Hyperuricaemia Prevalence Rates According to Their Physiochemical and Epidemiological Diagnostic Criteria and Their Associations with Cardio-Renal-Metabolic Factors: SIMETAP-HU Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions of HU, Medical Conditions and Comorbidities
2.3. Statistical Analysis
3. Results
3.1. General Clinical Characteristics of Overall Study Population
3.2. HU Prevalence Rates
3.3. Clinical Characteristics of Study Subjects According to Both Diagnostic Criteria
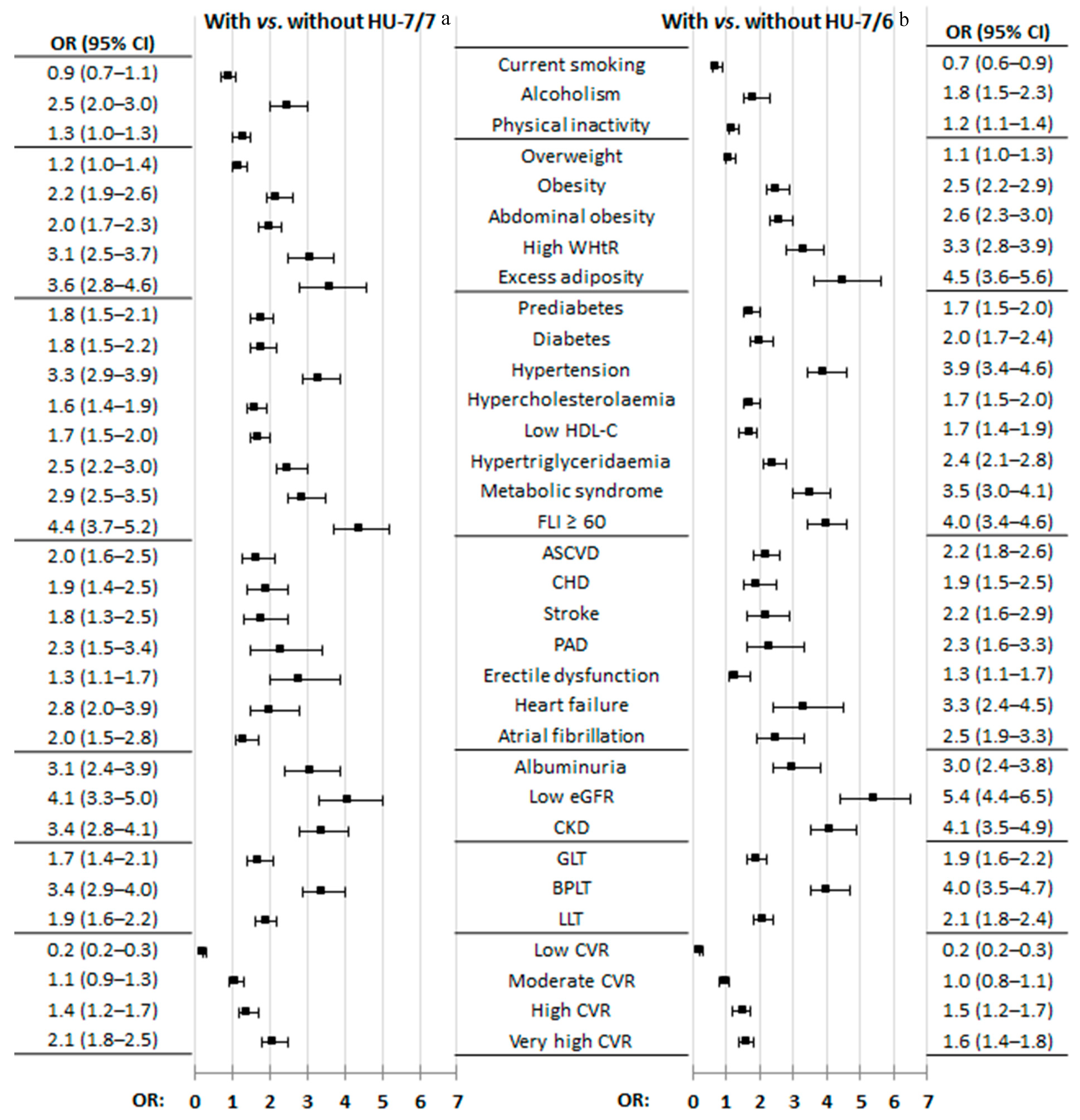
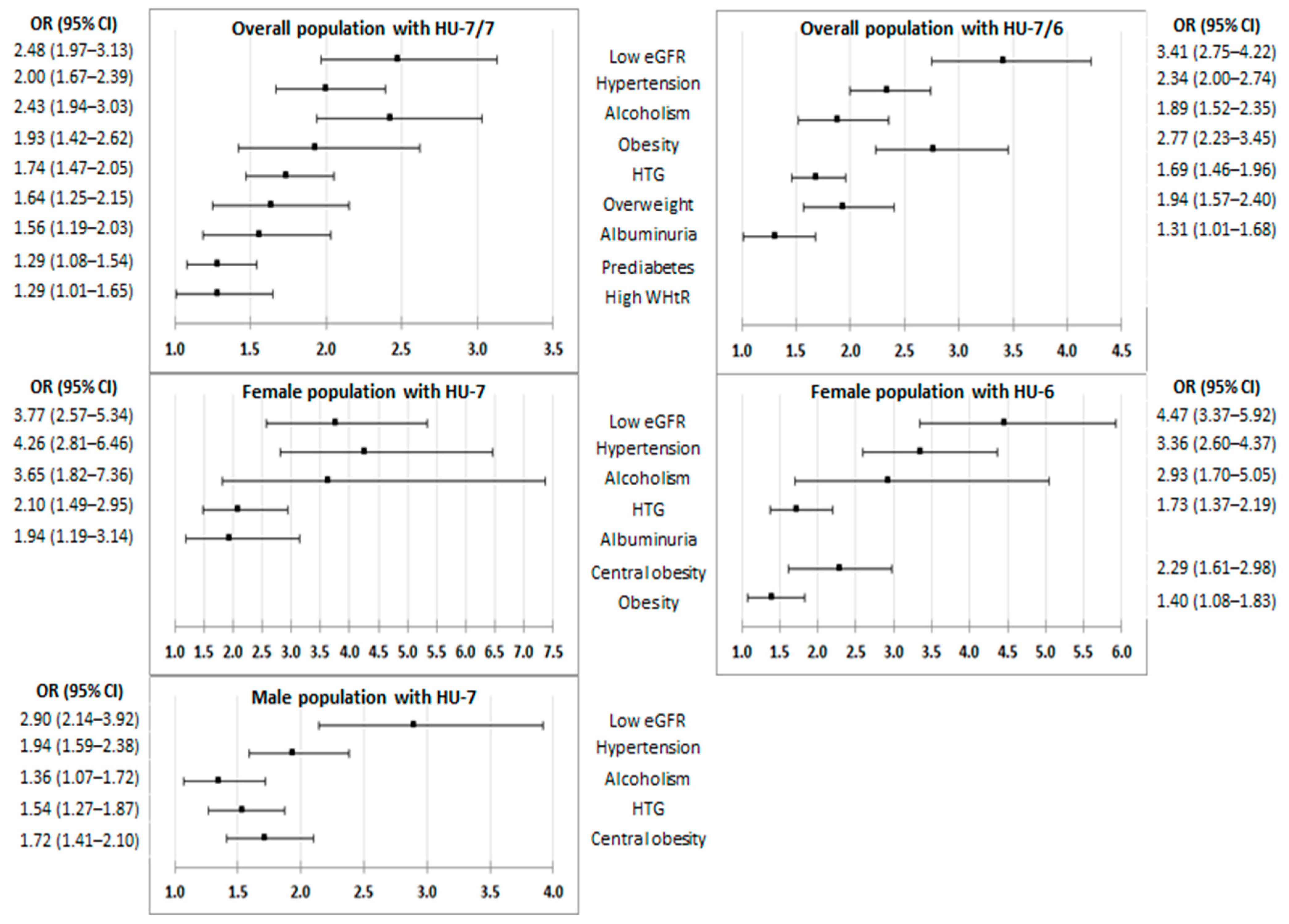
3.4. Effect of CKM Factors and Medical Conditions on HU
4. Discussion
4.1. Hyperuricaemia Prevalence Rates
4.2. CKM Factors and Medical Conditions Associated with HU
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| CKD | chronic kidney disease |
| CKM | cardiovascular–kidney–metabolic |
| CVD | cardiovascular disease |
| CVR | cardiovascular risk |
| DM | diabetes mellitus |
| eGFR | estimated glomerular filtration rate |
| FLI | fatty liver index |
| HF | heart failure |
| HTG | hypertriglyceridaemia |
| HTN | arterial hypertension |
| HU | hyperuricaemia |
| HU-6 | HU with SUA ≥ 6 mg/dL |
| HU-7 | HU with SUA ≥ 7 mg/dL |
| HU-7/6 | HU with SUA ≥ 7 mg/dL for men and ≥ 6.0 mg/dL for women |
| HU-7/7 | HU with SUA ≥ 7 mg/dL for both men and women |
| LDL-C | low-density lipoprotein cholesterol |
| MetS | metabolic syndrome |
| MSU | monosodium urate |
| SLD | steatotic liver disease |
| SUA | serum uric acid |
| ULT | urate-lowering drug therapy |
| WtHR | waist-to-height ratio |
References
- Kellgren, J.H.; Jeffrey, M.R.; Ball, J.; Council for International Organizations of Medical Sciences. The Epidemiology of Chronic Rheumatism: [Proceedings of] A Symposium Organized by the Council for International Organizations of Medical Sciences Established under the Joint Auspices of UNESCo & WHO with Partial Support by Grants from the National Institute of Arthritis & Metabolic Diseases and the Arthritis & Rheumatism Foundation; Blackwell Scientific Publications: Oxford, UK, 1963; Available online: https://ci.nii.ac.jp/ncid/BA33063611 (accessed on 10 July 2024).
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Lozada, L.G.S.; et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef]
- Valsaraj, R.; Singh, A.K.; Gangopadhyay, K.K.; Ghoshdastidar, B.; Goyal, G.; Batin, M.; Mukherjee, D.; Sengupta, U.; Chatterjee, S.; Sengupta, N. Management of asymptomatic hyperuricemia: Integrated Diabetes & Endocrine Academy (IDEA) consensus statement. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 93–100. [Google Scholar] [CrossRef]
- Yamanaka, H.; Japanese Society of Gout and Nucleic Acid Metabolism. Japanese guideline for the management of hyperuricemia and gout: Second edition. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology Guidelines for Management of Gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases. Chinese Multidisciplinary Expert Consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chin. Med J. 2017, 130, 2473–2488. [Google Scholar] [CrossRef]
- Chhana, A.; Lee, G.; Dalbeth, N. Factors influencing the crystallization of monosodium urate: A systematic literature review. BMC Musculoskelet. Disord. 2015, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shin, D. Concurrent presence of high serum uric acid and inflammation is associated with increased incidence of type 2 diabetes mellitus in Korean adult population. Sci. Rep. 2022, 12, 11000. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, J.E.; Jeon, C.H. Hyperuricemia is associated with an increased prevalence of metabolic syndrome in a general population and a decreased prevalence of diabetes in men. J. Rheum. Dis. 2020, 27, 247–260. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; E Kelley, E.; Nakagawa, T.; Madero, M.; I Feig, D.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric acid and hypertension: An update with recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Wang, J.; Qin, T.; Chen, J.; Li, Y.; Wang, L.; Huang, H.; Li, J. Hyperuricemia and risk of incident hypertension: A systematic review and meta-analysis of observational studies. PLoS ONE 2014, 9, e114259. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Association of hyperuricemia with cardiovascular diseases: Current evidence. Hosp. Pract. 2023, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Masi, S.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; Cicero, A.F.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid and fatal myocardial infarction: Detection of prognostic cut-off values: The URRAH (Uric Acid Right for Heart Health) study. J. Hypertens. 2020, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhong, X.; Xu, T.; Xu, T.; Zhang, Y. Sex-Specific relationship between serum uric acid and risk of stroke: A dose-response me-ta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, e005042. [Google Scholar] [CrossRef]
- Baker, J.F.; Schumacher, H.R.; Krishnan, E. Serum uric acid level and risk for peripheral arterial disease: Analysis of data from the multiple risk factor intervention trial. Angiology 2007, 58, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Salvetti, M.; Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.; Cirillo, M.; et al. Serum uric acid, predicts heart failure in a large Italian cohort: Search for a cut-off value the URic acid Right for heArt Health study. J. Hypertens. 2021, 39, 62–69. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Nishihara, S.; Nishi, Y.; Takahashi, O.; Kario, K.; Yamamoto, K.; Yamashita, T.; Hisatome, I. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int. J. Cardiol. 2017, 231, 137–142. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Kark, J.D. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: The Jerusalem Lipid Research Clinic cohort study. Nephrol. Dial. Transplant. 2011, 26, 2558–2566. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Morawiecka-Pietrzak, M.; Starzak, M.; Stanek, A.; Cieślar, G. Retrospective cross-sectional study of the relationship of thyroid volume and function with anthropometric measurements, body composition analysis parameters, and the diagnosis of metabolic syndrome in euthyroid people aged 18–65. Medicina 2024, 60, 1080. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. American Heart Association. Cardiovascular-kidney-metabolic health: A Presidential Advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation task force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, A.; Arranz-Martínez, E.; García-Álvarez, J.C.; Morales-Cobos, L.E.; García-Fernández, M.E.; de la Peña-Antón, N.; Calle, M.C.M.; Costa, A.M.; Martínez, D.P.; Villasur, M.P.G.; et al. Population and methodology of the SIMETAP study: Prevalence of cardiovascular risk factors, cardiovascular diseases, and related metabolic diseases. Clin. E Investig. En Arter. 2018, 30, 197–208. [Google Scholar] [CrossRef]
- Roddy, E.; Zhang, W.; Doherty, M. The changing epidemiology of gout. Nat. Clin. Pract. Rheumatol. 2007, 3, 443–449. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wang, J.; Liu, H.; Kwong, J.S.-W.; Chen, H.; Li, L.; Chung, S.-C.; Shah, A.; Chen, Y.; et al. Diagnosis and treatment for hyperuricemia and gout: A systematic review of clinical practice guidelines and consensus statements. BMJ Open 2019, 9, e026677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2001, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Trifirò, G.; Morabito, P.; Cavagna, L.; Ferrajolo, C.; Pecchioli, S.; Simonetti, M.; Bianchini, E.; Medea, G.; Cricelli, C.; Caputi, A.P.; et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: A nationwide population-based study. Ann. Rheum. Dis. 2013, 72, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Yeh, W.-T.; Chuang, S.-Y.; Wu, Y.-Y.; Pan, W.-H. Gender-specific risk factors for incident gout: A prospective cohort study. Clin. Rheumatol. 2012, 31, 239–245. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef]
- Higa, S.; Yoshida, M.; Shima, D.; Ii, Y.; Kitazaki, S.; Yamamoto, Y.; Fujimoto, Y. A retrospective, cross-sectional study on the prevalence of hyperuricemia using a Japanese healthcare database. Turk. J. Rheumatol. 2020, 35, 41–51. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Maloberti, A.; Qualliu, E.; Occhi, L.; Sun, J.; Grasso, E.; Tognola, C.; Tavecchia, G.; Cartella, I.; Milani, M.; Vallerio, P.; et al. Hyperuricemia prevalence in healthy subjects and its relationship with cardiovascular target organ damage. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Antelo-Pais, P.; Prieto-Díaz, M.; Micó-Pérez, R.M.; Pallarés-Carratalá, V.; Velilla-Zancada, S.; Polo-García, J.; Barquilla-García, A.; Ginel-Mendoza, L.; Segura-Fragoso, A.; Vitelli-Storelli, F.; et al. Prevalence of hyperuricemia and its association with cardiovascular risk factors and subclinical target organ damage. J. Clin. Med. 2023, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-B.; Zhang, W.-Q.; Tang, W.-W.; Liu, Y.; Ning, Y.; Huang, C.; Liu, J.-X.; Yi, Y.-J.; Xu, R.-H.; Wang, T.-D. Prevalence and associated factors of hyperuricemia among urban adults aged 35–79 years in southwestern China: A community-based cross-sectional study. Sci. Rep. 2020, 10, 15683. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-A.; Liu, T.; Ruan, G.-T.; Ge, Y.-Z.; Song, M.-M.; Xie, H.-L.; Lin, S.-Q.; Deng, L.; Zhang, H.-Y.; Zhang, Q.; et al. The relationship between fat distribution in central region and comorbidities in obese people: Based on NHANES 2011–2018. Front. Endocrinol. 2023, 14, 1114963. [Google Scholar] [CrossRef] [PubMed]
- Abdi, D.R.; Mohammadian, K.N.; Hosseinpour, A.; Asadi, S.; Ejtahed, H.S.; Qorbani, M. Waist to height ratio as a simple tool for predicting mortality: A systematic review and meta-analysis. Int. J. Obes. 2023, 47, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, T.; Ochiai, H.; Yoshimoto, T.; Nagahama, S.; Watanabe, A.; Yoshida, R.; Kokaze, A. Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr. Disord. 2020, 20, 2. [Google Scholar] [CrossRef]
- Romero-Saldaña, M.; Fuentes-Jiménez, F.J.; Vaquero-Abellán, M.; Álvarez-Fernández, C.; Aguilera-López, M.D.; Molina-Recio, G. Predictive capacity and cutoff value of waist-to-height ratio in the incidence of metabolic syndrome. Clin. Nurs. Res. 2019, 28, 676–691. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: Meta-analysis of population based cohorts. BMJ 2018, 363, k3951. [Google Scholar] [CrossRef]
- Kuwabara, M.; Borghi, C.; Cicero, A.F.; Hisatome, I.; Niwa, K.; Ohno, M.; Johnson, R.J.; Lanaspa, M.A. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int. J. Cardiol. 2018, 261, 183–188. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Gao, Y.-X.; Wang, X.; Chang, H.; Huang, G.-W. Serum uric acid and appropriate cutoff value for prediction of metabolic syndrome among Chinese adults. J. Clin. Biochem. Nutr. 2013, 52, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Overview and new insights into the metabolic syndrome: Risk factors and emerging variables in the development of type 2 diabetes and cerebrocardiovascular disease. Medicina 2023, 59, 561. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D’ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, pathophysiological basis and cardiovascular implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Gagliardi, A.C.; Miname, M.H.; Santos, R.D. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 2009, 202, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Krajčoviechová, A.; Wohlfahrt, P.; Bruthans, J.; Šulc, P.; Lánská, V.; Eremiášová, L.; Pudil, J.; Linhart, A.; Filipovský, J.; Mayer, O.; et al. Which serum uric acid levels are associated with increased cardiovascular risk in the general adult population? J. Clin. Hypertens. 2020, 22, 897–905. [Google Scholar] [CrossRef] [PubMed]
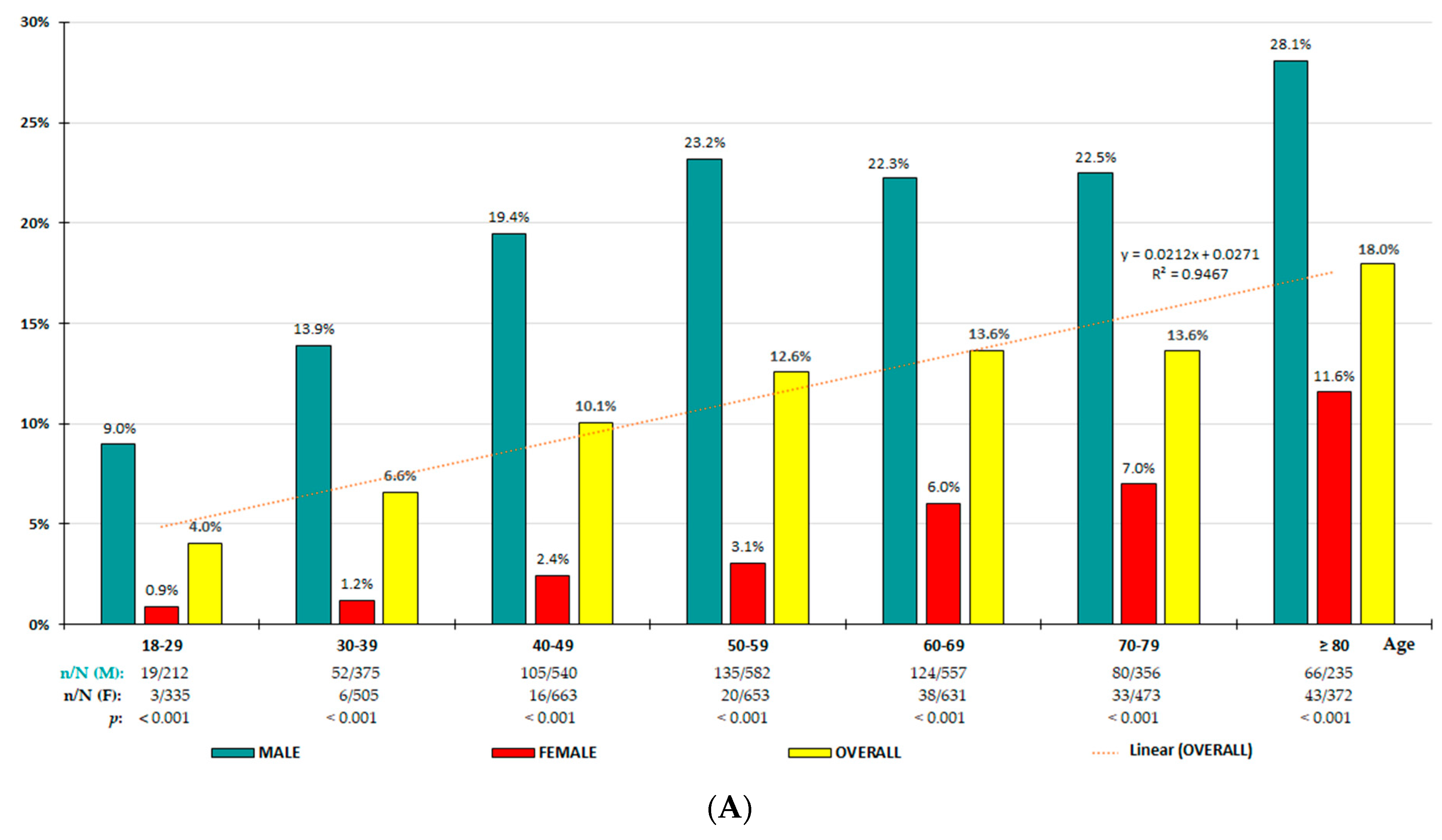
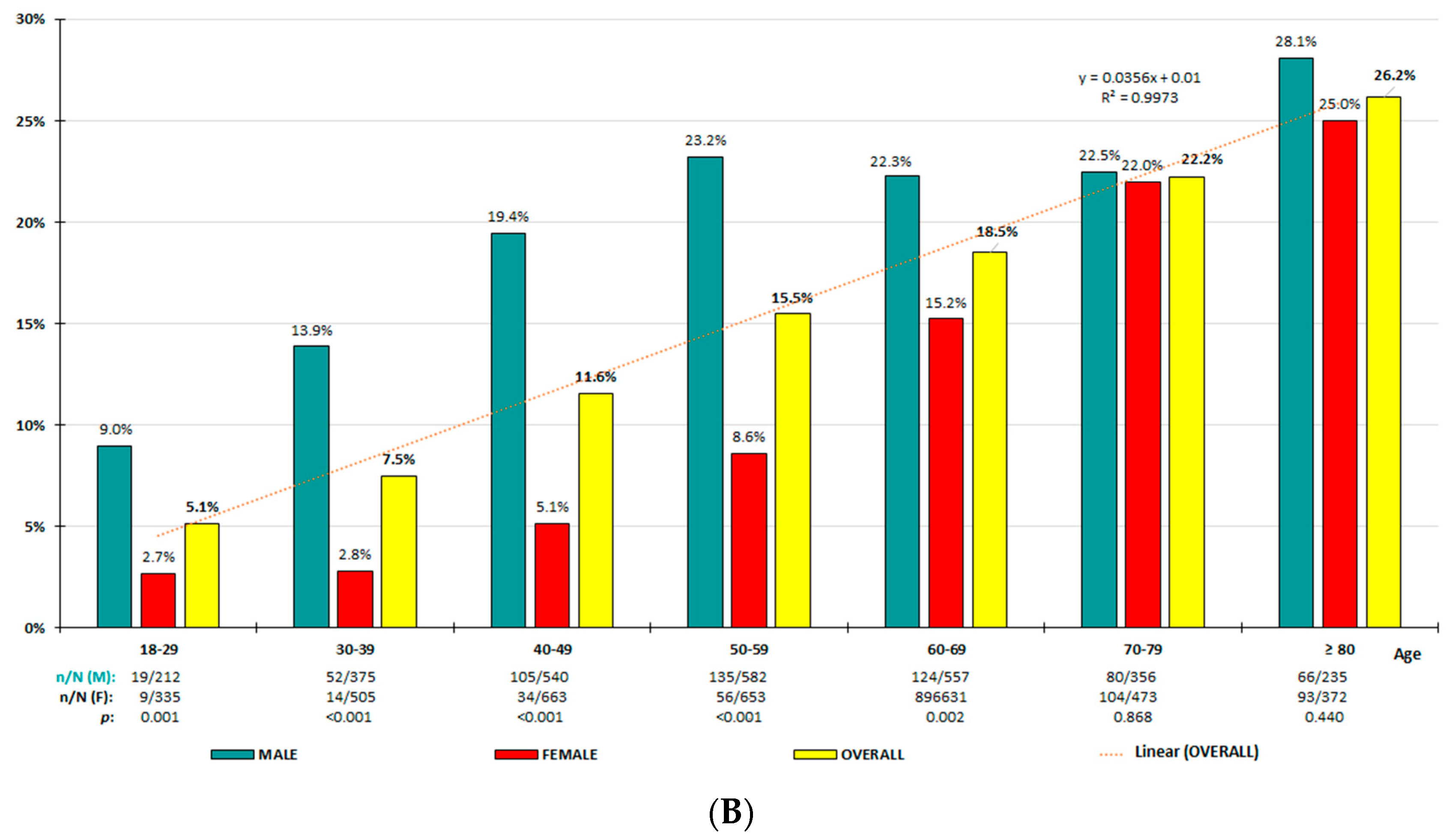
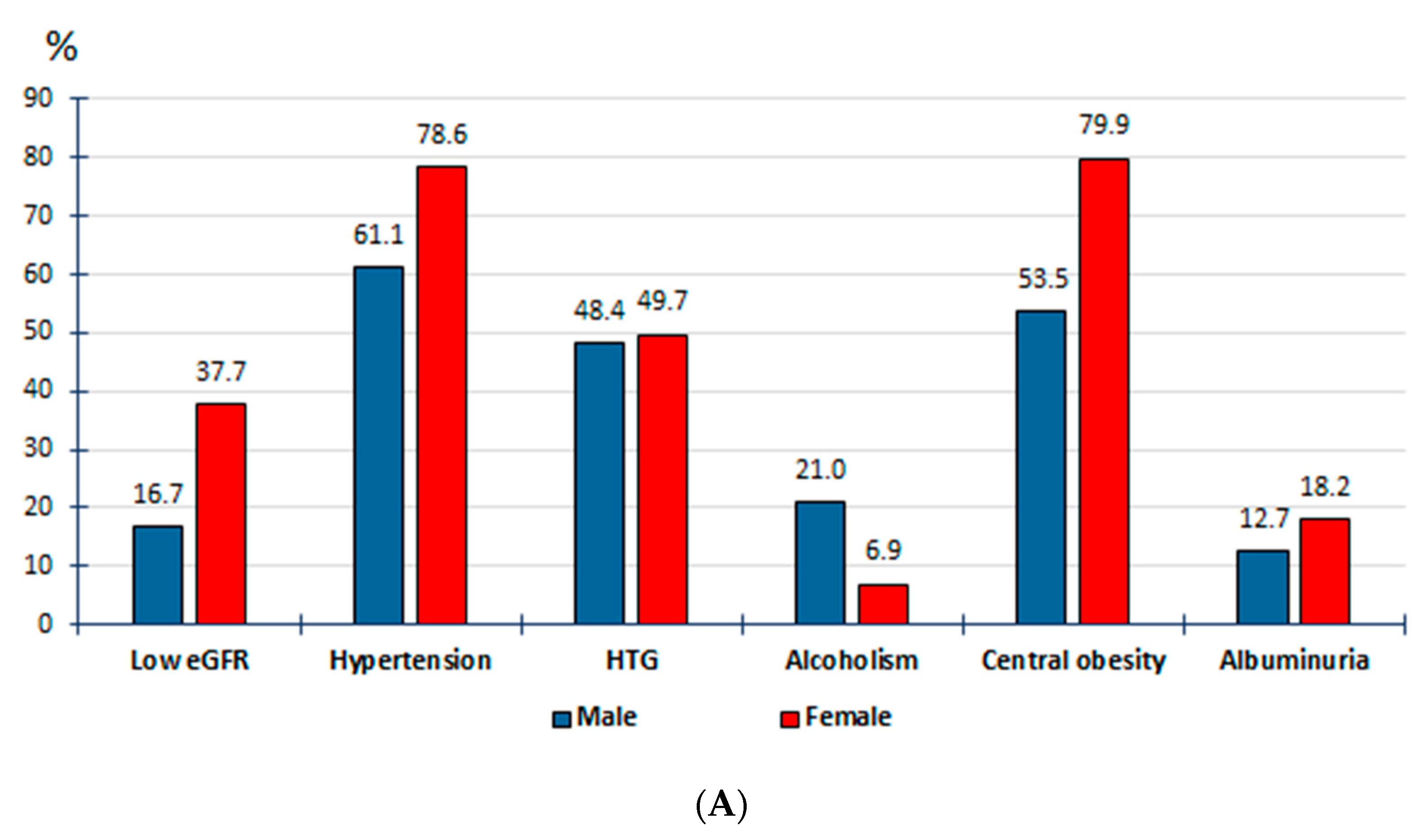
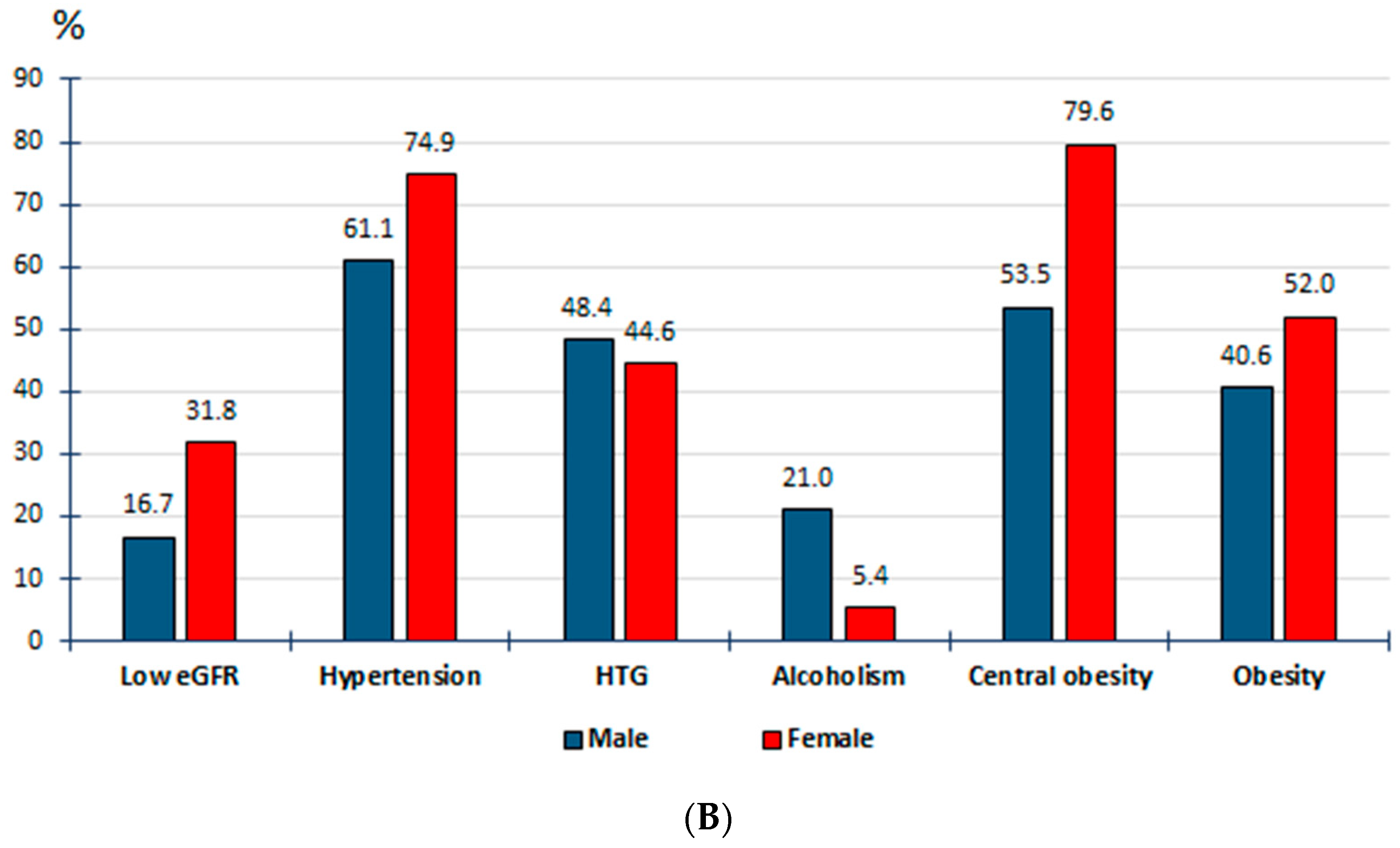
| Crude Prevalence Rates | Adjusted Prevalence Rates | |||||||
|---|---|---|---|---|---|---|---|---|
| Diagnostic Criteria | Age-Group (Years) | Overall % (95% CI) | Male % (95% CI) | Female % (95% CI) | p | Overall (%) | Male (%) | Female (%) |
| HU-7/7 HU-7/6 | <40 | 5.6 (4.4–6.8) 6.6 (5.3–7.9) | 12.1 (9.5–14.7) | 1.1 (0.4–1.7) 2.7 (1.6–3.8) | <0.001 | 5.4 6.4 | 11.7 | 1.1 2.7 |
| HU-7/7 HU-7/6 | <50 | 7.6 (6.6–8.7) 8.9 (7.8–10.0) | 15.6 (13.5–17.7) | 1.7 (1.0–2.3) 3.8 (2.8–4.8) | <0.001 | 7.1 8.3 | 14.5 | 1.6 3.6 |
| HU-7/7 HU-7/6 | 40–69 | 12.1 (11.0–13.1) 15.2 (14.0–16.3) | 21.7 (19.7–23.7) | 3.8 (3.0–4.7) 9.6 (8.3–10.9) | <0.001 | 11.8 14.7 | 21.4 | 3.6 9.0 |
| HU-7/7 HU-7/6 | 50–69 | 13.1 (11.7–14.4) 17.0 (15.4–18.5) | 22.7 (20.3–25.2) | 4.5 (3.4–5.7) 11.8 (10.1–13.6) | <0.001 | 13.0 16.8 | 22.8 | 4.4 11.5 |
| HU-7/7 HU-7/6 | ≥50 | 14.0 (12.9–15.1) 19.5 (18.3–20.8) | 23.4 (21.4–25.4) | 6.3 (5.3–7.3) 16.4 (14.8–18.0) | <0.001 | 13.9 19.3 | 23.4 | 6.2 16.1 |
| HU-7/7 HU-7/6 | ≥70 | 15.5 (13.6–17.3) 23.9 (21.2–28.2) | 24.7 (21.2–28.2) | 9.0 (7.1–10.9) 23.3 (20.5–26.2) | <0.001 0.543 | 15.5 23.9 | 24.7 | 9.1 23.4 |
| HU-7/7 HU-7/6 | ≥18 | 11.4 (10.6–12.2) 15.2 (14.3–16.1) | 20.3 (18.9–21.8) | 4.4 (3.7–5.0) 11.2 (10.2–12.2) | <0.001 | 10.2 13.4 | 18.4 | 3.8 9.6 |
| Male Populations | Female Populations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| With HU-7 No. 581 | Without HU-7 No. 2276 | Cohen’s d (95% CI) | With HU-7 No. 159 | Without HU-7 No. 3473 | Cohen’s d (95% IC) | With HU-6 No. 406 | Without HU-6 No. 3226 | Cohen’s d (95% CI) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age yr | 59.2 (15.5) | 54.3 (17.0) | 0.3 (0.2; 0.4) $$$ | 67.5 (15.8) | 54.5 (17.9) | 0.7 (0.6; 0.9) $$$ | 67.3 (15.4) | 53.5 (17.7) | 0.8 (0.7; 0.9) $$$ |
| BMI kg/m2 | 29.5 (4.6) | 27.5 (4.4) | 0.5 (0.4; 0.5) $$$ | 31.4 (6.1) | 27.0 (5.5) | 0.8 (0.6; 1.0) $$$ | 31.1 (6.0) | 26.7 (5.3) | 0.8 (0.7; 0.9) $$$ |
| WC cm | 102.4 (12.1) | 96.9 (12.5) | 0.4 (0.4; 0.5) $$$ | 100.6 (14.1) | 89.2 (13.9) | 0.8 (0.7; 1.0) $$$ | 99.6 (14.2) | 88.4 (13.6) | 0.8 (0.7; 0.9) $$$ |
| WtHR | 0.60 (0.07) | 0.57 (0.08) | 0.4 (0.3; 0.5) $$$ | 0.65 (0.09) | 0.56 (0.10) | 0.9 (0.7; 1.1) $$$ | 0.64 (0.09) | 0.56 (0.09) | 0.9 (0.8; 1.0) $$$ |
| CUN-BAE adiposity | 31.3 (5.6) | 28.3 (6.3) | 0.5 (0.4; 0.6) $$$ | 45.3 (5.9) | 39.0 (7.4) | 0.9 (0.7; 1.0) $$$ | 45.0 (5.9) | 38.6 (7.4) | 0.9 (0.8; 1.0) $$$ |
| SBP mmHg | 126.8 (15.0) | 123.4 (13.7) | 0.2 (0.2; 0.3) $$$ | 129.3 (16.2) | 119.8 (16.1) | 0.6 (0.4; 0.8) $$$ | 129.5 (15.6) | 119.1 (15.9) | 0.7 (0.6; 0.8) $$$ |
| DBP mmHg | 76.3 (10.0) | 74.5 (9.2) | 0.2 (0.1; 0.3) $$$ | 75.5 (9.4) | 72.0 (9.9) | 0.4 (0.2; 0.5) $$$ | 75.7 (9.6) | 71.7 (9.8) | 0.4 (0.3; 0.5) $$$ |
| FPG mg/dL a | 100.3 (22.6) | 99.5 (29.6) | 0.0 (−0.1; 0.1) NS | 101.0 (24.0) | 92.7 (23.2) | 0.4 (0.2; 0.5) $$$ | 101.3 (25.3) | 92.0 (22.8) | 0.4 (0.3; 0.5) $$$ |
| HbA1c % b # | 5.74 (0.75) | 5.70 (0.97) | 0.0 (−0.1; 0.1) NS | 5.99 (0.93) | 5.55 (0.85) | 0.5 (0.4; 0.7) $$$ | 5.95 (0.93) | 5.52 (0.83) | 0.5 (0.4; 0.6) $$$ |
| TC mg/dL c | 191.2 (39.9) | 187.5 (38.8) | 0.1 (0.0; 0.2) $ | 199.8 (42.2) | 196.3 (39.1) | 0.1 (−0.1; 0.3) NS | 198.8 (42.1) | 196.1 (38.9) | 0.1 (0.0; 0.2) NS |
| HDL-C mg/dL c | 47.1 (12.0) | 49.8 (12.7) | −0.2 (−0.3; −0.1) $$$ | 53.8 (13.4) | 59.5 (14.7) | −0.4 (−0.6; −0.2) $$$ | 56.0 (14.2) | 59.7 (14.7) | −0.3 (−0.4; −0.2) $$$ |
| Non-HDL-C mg/dL c | 144.1 (39.1) | 137.7 (38.7) | 0.2 (0.1; 0.3) $$$ | 146.0 (38.4) | 136.7 (38.1) | 0.2 (0.1; 0.4) $$ | 142.9 (40.5) | 136.4 (37.8) | 0.2 (0.1; 0.3) $$ |
| TG mg/dL d | 162.3 (132.0) | 129.1 (90.5) | 0.3 (0.2; 0.4) $$$ | 149.8 (108.5) | 106.6 (60.6) | 0.7 (0.5; 0.8) | 144.6 (93.2) | 103.9 (57.8) | 0.7 (0.6; 0.8) $$$ |
| SUA mg/dL e | 7.61 (1.16) | 5.21 (1.02) | 2.0 (1.9; 2.1) $$$ | 7.52 (1.02) | 4.25 (1.05) | 3.1 (3.0; 3.3) $$$ | 6.80 (0.89) | 4.09 (0.91) | 3.0 (2.9; 3.1) $$$ |
| Creatinine mg/dL f | 1.07 (0.38) | 0.93 (0.26) | 0.5 (0.4; 0.6) $$$ | 0.99 (0.52) | 0.7 (0.23) | 1.0 (0.8; 1.2) $$$ | 0.92 (0.48) | 0.73 (0.21) | 0.8 (0.7; 0.9) $$$ |
| eGFR mL/min/1.73 m2 | 80.7 (21.6) | 92.0 (18.5) | −0.6 (−0.7; −0.5) $$$ | 68.8 (25.1) | 92.2 (20.3) | −1.1 (−1.3; −1.0) $$$ | 72.5 (24.3) | 93.5 (19.4) | −1.1 (−1.2; −1.0) $$$ |
| uACR mg/g g | 34.1 (111.3) | 17.3 (69.2) | 0.2 (0.1; 0.3) $$$ | 33.7 (81.6) | 12.2 (36.1) | 0.6 (0.4; 0.7) $$$ | 25.4 (70.1) | 11.6 (33.3) | 0.4 (0.3; 0.5) $$$ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-García, A.; Serrano-Cumplido, A.; Arranz-Martínez, E.; Escobar-Cervantes, C.; Pallarés-Carratalá, V. Hyperuricaemia Prevalence Rates According to Their Physiochemical and Epidemiological Diagnostic Criteria and Their Associations with Cardio-Renal-Metabolic Factors: SIMETAP-HU Study. J. Clin. Med. 2024, 13, 4884. https://doi.org/10.3390/jcm13164884
Ruiz-García A, Serrano-Cumplido A, Arranz-Martínez E, Escobar-Cervantes C, Pallarés-Carratalá V. Hyperuricaemia Prevalence Rates According to Their Physiochemical and Epidemiological Diagnostic Criteria and Their Associations with Cardio-Renal-Metabolic Factors: SIMETAP-HU Study. Journal of Clinical Medicine. 2024; 13(16):4884. https://doi.org/10.3390/jcm13164884
Chicago/Turabian StyleRuiz-García, Antonio, Adalberto Serrano-Cumplido, Ezequiel Arranz-Martínez, Carlos Escobar-Cervantes, and Vicente Pallarés-Carratalá. 2024. "Hyperuricaemia Prevalence Rates According to Their Physiochemical and Epidemiological Diagnostic Criteria and Their Associations with Cardio-Renal-Metabolic Factors: SIMETAP-HU Study" Journal of Clinical Medicine 13, no. 16: 4884. https://doi.org/10.3390/jcm13164884





