continued? Bioengineering 2023, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Lo, K.W.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, J.-K.; Choi, J.-W.; Song, S.-M.; Yun, P.-Y.; Um, I.-W.; Leem, D.H. Demineralized Dentin Matrix Incorporated with rhBMP-2 Composite Graft for Treating Medication-Related Osteonecrosis of the Jaw. J. Clin. Med. 2024, 13, 4830. https://doi.org/10.3390/jcm13164830
Ku J-K, Choi J-W, Song S-M, Yun P-Y, Um I-W, Leem DH. Demineralized Dentin Matrix Incorporated with rhBMP-2 Composite Graft for Treating Medication-Related Osteonecrosis of the Jaw. Journal of Clinical Medicine. 2024; 13(16):4830. https://doi.org/10.3390/jcm13164830
Chicago/Turabian StyleKu, Jeong-Kui, Jin-Won Choi, Seung-Min Song, Pil-Young Yun, In-Woong Um, and Dae Ho Leem. 2024. "Demineralized Dentin Matrix Incorporated with rhBMP-2 Composite Graft for Treating Medication-Related Osteonecrosis of the Jaw" Journal of Clinical Medicine 13, no. 16: 4830. https://doi.org/10.3390/jcm13164830





