Nationwide Trends in Hospitalizations for Atrial Fibrillation and Flutter in the United States before and during the Outbreak of the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Patients and Variables
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
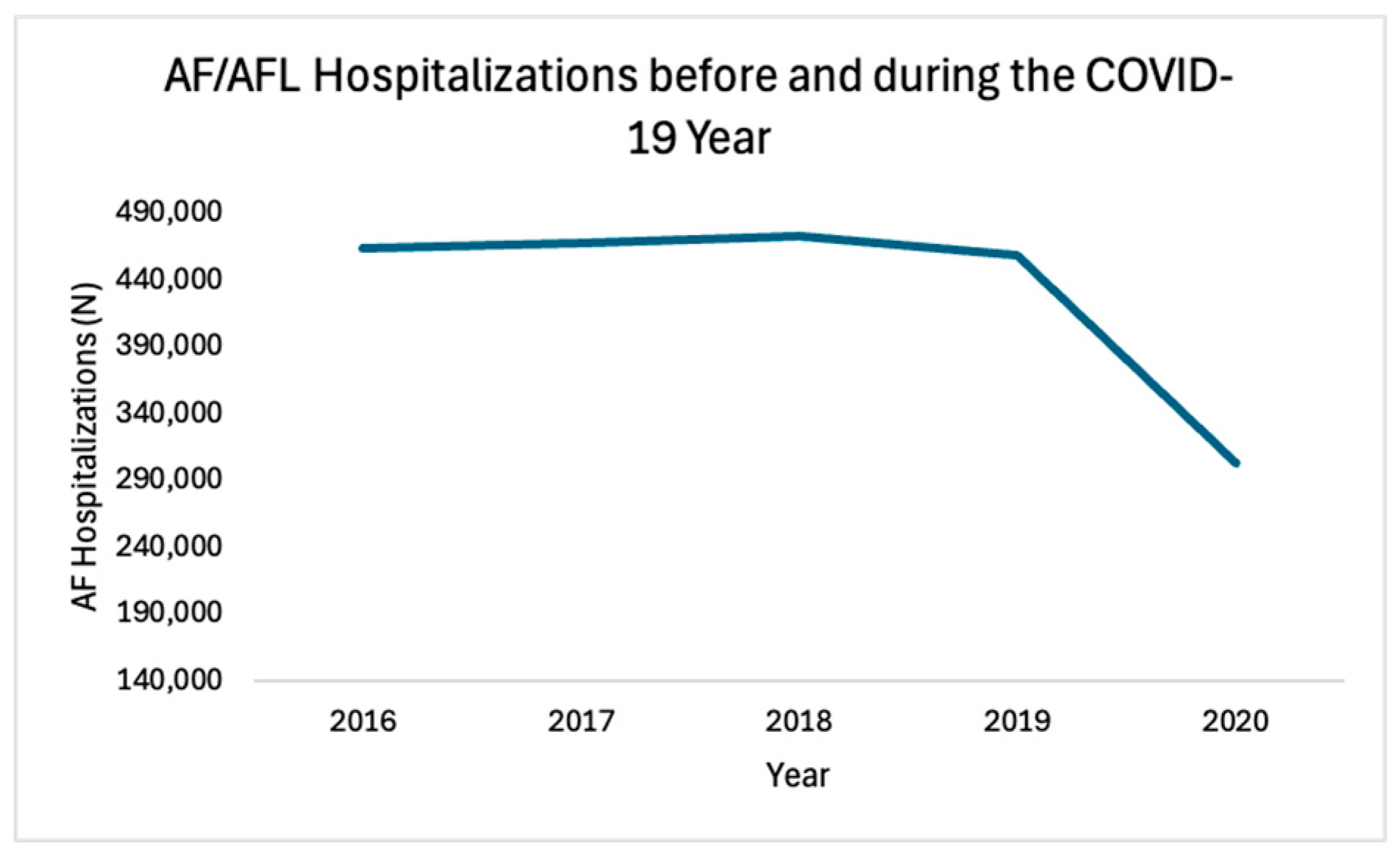
3.1. Trends in AF/AFL Hospitalizations
3.2. Trends in Adverse Endpoint Outcomes
3.3. Inpatient Catheter Ablation Procedure and Outcomes
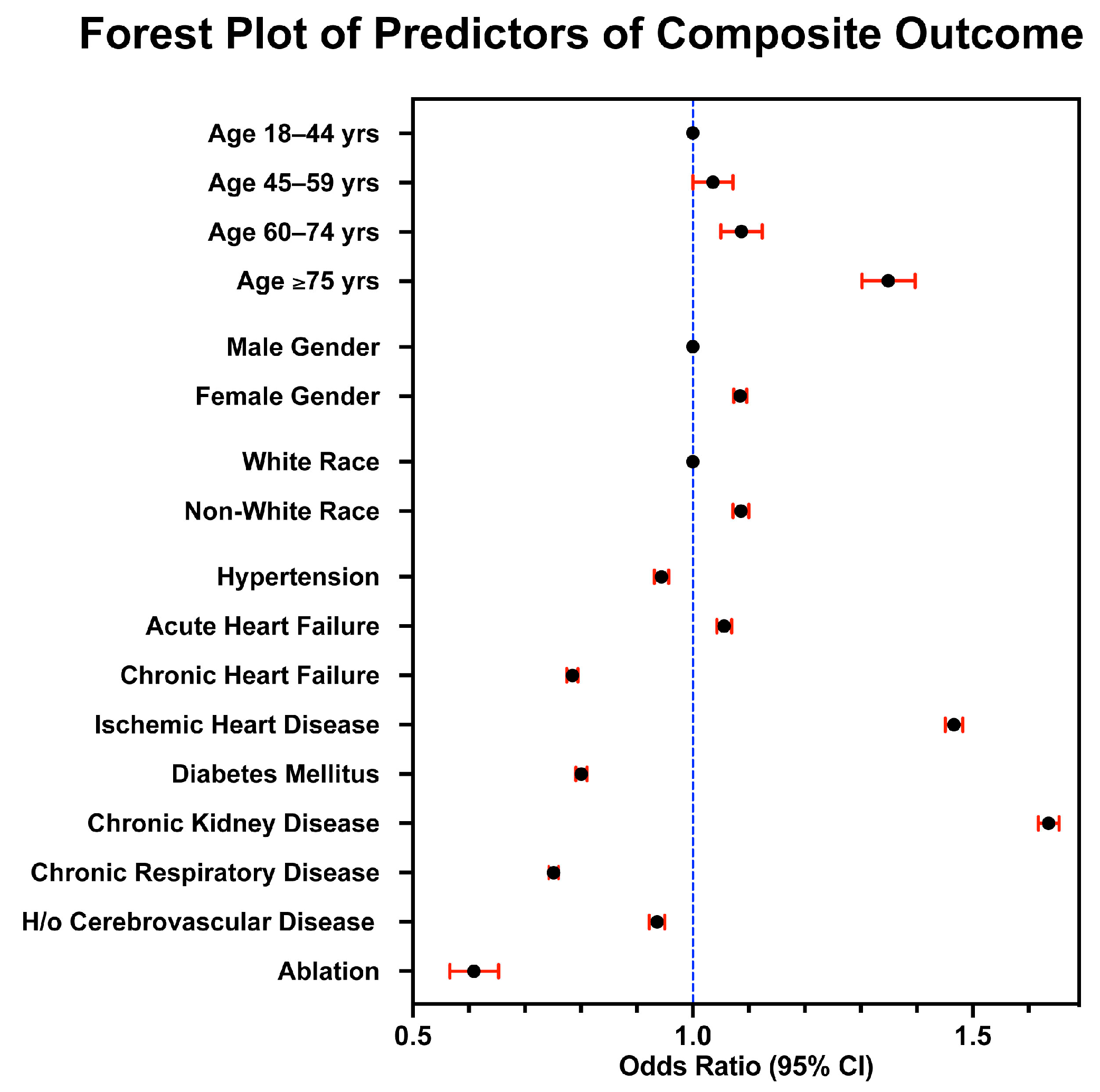
3.4. Multivariable Regression Analysis for Predictors of Endpoint Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. ICD-10 CM Codes Used in the Research
| Condition | ICD-10 CM Codes |
|---|---|
| AF/AFL | I48.0, I48.11, I48.19, I48.20, I48.21, I48.3, I48.4. I48.91, I48.92 |
| Hypertension | I10, I11.0, I11.9, I12.0, I12.9, I13.0, I13.10, I13.11, I13.2 I15.0, I15.1, I15.2, I15.8, I15.9, I16.0, I16.1, I16.9 |
| Chronic Heart Failure | I50.1, I50.20, I50.22, I50.30, I50.32, I50.40, I50.42, I50.810, I50.812, I50.814, I50.82, I50.83, I50.84, I50.89, I50.9 |
| Acute Heart Failure | I50.21, I50.23, I50.31, I50.33, I50.41, I50.43, I50.811, I50.813 |
| Chronic Ischemic Heart Disease | I25.1xx, I25.4x, I25.5, I25.7xx, I25.8xx, I25.9 |
| Diabetes Mellitus | E08.42, E08.649, E08.65, E08.9, E09.22, E09.649, E09.65, E09.8, E09.9, E10.0x, E10.1x, E10.6x, E10.8x, E10.9x, E11.0x, E11.1x, E11.6x, E11.8x, E11.9x, E13.0x, E13.1x, E13.6x, E13.8x, E13.9x |
| Chronic Kidney Disease | N17.0, N17.1, N17.2, N17.8, N17.9, N18.1, N18.2, N18.3, N18.30, N18.31, N18.32, N18.4, N18.5, N18.6, N18.9, N19 |
| Chronic Respiratory Disease | J40, J41.0, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, J44.9, J45.20, J45.21, J45.30, J45.40, J45.41, J45.42, J45.50, J45.51, J45.901, J45.902, J45.909, J45.990, J47.0, J47.1, J47.9 |
| History of Cerebrovascular Disease | I69.0x, I69.1x, I69.2x, I69.3x, I69.4x, I69.6x, I69.7x, I69.8x, I69.9x, Z86.73 |
| COVID-19 | U07.1, J12.82 |
| Acute Coronary Syndrome | I20.0, I20.1, I21.01, I21.02, I21.09, I21.11, I21.19 I21.21, I21.29, I21.3, I21.4, I21.9, I21.A1, I21.A9, I21.B, I22.0, I22.1, I22.2, I22.8, I22.9, I23.0, I24.0, I24.8, I24.9 |
| Stroke | I63.0x, I63.1x, I63.2x, I63.3x, I63.40, I63.41x, I63.42x, I63.43x, I63.44x, I63.49, I63.5x, I63.8x, I63.9, I69.05x |
| Catheter ablation | 025S0ZZ, 025S3ZZ, 025S4ZZ, 025T0ZZ, 025T3ZZ, 025T4ZZ, 02560ZZ, 02563ZZ, 02564ZZ, 02570ZK, 02570ZZ, 02573ZK, 02573ZZ, 02574ZK, 02574ZZ |
| Condition | ICD-10 CM Codes for Deyo-CCI Score | Score |
|---|---|---|
| Myocardial infarction | I21.x, I22.x, I25.2 | 1 |
| Congestive heart failure | I09.9, I11.0, I13.0, I13.2, I25.5, I42.0, I42.5–I42.9, I43.x, I50.x, P29.0 | 1 |
| Peripheral vascular disease | I70.x, I71.x, I73.1, I73.8, I73.9, I77.1, I79.0, I79.2, K55.1, K55.8, K55.9, Z95.8, Z95.9 | 1 |
| Cerebrovascular disease | G45.x, G46.x, H34.0, I60.x–I69.x | 1 |
| Dementia | F00.x–F03.x, F05.1, G30.x, G31.1 | 1 |
| Chronic pulmonary disease | I27.8, I27.9, J40.x–J47.x, J60.x–J67.x, J68.4, J70.1, J70.3 | 1 |
| Rheumatologic disease | M05.x, M06.x, M31.5, M32.x–M34.x, M35.1, M35.3, M36.0 | 1 |
| Peptic ulcer disease | K25.x–K28.x | 1 |
| Mild liver disease | B18.x, K70.0–K70.3, K70.9, K71.3–K71.5, K71.7, K73.x, K74.x, K76.0, K76.2–K76.4, K76.8, K76.9, Z94.4 | 1 |
| Diabetes | E10.0, E10.l, E10.6, E10.8, E10.9, E11.0, E11.1, E11.6, E11.8, E11.9, E12.0, E12.1, E12.6, E12.8, E12.9, E13.0, E13.1, E13.6, E13.8, E13.9, E14.0, E14.1, E14.6, E14.8, E14.9 | 1 |
| Diabetes with chronic complications | E10.2–E10.5, E10.7, E11.2–E11.5, E11.7, E12.2–E12.5, E12.7, E13.2–E13.5, E13.7, E14.2–E14.5, E14.7 | 2 |
| Hemiplegia or paraplegia | G04.1, G11.4, G80.1, G80.2, G81.x, G82.x, G83.0–G83.4, G83.9 | 2 |
| Renal disease | I12.0, I13.1, N03.2–N03.7, N05.2–N05.7, N18.x, N19.x, N25.0, Z49.0–Z49.2, Z94.0, Z99.2 | 2 |
| Any malignancy including leukemia and lymphoma | C00.x–C26.x, C30.x–C34.x, C37.x–C41.x, C43.x, C45.x–C58.x, C60.x–C76.x, C81.x–C85.x, C88.x, C90.x–C97.x | 2 |
| Moderate or severe liver disease | I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K72.1, K72.9, K76.5, K76.6, K76.7 | 3 |
| Metastatic solid tumor | C77.x–C80.x | 6 |
| Acquired immunodeficiency syndrome (AIDS) | B20.x–B22.x, B24.x | 6 |
Appendix B. Trends in Baseline Characteristics of Patients Hospitalized with Atrial Fibrillation in the U.S. between 2016 and 2020
| Total | 2016 | 2017 | 2018 | 2019 | 2020 | P (Trend) | ||
| N | Unweighted | 432,740 | 92,760 | 93,400 | 94,367 | 91,614 | 60,599 | 0.2301 |
| Weighted | 2,163,699 | 463,800 | 467,000 | 471,835 | 458,070 | 302,995 | ||
| Age (%) | 18–44 | 4 | 4.2 | 4 | 3.9 | 3.9 | 4.4 | <0.001 |
| 45–59 | 17.1 | 17.8 | 17.4 | 17 | 16.3 | 16.9 | ||
| 60–74 | 40 | 39 | 39.9 | 40.4 | 40.3 | 40.5 | ||
| 75 or older | 38.9 | 39 | 38.8 | 38.8 | 39.5 | 38.2 | ||
| Gender (%) | Male | 50.9 | 50.4 | 50.4 | 50.9 | 51.4 | 51.5 | <0.001 |
| Female | 49.1 | 49.6 | 49.6 | 49.1 | 48.6 | 48.5 | ||
| Race (%) | White | 81.5 | 81.8 | 81.6 | 81.2 | 81.8 | 81 | 0.024 |
| Black | 8.6 | 8.5 | 8.4 | 8.4 | 8.6 | 9.2 | ||
| Hispanic | 5.9 | 5.8 | 6 | 6.3 | 5.7 | 5.7 | ||
| Asian/Pacific Islander | 1.5 | 1.5 | 1.5 | 1.6 | 1.5 | 1.5 | ||
| Native American | 0.4 | 0.3 | 0.4 | 0.4 | 0.4 | 0.4 | ||
| Others | 2.1 | 2.1 | 2 | 2.1 | 2.1 | 2.1 | ||
| Comorbidities (%) | Hypertension | 79.4 | 77.5 | 79 | 80 | 80.5 | 80.3 | <0.001 |
| Chronic Heart Failure | 21.2 | 19.6 | 20.5 | 21.8 | 22.6 | 21.9 | <0.001 | |
| Ischemic Heart Disease | 32.6 | 32.3 | 32 | 32.7 | 33.1 | 33 | <0.001 | |
| Diabetes Mellitus | 21.1 | 24 | 20.8 | 20.3 | 20.1 | 20.3 | <0.001 | |
| Chronic Kidney Disease | 20.1 | 18.9 | 19.1 | 19.7 | 20.7 | 23.5 | <0.001 | |
| Chronic Respiratory Disease | 24.9 | 24.8 | 25.1 | 24.9 | 25.1 | 24.4 | 0.237 | |
| H/O Cerebrovascular Disease | 11.5 | 11 | 11.3 | 11.6 | 11.9 | 11.7 | <0.001 | |
| COVID-19 | 0.2 | 0 | 0 | 0 | 0 | 1.2 | <0.001 | |
| Disposition of Patients (%) | Routine | 74.6 | 74.1 | 74.2 | 74.9 | 75 | 74.6 | 0.21 |
| Transfer to Short term Hosp | 2.1 | 2.2 | 2.1 | 2 | 2.1 | 2 | ||
| Transfer other | 10.1 | 10.7 | 10.6 | 10.2 | 9.9 | 8.7 | ||
| Home Healthcare | 12 | 11.9 | 11.8 | 11.8 | 11.9 | 13.1 | ||
| Against Medical Advice | 1.2 | 1.1 | 1.2 | 1.1 | 1.1 | 1.5 | ||
| Deyo-CCI, % | 0 | 22.6 | 24.2 | 23.4 | 22.2 | 21.6 | 21.4 | <0.001 |
| 1 | 25.6 | 26 | 26.1 | 25.6 | 25.2 | 24.9 | ||
| 2 or higher | 51.8 | 49.8 | 50.5 | 52.2 | 53.2 | 53.7 | ||
| Status of hospital (%) | Rural | 10.6 | 10.9 | 10.8 | 10.5 | 10.2 | 10.7 | <0.001 |
| Urban non-teaching | 23.1 | 28.5 | 24.1 | 22.2 | 19.6 | 19.8 | ||
| Urban teaching | 66.3 | 60.6 | 65.1 | 67.3 | 70.1 | 69.5 | ||
| Hospital Bed Size (%) | Small | 21.1 | 19.1 | 20.3 | 21.3 | 22.2 | 23.6 | <0.001 |
| Medium | 30.1 | 29.7 | 30.8 | 30 | 30.2 | 29.4 | ||
| Large | 48.8 | 51.1 | 48.9 | 48.7 | 47.6 | 46.9 | ||
| Primary Expected Payer (%) | Medicare | 68.5 | 68 | 68.7 | 68.9 | 68.9 | 67.3 | 0.00548 |
| Medicaid | 6.3 | 6.2 | 6.3 | 6.2 | 6.2 | 6.9 | ||
| Private | 20.5 | 21.2 | 20.5 | 20.4 | 19.9 | 20.5 | ||
| Self-pay | 2.5 | 2.3 | 2.3 | 2.5 | 2.6 | 2.7 | ||
| No change | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| Other | 2.1 | 2.1 | 1.9 | 1.9 | 2.1 | 2.4 | ||
| Region of hospital (%) | Northeast | 19.7 | 20.5 | 20.1 | 19.3 | 19.3 | 19 | <0.001 |
| Midwest | 24.1 | 24.2 | 24.3 | 24.3 | 24.1 | 23.2 | ||
| South | 40.9 | 40.3 | 40.4 | 40.9 | 41.1 | 42.2 | ||
| West | 15.3 | 15 | 15.3 | 15.4 | 15.5 | 15.5 | ||
Appendix C. Trends in Outcomes of Patients Hospitalized with AF/AFL in the U.S. between 2016 and 2020
| Total | 2016 | 2017 | 2018 | 2019 | 2020 | p-Value | |
| Overall mortality (%) * | 0.9 | 0.9 | 0.9 | 0.8 | 0.8 | 1 | 0.644 |
| Acute Heart Failure (%) | 18.6 | 18.2 | 18.1 | 19.2 | 18.9 | 18.9 | <0.001 |
| Acute stroke (%) | 0.6 | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.175 |
| Acute Coronary Syndrome (%) ^ | 7.2 | 5.3 | 6.2 | 7.1 | 8.1 | 10.7 | <0.001 |
| Mortality/ACS/Stroke (%) | 8.4 | 6.5 | 7.4 | 8.2 | 9.2 | 11.8 | <0.001 |
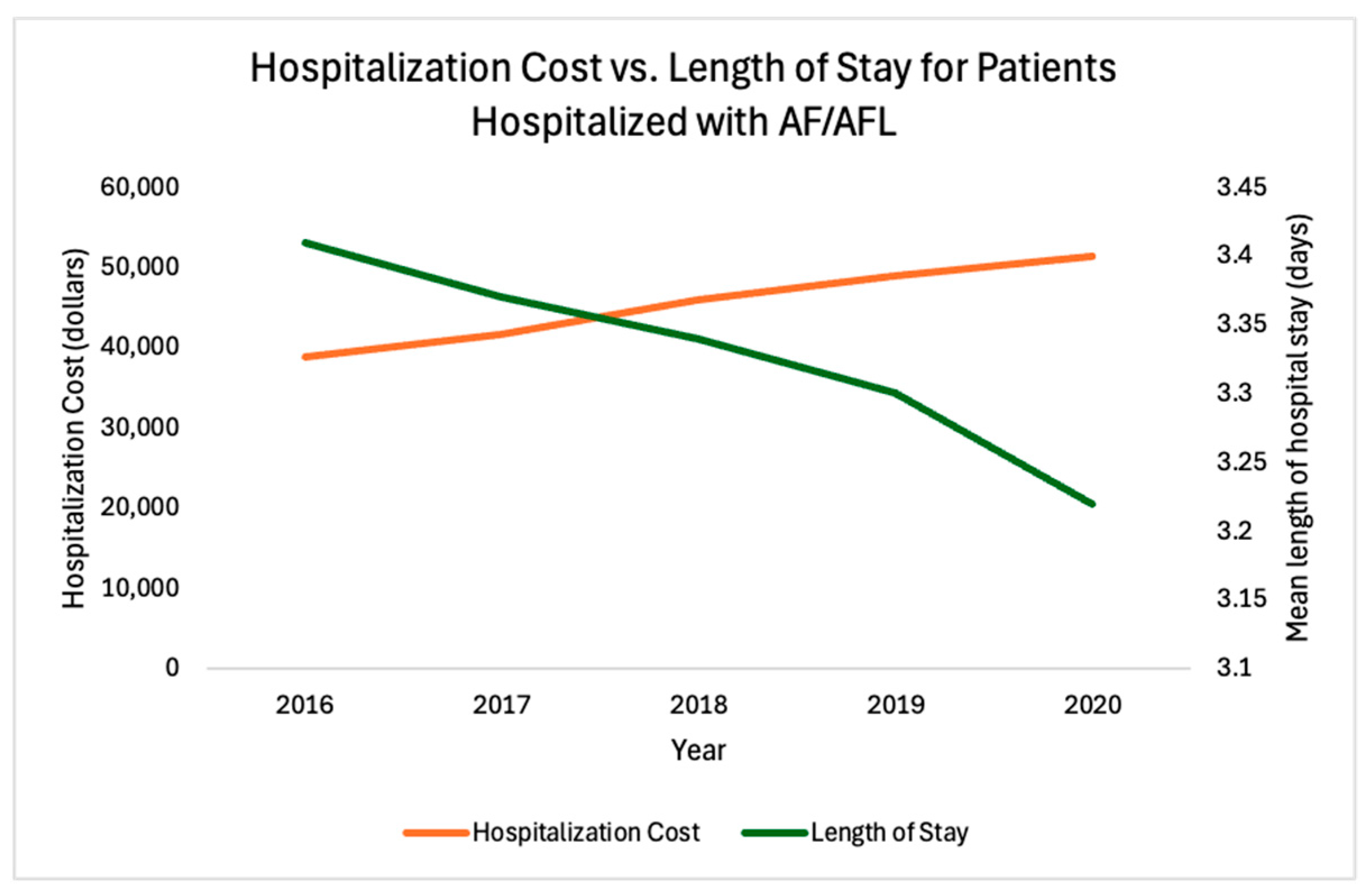
| Mean length of hospital stay, days | 3.34 | 3.41 | 3.37 | 3.34 | 3.3 | 3.22 | <0.001 |
| Mean hospitalization cost, USD | 44,908 | 38,841 | 41,620 | 46,067 | 48,913 | 51,347 | <0.001 |
| * Overall mortality refers to in-hospital mortality only. ^ Acute coronary syndrome refers to acute myocardial infarction, as well as other acute ischemic heart disease. | |||||||
References
- Patel, N.J.; Deshmukh, A.; Pant, S.; Singh, V.; Patel, N.; Arora, S.; Shah, N.; Chothani, A.; Savani, G.T.; Mehta, K.; et al. Contemporary Trends of Hospitalization for Atrial Fibrillation in the United States, 2000 Through 2010. Circulation 2014, 129, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Martínez, I.; Morales-Caba, L.; Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc. Med. 2023, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Terzic, C.M.; Medina-Inojosa, B.J. Cardiovascular Complications of Coronavirus Disease-2019. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef] [PubMed]
- Introduction to the HCUP National Inpatient Sample (NIS). Available online: https://hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2020.jsp (accessed on 30 December 2023).
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar] [PubMed]
- Radovanovic, D.; Seifert, B.; Urban, P.; Eberli, F.R.; Rickli, H.; Bertel, O.; Puhan, M.A.; Erne, P.; AMIS Plus Investigators. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014, 100, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Houchens, R.; Ross, D.; Elixhauser, A.; Jiang, J. Nationwide Inpatient Sample (NIS) Redesign Final Report. Available online: http://www.hcupus.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. (accessed on 30 December 2023).
- Cochran, W.G. Some Methods for Strengthening the Common χ2 Tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Armitage, P. Tests for Linear Trends in Proportions and Frequencies. Biometrics 1955, 11, 375–386. [Google Scholar] [CrossRef]
- Cuzick, J. A wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S.M. Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Wong, C.X.; Brooks, A.G.; Leong, D.P.; Roberts-Thomson, K.C.; Sanders, P. The Increasing Burden of Atrial Fibrillation Compared with Heart Failure and Myocardial Infarction: A 15-Year Study of All Hospitalizations in Australia. Arch. Intern. Med. 2012, 172, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; Joung, B.; et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am. Heart J. 2018, 202, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, T.-H.; Baek, Y.-S.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. The Trends of Atrial Fibrillation-Related Hospital Visit and Cost, Treatment Pattern and Mortality in Korea: 10-Year Nationwide Sample Cohort Data. Korean Circ. J. 2017, 47, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.U.; Munir, M.B.; Sharbaugh, M.S.; Thoma, F.W.; Althouse, A.D.; Saba, S. Trends in atrial fibrillation hospitalizations in the United States: A report using data from the National Hospital Discharge Survey. Indian Pacing Electrophysiol. J. 2018, 18, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, M.É.; Marynak, K.; Clarke, K.E.; Zainab, S.; Iju, S.; Thierry, J.M.; Ali, N.; McMillan, H.; Wiley, J.F.; Weaver, M.D.; et al. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns—United States, June 2020. 2020. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6936a4.htm?s_cid=mm6936a4_w (accessed on 16 April 2024).
- Mafham, M.M.; Spata, E.; Goldacre, R.; Gair, D.; Curnow, P.; Bray, M.; Hollings, S.; Roebuck, C.; Gale, C.P.; Mamas, M.A.; et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020, 396, 381–389. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, F.; Du, X.; Long, D.; Sang, C.; Tang, R.; Dong, J.; Guo, M.; Ma, C. Impact of COVID-19 Pandemic on Hospital Admissions of Acute Coronary Syndrome: A Beijing Inpatient Database Study. Lancet Reg. Health–West. Pac. 2022, 19, 100335. [Google Scholar] [CrossRef]
- Stewart, S.; Hart, C.L.; Hole, D.J.; McMurray, J.J.V. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001, 86, 516–521. [Google Scholar] [CrossRef]
- Khairallah, F.; Ezzedine, R.; Ganz, L.I.; London, B.; Saba, S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am. J. Cardiol. 2004, 94, 500–504. [Google Scholar] [CrossRef]
- Mujib, M.; Zhang, Y.; Feller, M.A.; Ahmed, A. Evidence of a “Heart Failure Belt” in the Southeastern United States. Am. J. Cardiol. 2011, 107, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Gillum, R.; Ingram, D. Relation between Residence in the Southeast Region of the United States and Stroke Incidence The NHANES I Epidemiologic Followup Study. Am. J. Epidemiol. 1996, 144, 665–673. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Gelder, I.C.v.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Rozen, G.; Elbaz-Greener, G.; Marai, I.; Heist, E.K.; Ruskin, J.N.; Carasso, S.; Birati, E.Y.; Amir, O. The relationship between the body mass index and in-hospital mortality in patients admitted for sudden cardiac death in the United States. Clin. Cardiol. 2021, 44, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Rozen, G.; Elbaz-Greener, G.; Margolis, G.; Marai, I.; Heist, E.K.; Ruskin, J.N.; Carasso, S.; Roguin, A.; Birati, E.Y.; Amir, O. The Obesity Paradox in Real-World Nation-Wide Cohort of Patients Admitted for a Stroke in the U.S. J. Clin. Med. 2022, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A.; Verma, A.; Kowey, P.R.; Halperin, J.L.; Gersh, B.J.; Wachter, R.; Pouliot, E.; Ziegler, P.D.; REVEAL AF Investigators. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol. 2017, 2, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Lubitz, S.A.; Mahida, S.; Magnani, J.W.; Fontes, J.D.; Sinner, M.F.; Van Gelder, I.C.; Ellinor, P.T.; Benjamin, E.J. Symptoms and Functional Status of Patients With Atrial Fibrillation. Circulation 2012, 125, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; Biviano, A.; Garan, H.; Goldbarg, S.; Kim, J.-H.; Yeo, I.; Tracy, C.; Ayanian, S.; et al. Worldwide Survey of COVID-19—Associated Arrhythmias. Circ. Arrhythmia Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Alfatlawi, H.; Mohamed, A.; Mhanna, M.; Mahmoud, M.; Elsheik, R.; Moukarbel, G.; Assaly, R. Outcomes of Heart Failure Related Hospitalizations During the COVID-19 Pandemic. Cureus 2023, 15, e36935. [Google Scholar] [CrossRef]
- Decker, W.W.; Smars, P.A.; Vaidyanathan, L.; Goyal, D.G.; Boie, E.T.; Stead, L.G.; Packer, D.L.; Meloy, T.D.; Boggust, A.J.; Haro, L.H.; et al. A Prospective, Randomized Trial of an Emergency Department Observation Unit for Acute Onset Atrial Fibrillation. Ann. Emerg. Med. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Kim, M.H.; Decena, B.F.; Bruckman, D.; Eagle, K.A. Use patterns of low-molecular weight heparin and the impact on length of stay in patients hospitalized for atrial fibrillation. Am. Heart J. 2003, 145, 665–669. [Google Scholar] [CrossRef] [PubMed]

| Total | 2016–2019 (Mean) | 2020 | ||
|---|---|---|---|---|
| N | Unweighted | 432,740 | 93,035 | 60,599 |
| Weighted | 2,163,699 | 465,176 | 302,995 | |
| Age (%) | 18–44 | 4 | 4 | 4.4 |
| 45–59 | 17.1 | 17.1 | 16.9 | |
| 60–74 | 40 | 39.9 | 40.5 | |
| 75 or older | 38.9 | 39 | 38.2 | |
| Gender (%) | Male | 50.9 | 50.8 | 51.5 |
| Female | 49.1 | 49.2 | 48.5 | |
| Race (%) | White | 81.5 | 81.6 | 81 |
| Black | 8.6 | 8.5 | 9.2 | |
| Hispanic | 5.9 | 6 | 5.7 | |
| Asian/Pacific Islander | 1.5 | 1.5 | 1.5 | |
| Native American | 0.4 | 0.4 | 0.4 | |
| Others | 2.1 | 2.1 | 2.1 | |
| Comorbidities (%) | Hypertension | 79.4 | 79.2 | 80.3 |
| Chronic Heart Failure | 21.2 | 21.1 | 21.9 | |
| Ischemic Heart Disease | 32.6 | 32.5 | 33 | |
| Diabetes Mellitus | 21.1 | 21.3 | 20.3 | |
| Chronic Kidney Disease | 20.1 | 19.6 | 23.5 | |
| Chronic Respiratory Disease | 24.9 | 25 | 24.4 | |
| H/O Cerebrovascular Disease | 11.5 | 11.5 | 11.7 | |
| COVID-19 | 0.2 | 0 | 1.2 | |
| Deyo-CCI, % | 0 | 22.6 | 22.8 | 21.4 |
| 1 | 25.6 | 25.7 | 24.9 | |
| 2 or higher | 51.8 | 51.4 | 53.7 | |
| Disposition of Patients (%) | Routine | 74.6 | 74.6 | 74.6 |
| Transfer to Short- term Hosp | 2.1 | 2.1 | 2 | |
| Transfer other | 10.1 | 10.4 | 8.7 | |
| Home Healthcare | 12 | 11.8 | 13.1 | |
| Against Medical Advice | 1.2 | 1.1 | 1.5 | |
| Status of hospital (%) | Rural | 10.6 | 10.6 | 10.7 |
| Urban non-teaching | 23.1 | 23.6 | 19.8 | |
| Urban teaching | 66.3 | 65.8 | 69.5 | |
| Hospital Bed Size (%) | Small | 21.1 | 20.7 | 23.6 |
| Medium | 30.1 | 30.2 | 29.4 | |
| Large | 48.8 | 49.1 | 46.9 | |
| Primary Expected Payer (%) | Medicare | 68.5 | 68.6 | 67.3 |
| Medicaid | 6.3 | 6.2 | 6.9 | |
| Private | 20.5 | 20.5 | 20.5 | |
| Self-pay | 2.5 | 2.4 | 2.7 | |
| No change | 0.2 | 0.2 | 0.2 | |
| Other | 2.1 | 2 | 2.4 | |
| Region of hospital (%) | Northeast | 19.7 | 19.8 | 19 |
| Midwest | 24.1 | 24.2 | 23.2 | |
| South | 40.9 | 40.7 | 42.2 | |
| West | 15.3 | 15.3 | 15.5 |
| Total | 2016–2019 | 2020 | Percent Change | p-Value | |
|---|---|---|---|---|---|
| Overall mortality (%) * | 0.9 | 0.8 | 1 | 25% | 0.007 |
| Acute Heart Failure | 18.6 | 18.6 | 18.9 | 1.61% | 0.0347 |
| Acute stroke (%) | 0.6 | 0.6 | 0.6 | 0% | 0.3816 |
| Acute Coronary Syndrome (%) ^ | 7.2 | 6.7 | 10.7 | 60% | <0.001 |
| Mortality/ACS/Stroke (%) | 8.4 | 7.8 | 11.8 | 51% | <0.001 |
| Mean length of hospital stay, days | 3.34 | 3.35 | 3.22 | −3.88% | <0.001 |
| Mean hospitalization cost, USD | 44,908 | 43,860 | 51,347 | 17% | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoudi, S.; John, K.; Chalhoub, F.; Chee, J.; Infeld, M.; Elbaz-Greener, G.; Homoud, M.; Ruskin, J.N.; Heist, E.K.; Madias, C.; et al. Nationwide Trends in Hospitalizations for Atrial Fibrillation and Flutter in the United States before and during the Outbreak of the COVID-19 Pandemic. J. Clin. Med. 2024, 13, 4883. https://doi.org/10.3390/jcm13164883
Daoudi S, John K, Chalhoub F, Chee J, Infeld M, Elbaz-Greener G, Homoud M, Ruskin JN, Heist EK, Madias C, et al. Nationwide Trends in Hospitalizations for Atrial Fibrillation and Flutter in the United States before and during the Outbreak of the COVID-19 Pandemic. Journal of Clinical Medicine. 2024; 13(16):4883. https://doi.org/10.3390/jcm13164883
Chicago/Turabian StyleDaoudi, Sarah, Kevin John, Fadi Chalhoub, Jennifer Chee, Margaret Infeld, Gabby Elbaz-Greener, Munther Homoud, Jeremy N. Ruskin, E. Kevin Heist, Christopher Madias, and et al. 2024. "Nationwide Trends in Hospitalizations for Atrial Fibrillation and Flutter in the United States before and during the Outbreak of the COVID-19 Pandemic" Journal of Clinical Medicine 13, no. 16: 4883. https://doi.org/10.3390/jcm13164883





