Gait Performance and Brain Activity Are Improved by Gait Automatization during Robot-Assisted Gait Training in Patients with Burns: A Prospective, Randomized, Single-Blinded Study
Abstract
1. Introduction
2. Methods
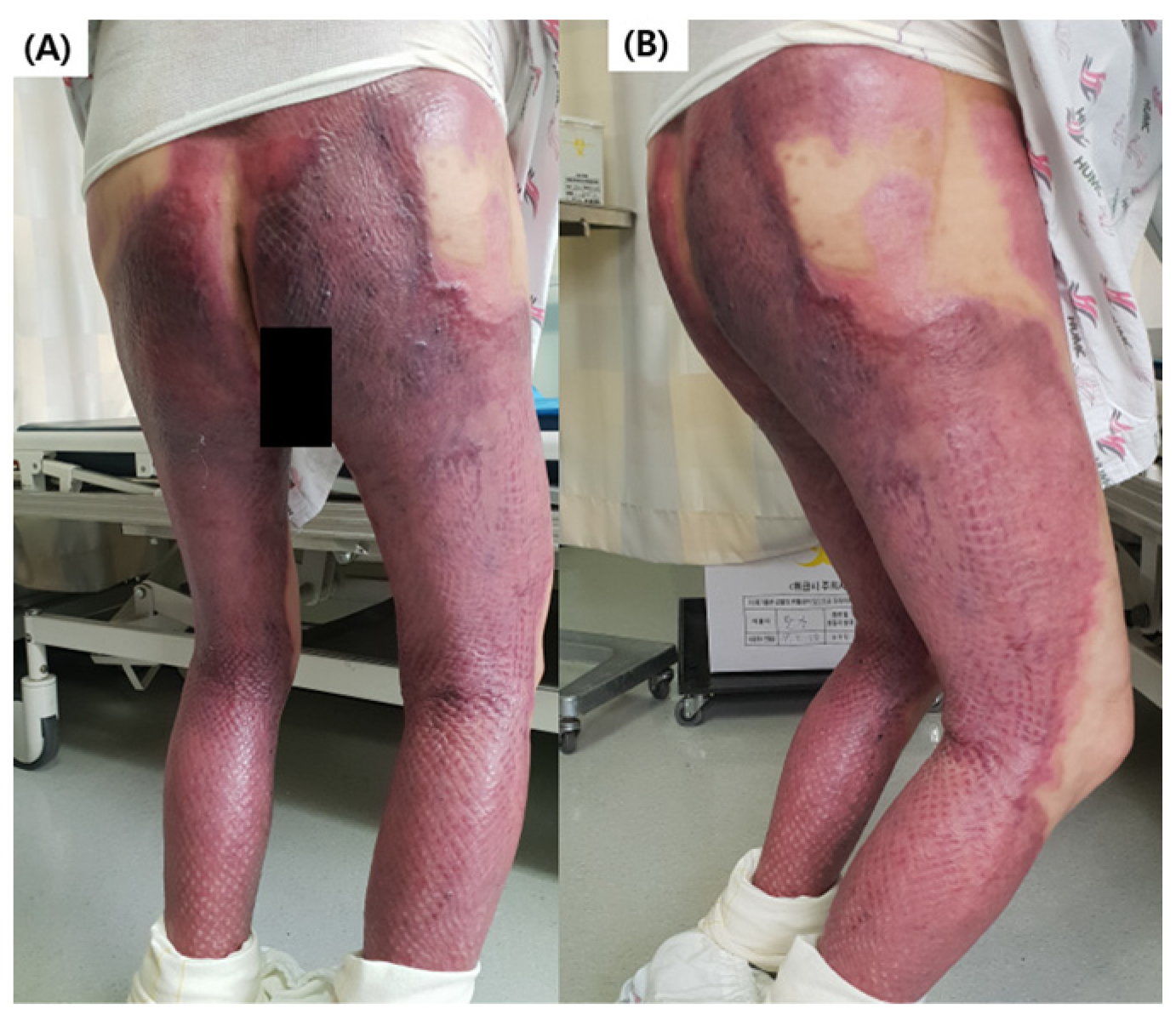
2.1. Patient Selection and Ethical Considerations
2.2. Interventions
2.2.1. CON Group Training
2.2.2. RAGT Group Training
2.3. Measuring the Cortical Activity
2.4. Assessing Treatment Effects and Functional Recovery
2.5. Statistical Analyses
3. Results
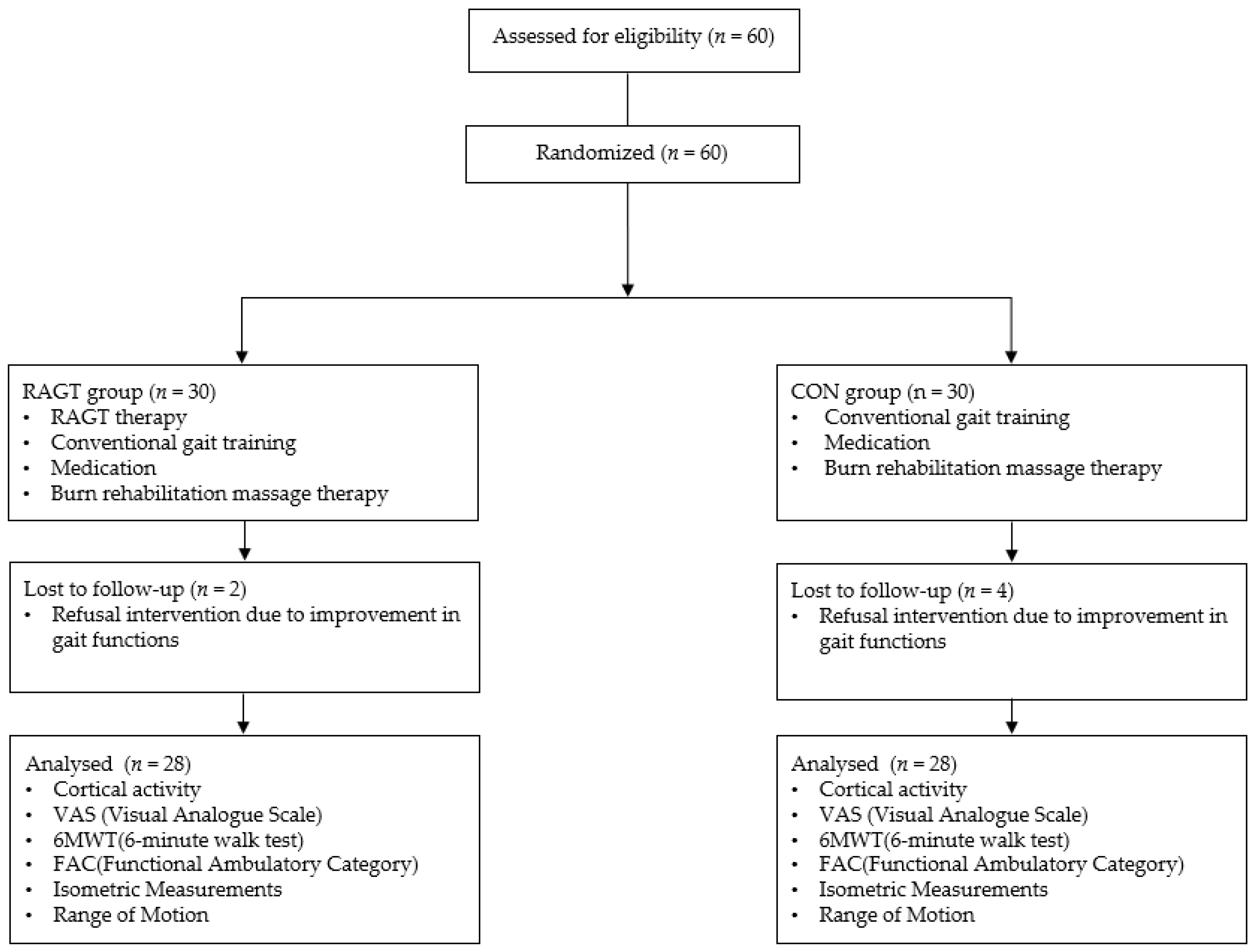
3.1. Patient Demographics
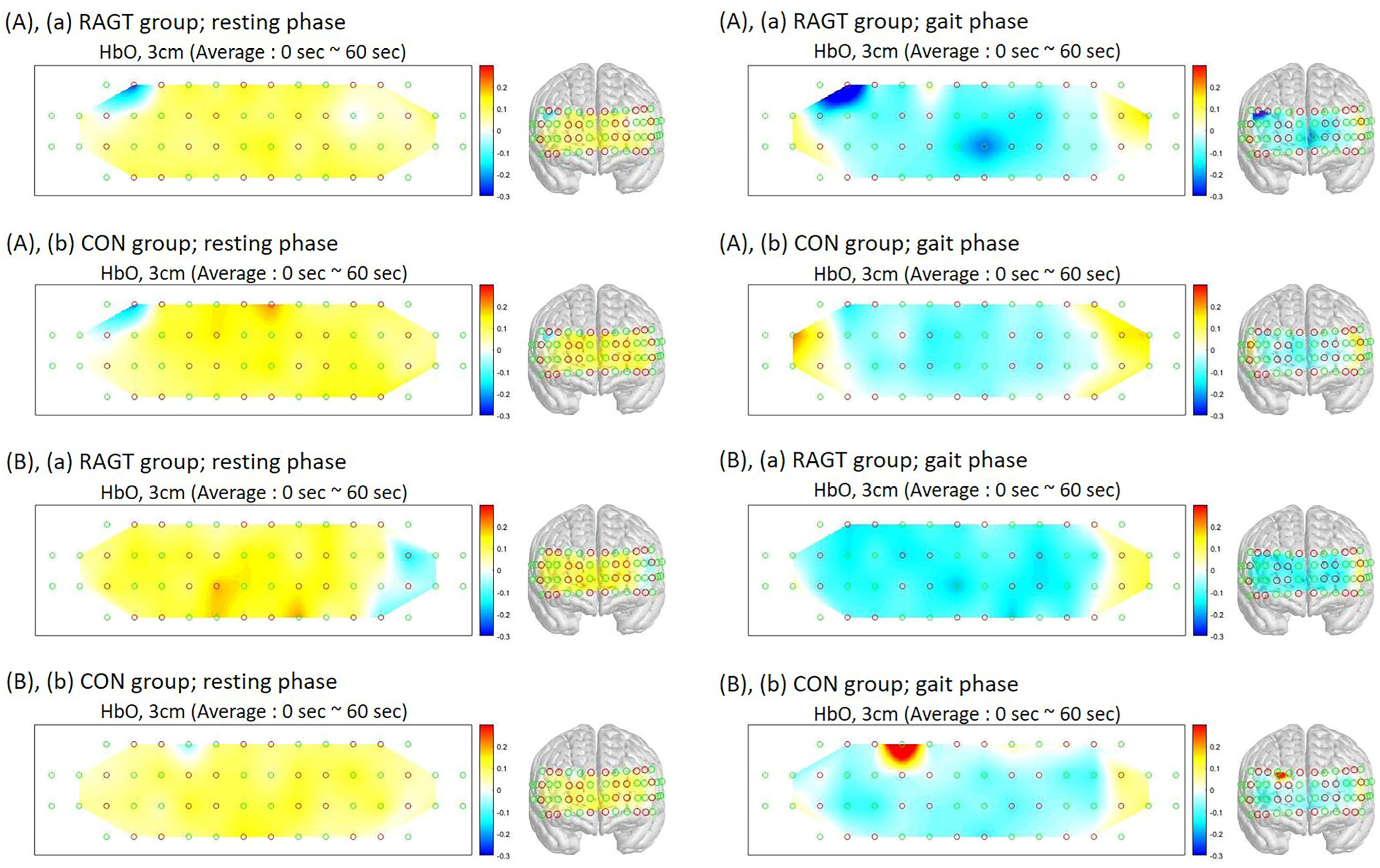
3.2. Outcomes of PFC Activation
3.3. Outcomes of Physical Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISBI Practice Guidelines Committee. ISBI Practice Guidelines for Burn Care. Burns 2016, 42, 953–1021. [Google Scholar] [CrossRef]
- Yun, S.J.; Lee, H.H.; Lee, W.H.; Lee, S.H.; Oh, B.M.; Seo, H.G. Effect of robot-assisted gait training on gait automaticity in Parkinson disease: A prospective, open-label, single-arm, pilot study. Medicine 2021, 100, e24348. [Google Scholar] [CrossRef]
- Gawaziuk, J.P.; Peters, B.; Logsetty, S. Early ambulation after-grafting of lower extremity burns. Burns 2018, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Sugaya, H.; Kubota, S.; Onishi, M.; Kanamori, A.; Sankai, Y.; Yamazaki, M. Knee-Extension Training with a Single-Joint Hybrid Assistive Limb during the Early Postoperative Period after Total Knee Arthroplasty in a Patient with Osteoarthritis. Case Rep. Orthop. 2016, 2016, 9610745. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Ahn, N.E.; Kim, D.H.; Kim, D.Y. Plantar Pressure Distribution During Robotic-Assisted Gait in Post-stroke Hemiplegic Patients. Ann. Rehabil. Med. 2014, 38, 145–152. [Google Scholar] [CrossRef]
- Goto, K.; Morishita, T.; Kamada, S.; Saita, K.; Fukuda, H.; Shiota, E.; Sankai, Y.; Inoue, T. Feasibility of rehabilitation using the single-joint hybrid assistive limb to facilitate early recovery following total knee arthroplasty: A pilot study. Assist Technol. 2017, 29, 197–201. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kubota, S.; Sugaya, H.; Hyodo, K.; Ogawa, K.; Taniguchi, Y.; Kanamori, A.; Sankai, Y.; Yamazaki, M. Robotic device-assisted knee extension training during the early postoperative period after opening wedge high tibial osteotomy: A case report. J. Med. Case Rep. 2017, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Lee, K.J.; Kim, S.H.; Seo, C.H. Effectiveness of robot-assisted gait training on patients with burns: A preliminary study. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 888–893. [Google Scholar] [CrossRef]
- Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Lee, K.J.; Seo, C.H. Effects of Robot-Assisted Gait Training in Patients with Burn Injury on Lower Extremity: A Single-Blind, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2813. [Google Scholar] [CrossRef]
- Smania, N.; Bonetti, P.; Gandolfi, M.; Cosentino, A.; Waldner, A.; Hesse, S.; Werner, C.; Bisoffi, G.; Geroin, C.; Munari, D. Improved gait after repetitive locomotor training in children with cerebral palsy. Am. J. Phys. Med. Rehabil. 2011, 90, 137–149. [Google Scholar] [CrossRef]
- Munari, D.; Fonte, C.; Varalta, V.; Battistuzzi, E.; Cassini, S.; Montagnoli, A.P.; Gandolfi, M.; Modenese, A.; Filippetti, M.; Smania, N.; et al. Effects of robot-assisted gait training combined with virtual reality on motor and cognitive functions in patients with multiple sclerosis: A pilot, single-blind, randomized controlled trial. Restor. Neurol. Neurosci. 2020, 38, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vitorio, R.; Stuart, S.; Gobbi, L.T.B.; Rochester, L.; Alcock, L.; Pantall, A. Reduced Gait Variability and Enhanced Brain Activity in Older Adults with Auditory Cues: A Functional Near-Infrared Spectroscopy Study. Neurorehabilit. Neural Repair 2018, 32, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.A.; Fox, E.J.; Daly, J.J.; Rose, D.K.; Christou, E.A.; McGuirk, T.E.; Otzel, D.M.; Butera, K.A.; Chatterjee, S.A.; Clark, D.J. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum. Mov. Sci. 2018, 59, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cha, J.Y.; Yoo, J.W.; Nazareno, M.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Effect of the Application of Virtual Reality on Pain Reduction and Cerebral Blood Flow in Robot-Assisted Gait Training in Burn Patients. J. Clin. Med. 2022, 11, 3762. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Cho, Y.S.; Lee, K.J.; Lee, S.Y.; Seo, C.H. Frontal lobe oxyhemoglobin levels in patients with lower extremity burns assessed using a functional near-Infrared spectroscopy device during usual walking: A pilot study. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Beyer, N.; Frolich, A.; Godtfredsen, N.; Bieler, T. Intra- and inter-rater reproducibility of the 6-minute walk test and the 30-second sit-to-stand test in patients with severe and very severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3447–3457. [Google Scholar] [CrossRef]
- Jackson, S.M.; Cheng, M.S.; Smith, A.R., Jr.; Kolber, M.J. Intrarater reliability of hand held dynamometry in measuring lower extremity isometric strength using a portable stabilization device. Musculoskelet. Sci. Pract. 2017, 27, 137–141. [Google Scholar] [CrossRef]
- Maggioni, S.; Melendez-Calderon, A.; van Asseldonk, E.; Klamroth-Marganska, V.; Lünenburger, L.; Riener, R.; van der Kooij, H. Robot-aided assessment of lower extremity functions: A review. J. Neuroeng. Rehabil. 2016, 13, 72. [Google Scholar] [CrossRef]
- Vitorio, R.; Stuart, S.; Mancini, M. Executive Control of Walking in People With Parkinson’s Disease with Freezing of Gait. Neurorehabilit. Neural Repair 2020, 34, 1138–1149. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Muir, S.W.; Hall, M.; Doherty, T.J.; Kloseck, M.; Beauchet, O.; Speechley, M. Gait variability is associated with frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Hermand, E.; Compagnat, M.; Dupuy, O.; Salle, J.Y.; Daviet, J.C.; Perrochon, A. Functional Status Is Associated with Prefrontal Cortex Activation in Gait in Subacute Stroke Patients: A Functional Near-Infrared Spectroscopy Study. Front. Neurol. 2020, 11, 559227. [Google Scholar] [CrossRef] [PubMed]
- Caliandro, P.; Molteni, F.; Simbolotti, C.; Guanziroli, E.; Iacovelli, C.; Reale, G.; Giovannini, S.; Padua, L. Exoskeleton-assisted gait in chronic stroke: An EMG and functional near-infrared spectroscopy study of muscle activation patterns and prefrontal cortex activity. Clin. Neurophysiol. 2020, 131, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Belli, V.; Orcioli-Silva, D.; Beretta, V.S.; Vitório, R.; Zampier, V.C.; Nóbrega-Sousa, P.; Conceição, N.R.D.; Gobbi, L.T.B. Prefrontal Cortical Activity during Preferred and Fast Walking in Young and Older Adults: An fNIRS Study. Neuroscience 2021, 473, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Chun, M.H.; Lee, J.; Lee, C. The effect of robot-assisted gait training on cortical activation in stroke patients: A functional near-infrared spectroscopy study. NeuroRehabilitation 2021, 49, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Franceschini, M.; Waldner, A.; Hesse, S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur. J. Phys. Rehabil. Med. 2012, 48, 111–121. [Google Scholar] [PubMed]
- Chang, W.H.; Kim, Y.H. Robot-assisted Therapy in Stroke Rehabilitation. J. Stroke 2013, 15, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Lee, S.; Wikoff, A.; Packel, A.; Toczylowski, T.; Feeley, J. A Comparison of Locomotor Therapy Interventions: Partial-Body Weight-Supported Treadmill, Lokomat, and G-EO Training in People with Traumatic Brain Injury. PM R 2017, 9, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Davies, T.C.; Xie, S. Effectiveness of robot-assisted therapy on ankle rehabilitation—A systematic review. J. Neuroeng. Rehabil. 2013, 10, 30. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef]
- Sale, P.; Stocchi, F.; Galafate, D.; De Pandis, M.F.; Le Pera, D.; Sova, I.; Galli, M.; Foti, C.; Franceschini, M. Effects of robot assisted gait training in progressive supranuclear palsy (PSP): A preliminary report. Front. Hum. Neurosci. 2014, 8, 207. [Google Scholar] [CrossRef]
- Sung, J.; Choi, S.; Kim, H.; Lee, G.; Han, C.; Ji, Y.; Shin, D.; Hwang, S.; Yun, D.; Jang, H.; et al. Feasibility of Rehabilitation Training with a Newly Developed, Portable, Gait Assistive Robot for Balance Function in Hemiplegic Patients. Ann. Rehabil. Med. 2017, 41, 178–187. [Google Scholar] [CrossRef]
- Lam, T.; Pauhl, K.; Ferguson, A.; Malik, R.N.; Bkin; Krassioukov, A.; Eng, J.J. Training with robot-applied resistance in people with motor-incomplete spinal cord injury: Pilot study. J. Rehabil. Res. Dev. 2015, 52, 113–129. [Google Scholar] [CrossRef]
- Johnson, M.J. Recent trends in robot-assisted therapy environments to improve real-life functional performance after stroke. J. Neuroeng. Rehabil. 2006, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Ko, Y.J.; Chang, W.H.; Lee, J.H.; Lee, K.B.; Park, Y.J.; Ha, H.G.; Kim, Y.H. Effects of Robot-assisted Gait Training Combined with Functional Electrical Stimulation on Recovery of Locomotor Mobility in Chronic Stroke Patients: A Randomized Controlled Trial. J. Phys. Ther. Sci. 2014, 26, 1949–1953. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Alamro, R.A.; Williams, A.M.; Lam, T. Overground vs. treadmill-based robotic gait training to improve seated balance in people with motor-complete spinal cord injury: A case report. J. Neuroeng. Rehabil. 2017, 14, 27. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, H.J.; Hwang, S.W.; Pyo, H.; Yang, S.P.; Lim, M.H.; Park, G.L.; Kim, E.J. Clinical Characteristics of Proper Robot-Assisted Gait Training Group in Non-ambulatory Subacute Stroke Patients. Ann. Rehabil Med. 2016, 40, 183–189. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Xu, Z.; Gu, X. A pilot study of post-total knee replacement gait rehabilitation using lower limbs robot-assisted training system. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 203–208. [Google Scholar] [CrossRef]
- Ziegler, M.D.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Why variability facilitates spinal learning. J. Neurosci. 2010, 30, 10720–10726. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, J.Y.; Park, H.K.; Kim, N.Y. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann. Rehabil. Med. 2014, 38, 719–725. [Google Scholar] [CrossRef]


| RAGT Group (n = 28) | CON Group (n = 26) | p-Value | |
|---|---|---|---|
| Male: female | 24:4 | 22:4 | 0.91 |
| Age (years) | 51.21 ± 11.45 | 51.85 ± 12.34 | 0.84 |
| TBSA (%) | 32.57 ± 19.36 | 30.38 ± 15.67 | 0.76 |
| Mechanism of burn, n FB: SB: CB: EB | 18:4:4:2 | 14:6:2:4 | 0.48 |
| Duration (days) between burn injury and therapy | 93.50 ± 37.83 | 81.77 ± 28.86 | 0.18 |
| VAS | 8.43 ± 0.92 | 8.54 ± 0.86 | 0.59 |
| FAC | 0.79 ± 0.42 | 0.62 ± 0.50 | 0.18 |
| 6 MWT (m) | 283.57 ± 112.98 | 265.38 ± 119.37 | 0.73 |
| Isometric measurements (Nm) | |||
| Hip flexor, right | 25.29 ± 7.17 | 26.69 ± 4.71 | 0.31 |
| Hip flexor, left | 23.86 ± 7.14 | 25.38 ± 5.61 | 0.47 |
| Hip extensor, right | 18.64 ± 7.79 | 17.46 ± 7.08 | 0.47 |
| Hip extensor, left | 17.62 ± 8.37 | 15.62 ± 8.18 | 0.33 |
| Knee flexor, right | 17.36 ± 7.40 | 18.85 ± 4.66 | 0.16 |
| Knee flexor, left | 17.43 ± 6.86 | 18.62 ± 7.45 | 0.51 |
| Knee extensor, right | 22.17 ± 9.64 | 24.77 ± 6.07 | 0.16 |
| Knee extensor, left | 22.64 ± 7.35 | 23.38 ± 7.46 | 0.47 |
| Ankle dorsiflexor, right | 17.21 ± 8.80 | 18.23 ± 6.03 | 0.51 |
| Ankle dorsiflexor, left | 16.41 ± 8.89 | 16.31 ± 8.18 | 0.90 |
| Ankle plantarflexor, right | 16.00 ± 6.99 | 15.77 ± 4.67 | 0.78 |
| Ankle plantarflexor, left | 15.01 ± 7.87 | 15.23 ± 6.39 | 0.92 |
| Range of Motion (degree) | |||
| Hip flexion, right | 94.57 ± 15.69 | 99.62 ± 1.36 | 0.12 |
| Hip flexion, left | 95.71 ± 15.74 | 98.85 ±11.34 | 0.47 |
| Hip extension, right | 19.93 ± 7.06 | 22.62 ± 4.59 | 0.87 |
| Hip extension, left | 21.57 ± 5.68 | 22.23 ± 5.63 | 0.70 |
| Knee flexion, right | 122.29 ± 19.62 | 123.85 ± 17.37 | 0.33 |
| Knee flexion, left | 117.29 ± 27.10 | 124.31 ± 21.14 | 0.44 |
| Knee extension, right | −3.43 ± 7.05 | −1.15 ± 3.29 | 0.09 |
| Knee extension, left | −5.14 ± 13.51 | −4.62 ± 7.61 | 0.73 |
| Ankle dorsiflexion, right | 16.07 ± 5.39 | 16.92 ± 5.48 | 0.45 |
| Ankle dorsiflexion, left | 16.36 ± 5.88 | 14.85 ± 6.36 | 0.49 |
| Ankle plantarflexion, right | 37.50 ± 4.91 | 38.46 ± 3.77 | 0.47 |
| Ankle plantarflexion, left | 37.14 ± 6.30 | 34.92 ± 8.35 | 0.37 |
| Before Training | After 8 Weeks of Training | |||||
|---|---|---|---|---|---|---|
| RAGT Group | CON Group | p-Value | RAGT Group | CON Group | p-Value | |
| Resting phase | ||||||
| HbO2 | 0.00150 ± 0.00454 | 0.00046 ±0.00047 | 0.95 | 0.00046 ± 0.00062 | 0.00024 ± 0.00061 | 0.30 |
| HbR | −0.00032 ± 0.00094 | 0.00000 ± 0.00015 | 0.17 | −0.00011 ± 0.00016 | 0.00001 ± 0.00039 | 0.19 |
| Gait phase | ||||||
| HbO2 | −0.00060 ± 0.00076 | −0.00032 ± 0.00121 | 0.10 | −0.00079 ± 0.00062 | −0.00013 ± 0.00074 | 0.002 * |
| HbR | 0.00001 ± 0.00036 | 0.00020 ± 0.00048 | 0.21 | 0.00013 ± 0.00042 | 0.00005 ± 0.00030 | 0.89 |
| RAGT Group (n = 28) | CON Group (n = 26) | ||||||
|---|---|---|---|---|---|---|---|
| Before Training | After Training | p-Value | Before Training | After Training | p-Value | Intergroup p-Value after Training | |
| VAS | 8.43 ± 0.92 | 5.50 ± 1.62 | <0.01 * | 8.54 ± 0.86 | 5.69 ± 1.16 | <0.01 * | 0.26 |
| FAC | 0.79 ± 0.42 | 3.29 ± 0.71 | <0.01 * | 0.62 ± 0.50 | 3.08 ± 0.74 | <0.01 * | 0.30 |
| 6 MWT | 283.57 ± 112.98 | 377.86 ± 80.39 | <0.01 * | 265.38 ± 119.37 | 323.85 ± 92.57 | 0.13 | 0.02 ** |
| Isometric measurements (Nm) | |||||||
| Hip flexor, right | 25.29 ± 7.17 | 25.32 ± 10.09 | 0.99 | 26.69 ± 4.71 | 21.65 ± 5.69 | <0.01 * | 0.09 |
| Hip flexor, left | 23.86 ± 7.14 | 23.00 ± 10.67 | 0.68 | 25.38 ± 5.61 | 23.42 ± 7.04 | 0.30 | 0.34 |
| Hip Extensor, right | 18.64 ± 7.79 | 20.11 ± 8.91 | 0.51 | 17.46 ± 7.08 | 17.12 ± 4.11 | 0.84 | 0.12 |
| Hip extensor, left | 17.62 ± 8.37 | 16.29 ± 7.24 | 0.98 | 15.62 ± 8.18 | 19.46 ± 6.91 | 0.13 | 0.12 |
| Knee flexor, right | 17.36 ± 7.40 | 22.00 ± 7.28 | 0.05 | 18.85 ± 4.66 | 18.92 ± 8.20 | 0.60 | 0.06 |
| Knee flexor, left | 17.43 ± 6.86 | 23.14 ± 8.49 | 0.03 * | 18.62 ± 7.45 | 18.46 ± 7.06 | 0.73 | 0.04 * |
| Knee extensor, right | 22.17 ± 9.64 | 25.32 ± 6.80 | 0.16 | 24.77 ± 6.07 | 23.73 ± 10.53 | 0.35 | 0.27 |
| Knee extensor, left | 22.64 ± 7.35 | 24.75 ± 6.02 | 0.15 | 23.38 ± 7.46 | 22.58 ± 8.55 | 0.19 | 0.23 |
| Ankle dorsiflexor, right | 17.21 ± 8.80 | 21.21 ± 7.24 | 0.08 | 18.23 ± 6.03 | 17.23 ± 9.76 | 0.49 | 0.06 |
| Ankle dorsiflexor, left | 16.41 ± 8.89 | 20.11 ± 8.90 | 0.12 | 16.31 ± 8.18 | 16.19 ± 10.00 | 0.63 | 0.13 |
| Ankle plantarflexor, right | 16.00 ± 6.99 | 21.50 ± 9.09 | 0.01 * | 15.77 ± 4.67 | 16.46 ± 7.13 | 0.77 | 0.03 ** |
| Ankle plantarflexor, left | 15.01 ± 7.87 | 21.71 ± 8.78 | 0.01 * | 15.23 ± 6.39 | 15.69 ± 7.15 | 0.93 | 0.01 ** |
| Range of motion (degree) | |||||||
| Hip flexion, right | 94.57 ± 15.69 | 93.93 ± 19.64 | 0.96 | 99.62 ± 1.36 | 99.00 ± 2.77 | 0.28 | 0.96 |
| Hip flexion, left | 95.71 ± 15.74 | 94.43 ± 19.52 | 0.67 | 98.85 ±11.34 | 96.31 ± 6.94 | 0.33 | 0.28 |
| Hip extension, right | 19.93 ± 7.06 | 23.54 ± 5.67 | 0.04 * | 22.62 ± 4.59 | 18.81 ± 6.57 | 0.02 * | 0.01 ** |
| Hip extension, left | 21.57 ± 5.68 | 24.46 ± 5.44 | 0.11 | 22.23 ± 5.63 | 19.58 ± 6.11 | 0.10 | 0.03 ** |
| Knee flexion, right | 122.29 ± 19.62 | 128.32 ± 16.75 | 0.18 | 123.85 ± 17.37 | 121.96 ± 25.02 | 0.77 | 0.50 |
| Knee flexion, left | 117.29 ± 27.10 | 130.43 ± 20.56 | 0.10 | 124.31 ± 21.14 | 116.85 ± 29.13 | 0.35 | 0.06 |
| Knee extension, right | −3.43 ± 7.05 | 1.93 ± 7.97 | 0.01 * | −1.15 ± 3.29 | −2.08 ± 4.81 | 0.29 | 0.01 ** |
| Knee extension, left | −5.14 ± 13.51 | 1.93 ± 7.97 | 0.03 * | −4.62 ± 7.61 | −2.38 ± 4.81 | 0.17 | 0.01 ** |
| Ankle dorsiflexion, right | 16.07 ± 5.39 | 23.43 ± 28.06 | 0.35 | 16.92 ± 5.48 | 14.00 ± 8.20 | 0.12 | 0.30 |
| Ankle dorsiflexion, left | 16.36 ± 5.88 | 22.18 ± 28.52 | 0.99 | 14.85 ± 6.36 | 13.96 ± 8.06 | 0.38 | 0.51 |
| Ankle plantarflexion, right | 37.50 ± 4.91 | 36.71 ± 10.49 | 0.77 | 38.46 ± 3.77 | 36.15 ± 6.46 | 0.11 | 0.15 |
| Ankle plantarflexion, left | 37.14 ± 6.30 | 35.14 ± 11.20 | 0.48 | 34.92 ± 8.35 | 36.77 ± 5.85 | 0.33 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Seo, J.; Seo, C.H.; Cho, Y.S.; Joo, S.Y. Gait Performance and Brain Activity Are Improved by Gait Automatization during Robot-Assisted Gait Training in Patients with Burns: A Prospective, Randomized, Single-Blinded Study. J. Clin. Med. 2024, 13, 4838. https://doi.org/10.3390/jcm13164838
Lee SY, Seo J, Seo CH, Cho YS, Joo SY. Gait Performance and Brain Activity Are Improved by Gait Automatization during Robot-Assisted Gait Training in Patients with Burns: A Prospective, Randomized, Single-Blinded Study. Journal of Clinical Medicine. 2024; 13(16):4838. https://doi.org/10.3390/jcm13164838
Chicago/Turabian StyleLee, Seung Yeol, Jisu Seo, Cheong Hoon Seo, Yoon Soo Cho, and So Young Joo. 2024. "Gait Performance and Brain Activity Are Improved by Gait Automatization during Robot-Assisted Gait Training in Patients with Burns: A Prospective, Randomized, Single-Blinded Study" Journal of Clinical Medicine 13, no. 16: 4838. https://doi.org/10.3390/jcm13164838
APA StyleLee, S. Y., Seo, J., Seo, C. H., Cho, Y. S., & Joo, S. Y. (2024). Gait Performance and Brain Activity Are Improved by Gait Automatization during Robot-Assisted Gait Training in Patients with Burns: A Prospective, Randomized, Single-Blinded Study. Journal of Clinical Medicine, 13(16), 4838. https://doi.org/10.3390/jcm13164838







