Bone Health Optimization in Adult Spinal Deformity Patients: A Narrative Review
Abstract
:1. Introduction
2. Osteoporosis and Osteopenia
3. Risks and Complications in Osteoporotic Patients
3.1. Vertebral Compression Fractures
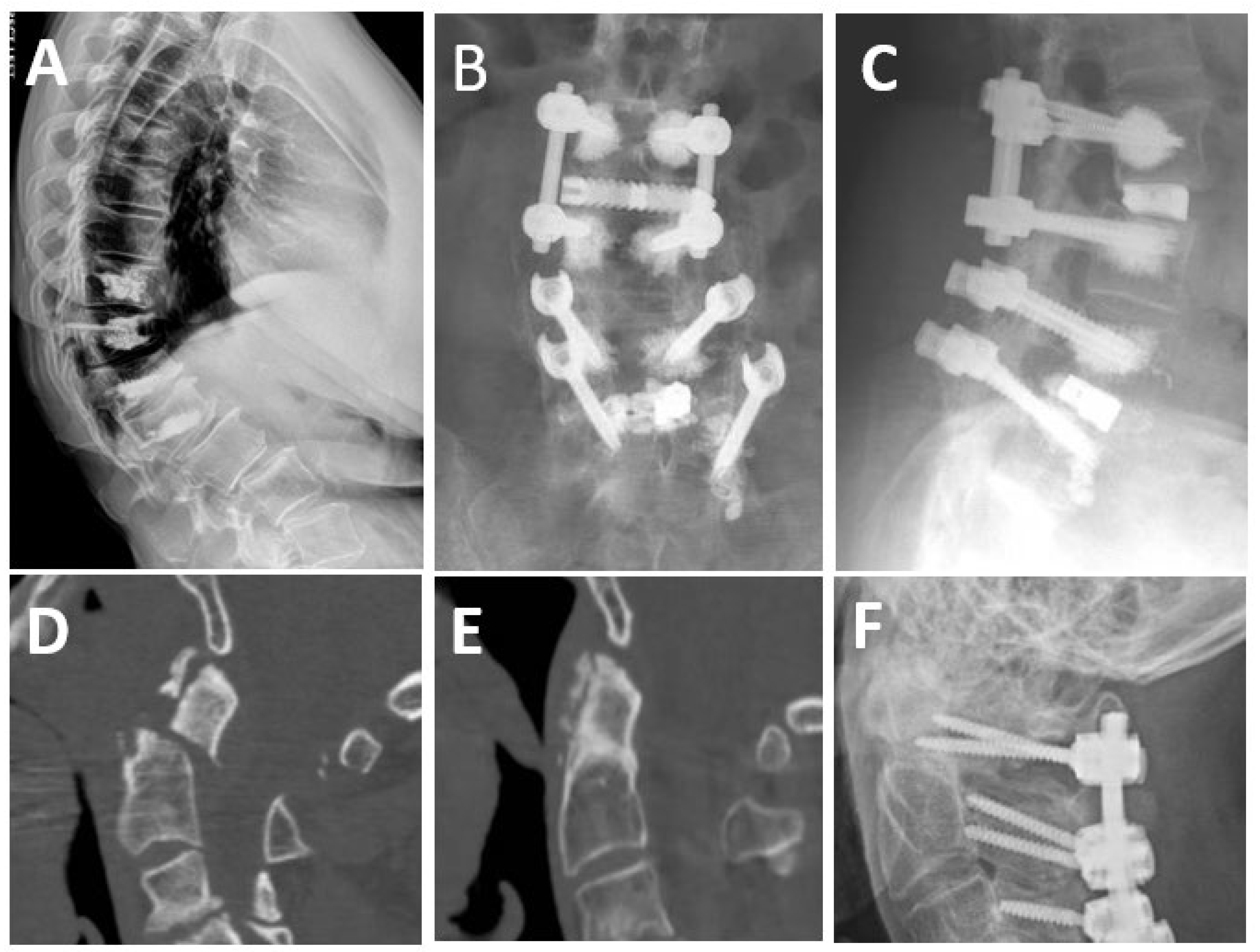
3.2. Hardware Failure
3.3. Proximal Junctional Kyphosis and Failure
4. Osteoporosis Treatment and Optimization for Spine Surgery
4.1. Vitamin and Mineral Supplementation
4.2. Antiresorptive Agents
Bisphosphonates
4.3. Anabolic Agents
4.3.1. Denosumab
4.3.2. Romosozumab
4.4. Parathyroid Hormone (PTH) Analogs
4.4.1. Teriparatide
4.4.2. Abaloparatide
5. Future Research Plans
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Safaee, M.M.; Ames, C.P.; Smith, J.S. Epidemiology and Socioeconomic Trends in Adult Spinal Deformity Care. Neurosurgery 2020, 87, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, J.H.; Chang, D.G.; Suk, S.I.; Suh, S.W.; Song, K.S.; Park, J.B.; Cho, W. Adult Spinal Deformity: Current Concepts and Decision-Making Strategies for Management. Asian Spine J. 2020, 14, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Scheer, J.K.; Lafage, V.; Smith, J.S.; Bess, S.; Berven, S.H.; Mundis, G.M.; Sethi, R.K.; Deinlein, D.A.; Coe, J.D. Adult Spinal Deformity: Epidemiology, Health Impact, Evaluation, and Management. Spine Deform. 2016, 4, 310–322. [Google Scholar] [CrossRef]
- Tsirikos, A.I.; Roberts, S.B.; Bhatti, E. Incidence of Spinal Deformity Surgery in a National Health Service from 2005 to 2018: An Analysis of 2,205 Children and Adolescents. Bone Jt. Open 2020, 1, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dietz, N.; Hollis, P.; Fortuny, E.; Gruter, B.; Virojanapa, J.; Williams, B.; Spiessberger, A. Systemic Risk Factors for Adult Spinal Deformity (ASD): A Retrospective Analysis of 48 Patients. Cureus 2022, 14, e25214. [Google Scholar] [CrossRef]
- Good, C.R.; Auerbach, J.D.; O’Leary, P.T.; Schuler, T.C. Adult Spine Deformity. Curr. Rev. Musculoskelet Med. 2011, 4, 159–167. [Google Scholar] [CrossRef]
- Mansfield, J.T.; Bennett, M. Scheuermann Disease; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Williamson, T.K.; Passfall, L.; Ihejirika-Lomedico, R.; Espinosa, A.; Owusu-Sarpong, S.; Lanre-Amos, T.; Schoenfeld, A.J.; Passias, P.G. Assessing the influence of modifiable patient-related factors on complication rates after adult spinal deformity surgery. Bone Jt. J. 2022, 104, 1249–1255. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Gunjotikar, S.; Tanaka, M.; Komatsubara, T.; Latka, K.; Ekade, S.J.; Prabhu, S.P.; Takamatsu, K.; Yasuda, Y.; Nakagawa, M. Evaluation and Rehabilitation after Adult Lumbar Spine Surgery. J. Clin. Med. 2024, 13, 2915. [Google Scholar] [CrossRef]
- Ton, A.; Shahrestani, S.; Chen, X.T.; Ballatori, A.M.; Wang, J.C.; Buser, Z. The Effect of Modifiable Risk Factors on Postoperative Complications in Lumbar Spine Fusions. Glob. Spine J. 2021, 13, 1212–1222. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, Etiology, and Diagnosis of Osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, 3–11. [Google Scholar] [CrossRef]
- Xiao, P.L.; Cui, A.Y.; Hsu, C.J.; Peng, R.; Jiang, N.; Xu, X.H.; Ma, Y.G.; Liu, D.; Lu, H.D. Global Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 1–20. [Google Scholar] [CrossRef]
- (US) Office of the Surgeon General. Bone Health and Osteoporosis; US Health and Human Services: Washington, DC, USA, 2004; Volume 437. [Google Scholar]
- Mundy, G.R. Osteopenia. Dis.-A-Mon. 1987, 33, 537–600. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, G.; Holick, M.F. Diagnosis and Treatment of Osteopenia. Rev. Endocr. Metab. Disord. 2010, 11, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Varacallo, M.; Seaman, T.J.; Jandu, J.S.; Pizzutillo, P. Osteopenia; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zhang, J.; Morgan, S.L.; Saag, K.G. Osteopenia: Debates and Dilemmas. Curr. Rheumatol. Rep. 2013, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Sidon, E.; Stein, M.; Ramalingam, G.; Shemesh, S.; Benharroch, D.; Ohana, N. Gender Differences in Spinal Injuries: Causes and Location of Injury. J. Womens Health 2018, 27, 946–951. [Google Scholar] [CrossRef]
- Bilston, L.E.; Brown, J. Pediatric Spinal Injury Type and Severity Are Age and Mechanism Dependent. Spine 2007, 32, 2339–2347. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92. [Google Scholar]
- Ogiri, M.; Nishida, K.; Park, H.J.; Rossi, A. Systematic Literature Review and Meta-Analysis on the Clinical Outcomes of Spine Surgeries in Patients with Concurrent Osteoporosis. Spine Surg. Relat. Res. 2023, 7, 200. [Google Scholar] [CrossRef]
- Bjerke, B.T.; Zarrabian, M.; Aleem, I.S.; Fogelson, J.L.; Currier, B.L.; Freedman, B.A.; Bydon, M.; Nassr, A. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 563. [Google Scholar] [CrossRef]
- DeWald, C.J.; Stanley, T. Instrumentation-Related Complications of Multilevel Fusions for Adult Spinal Deformity Patients over Age 65: Surgical Considerations and Treatment Options in Patients with Poor Bone Quality. Spine 2006, 30 (Suppl. 19), 573–579. [Google Scholar] [CrossRef]
- Wolfert, A.J.; Rompala, A.; Beyer, G.A.; Shah, N.V.; Ikwuazom, C.P.; Kim, D.; Shah, S.T.; Passias, P.G.; Lafage, V.; Schwab, F.J.; et al. The Impact of Osteoporosis on Adverse Outcomes After Short Fusion for Degenerative Lumbar Disease. J. Am. Acad. Orthop. Surg. 2022, 30, 573–579. [Google Scholar] [CrossRef]
- Chin, D.K.; Park, J.Y.; Yoon, Y.S.; Kuh, S.U.; Jin, B.H.; Kim, K.S.; Cho, Y.E. Prevalence of Osteoporosis in Patients Requiring Spine Surgery: Incidence and Significance of Osteoporosis in Spine Disease. Osteoporos. Int. 2007, 18, 1219–1224. [Google Scholar] [CrossRef]
- Lubelski, D.; Choma, T.J.; Steinmetz, M.P.; Harrop, J.S.; Mroz, T.E. Perioperative Medical Management of Spine Surgery Patients with Osteoporosis. Neurosurgery 2015, 77, 92–97. [Google Scholar] [CrossRef]
- Kuo, C.C.; Soliman, M.A.R.; Aguirre, A.O.; Ruggiero, N.; Kruk, M.; Khan, A.; Ghannam, M.M.; Almeida, N.D.; Jowdy, P.K.; Smolar, D.E.; et al. Vertebral Bone Quality Score Independently Predicts Proximal Junctional Kyphosis and/or Failure After Adult Spinal Deformity Surgery. Neurosurgery 2023, 92, 945–954. [Google Scholar] [CrossRef]
- Sing, D.C.; Berven, S.H.; Burch, S.; Metz, L.N. Increase in Spinal Deformity Surgery in Patients Age 60 and Older Is Not Associated with Increased Complications. Spine J. 2017, 17, 627–635. [Google Scholar] [CrossRef]
- Diebo, B.G.; Sheikh, B.; Freilich, M.; Shah, N.V.; Redfern, J.A.I.; Tarabichi, S.; Shepherd, E.M.; Lafage, R.; Passias, P.G.; Najjar, S.; et al. Osteoporosis and Spine Surgery: A Critical Analysis Review. JBJS Rev. 2020, 8, e0160. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Gupta, A.; Cha, T.; Schwab, J.; Fogel, H.; Tobert, D.; Razi, A.E.; Hecht, A.; Bono, C.M.; Hershman, S. Osteoporosis Increases the Likelihood of Revision Surgery Following a Long Spinal Fusion for Adult Spinal Deformity. Spine J. 2021, 21, 134–140. [Google Scholar] [CrossRef]
- Thomas, K.; Wong, K.H.; Steelman, S.C.; Rodriguez, A. Surgical Risk Assessment and Prevention in Elderly Spinal Deformity Patients. Geriatr. Orthop. Surg. Rehabil. 2019, 10, 2151459319851681. [Google Scholar] [CrossRef]
- Pappou, I.P.; Girardi, F.P.; Sandhu, H.S.; Parvataneni, H.K.; Cammisa, F.P.; Schneider, R.; Frelinghuysen, P.; Lane, J.M. Discordantly High Spinal Bone Mineral Density Values in Patients with Adult Lumbar Scoliosis. Spine 2006, 31, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.; Urits, I.; Orhurhu, V.; Orhurhu, M.S.; Callan, J.; Powell, J.; Manchikanti, L.; Kaye, A.D.; Kaye, R.J.; Viswanath, O. Current Concepts in the Management of Vertebral Compression Fractures. Curr. Pain Headache Rep. 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Schousboe, J.T. Clinical Practice. Vertebr. Fractures. N. Engl. J. Med. 2011, 364, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; McGirt, M.J. Vertebral Compression Fractures: A Review of Current Management and Multimodal Therapy. J. Multidiscip. Healthc. 2013, 6, 205–214. [Google Scholar] [CrossRef]
- Alexandru, D.; So, W.E. Vertebral Compression Fractures. Perm. J. 2012, 16, 46. [Google Scholar] [CrossRef]
- Zohar, A.; Getzler, I.; Behrbalk, E. Higher Mortality Rate in Patients with Vertebral Compression Fractures Is Due to Deteriorated Medical Status Prior to the Fracture Event. Geriatr. Orthop. Surg. Rehabil. 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Karikari, I.O.; Metz, L.N. Preventing Pseudoarthrosis and Proximal Junctional Kyphosis: How to Deal with the Osteoporotic Spine. Neurosurg. Clin. North Am. 2018, 29, 365–374. [Google Scholar] [CrossRef]
- Rometsch, E.; Spruit, M.; Zigler, J.E.; Menon, V.K.; Ouellet, J.A.; Mazel, C.; Härtl, R.; Espinoza, K.; Kandziora, F. Screw-Related Complications After Instrumentation of the Osteoporotic Spine: A Systematic Literature Review With Meta-Analysis. Glob. Spine J. 2020, 10, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.I.; Nunna, R.S.; Maasarani, S.; Belmont, E.; Deme, P.; Chilakapati, S.; Eldridge, C.; Singh, R.; Bagley, C.A.; Adogwa, O. Association of Osteopenia and Osteoporosis with Higher Rates of Pseudarthrosis and Revision Surgery in Adult Patients Undergoing Single-Level Lumbar Fusion. Neurosurg. Focus 2020, 49, 6. [Google Scholar] [CrossRef]
- Moldovan, F. Bone Cement Implantation Syndrome: A Rare Disaster Following Cemented Hip Arthroplasties—Clinical Considerations Supported by Case Studies. J. Pers. Med. 2023, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Yaltirik, K.; Ashour, A.M.; Reis, C.R.; Ozdogan, S.; Atalay, B. Vertebral augmentation by kyphoplasty and vertebroplasty: 8 years experience outcomes and complications. J. Craniovertebral Junction Spine 2016, 7, 153–160. [Google Scholar] [CrossRef]
- Yagi, M.; Fujita, N.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Ishii, K.; Nakamura, M.; Matsumoto, M.; Watanabe, K. Low Bone-Mineral Density Is a Significant Risk for Proximal Junctional Failure after Surgical Correction of Adult Spinal Deformity. Spine 2018, 43, 485–491. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, J.H.; Chang, D.G.; Suk, S.I.; Suh, S.W.; Kim, S.I.; Song, K.S.; Park, J.B.; Cho, W. Proximal Junctional Kyphosis in Adult Spinal Deformity: Definition, Classification, Risk Factors, and Prevention Strategies. Asian Spine J. 2022, 16, 440. [Google Scholar] [CrossRef]
- Judy, B.F.; Tracz, J.A.; Alomari, S.; Witham, T.F. Patient Optimization for the Prevention of Proximal Junctional Kyphosis. Int. J. Spine Surg. 2023, 60504, 8510. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Lenke, L.G.; Bridwell, K.H.; Kim, Y.J.; Koester, L.; Hensley, M. Proximal Junctional Vertebral Fracture in Adults after Spinal Deformity Surgery Using Pedicle Screw Constructs: Analysis of Morphological Features. Spine 2010, 1976, 138–145. [Google Scholar] [CrossRef]
- Doodkorte, R.J.P.; Vercoulen, T.F.G.; Roth, A.K.; Bie, R.A.; Willems, P.C. Instrumentation Techniques to Prevent Proximal Junctional Kyphosis and Proximal Junctional Failure in Adult Spinal Deformity Correction—A Systematic Review of Biomechanical Studies. Spine J. 2021, 21, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.D.; Warden, K.E.; Biomed, M.; Herzig, M.A.; McAfee, P.C. Influence of Bone Mineral Density on the Fixation of Thoracolumbar Implants. A Comparative Study of Transpedicular Screws, Laminar Hooks, and Spinous Process Wires. Spine 1990, 15, 902–907. [Google Scholar] [CrossRef]
- Hohn, E.A.; Chu, B.; Martin, A.; Yu, E.; Telles, C.; Leasure, J.; Lynch, T.L.; Kondrashov, D. The Pedicles Are Not the Densest Regions of the Lumbar Vertebrae: Implications for Bone Quality Assessment and Surgical Treatment Strategy. Glob. Spine J. 2017, 7, 567. [Google Scholar] [CrossRef]
- Ward, K.A.; Pearse, C.M.; Madanhire, T.; Wade, A.N.; Fabian, J.; Micklesfield, L.K.; Gregson, C.L. Disparities in the Prevalence of Osteoporosis and Osteopenia in Men and Women Living in Sub-Saharan Africa, the UK, and the USA. Curr. Osteoporos. Rep. 2023, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef]
- Hryvniak, D.; Frost, C.D. Spine Injury Prevention. Clin. Sports Med. 2021, 40, 429–444. [Google Scholar] [CrossRef]
- Bernatz, J.T.; Winzenried, A.E.; Hare, K.J.; Mikula, A.L.; Williams, S.K.; Binkley, N.C.; Anderson, P.A. Effect of Bone Health Optimization on Osteoporosis Screening and Treatment Before Thoracolumbar Fusion. JAAOS Glob. Res. Rev. 2022, 6, 2151459319851681. [Google Scholar] [CrossRef]
- Chung, A.; Robinson, J.; Gendelberg, D.; Jimenez, J.; Anand, A.; Rao, A.; Khandehroo, B.; Kahwaty, S.; Anand, N. Do Peri-Operative Parathyroid Hormone (PTH) Analogues Improve Bone Density and Decrease Mechanical Complications in Spinal Deformity Correction?—A Minimum 2-Year Radiological Study Measuring Hounsfield Units. Eur. Spine J. 2023, 32, 3651–3658. [Google Scholar] [CrossRef]
- Cho, J.H.; Cho, D.C.; Yu, S.H.; Jeon, Y.H.; Sung, J.K.; Kim, K.T. Effect of Dietary Calcium on Spinal Bone Fusion in an Ovariectomized Rat Model. J. Korean Neurosurg. Soc. 2012, 52, 281–287. [Google Scholar] [CrossRef]
- Metzger, M.F.; Kanim, L.E.A.; Zhao, L.; Robinson, S.T.; Delamarter, R.B. The Relationship between Serum Vitamin D Levels and Spinal Fusion Success: A Quantitative Analysis. Spine 2015, 40, E458–E468. [Google Scholar] [CrossRef]
- Stoker, G.E.; Buchowski, J.M.; Bridwell, K.H.; Lenke, L.G.; Riew, K.D.; Zebala, L.P. Preoperative Vitamin D Status of Adults Undergoing Surgical Spinal Fusion. Spine 2013, 38, 507–515. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoon, J.Y.; Lee, B.H.; Jung, H.S.; Park, M.S.; Park, J.O.; Moon, E.S.; Kim, H.S.; Lee, H.M.; Moon, S.H. Changes in Vitamin D Status after Surgery in Female Patients with Lumbar Spinal Stenosis and Its Clinical Significance. Spine 1976, 37, E1326–E1330. [Google Scholar] [CrossRef]
- Schwalfenberg, G. Improvement of Chronic Back Pain or Failed Back Surgery with Vitamin D Repletion: A Case Series. J. Am. Board Fam. Med. 2009, 22, 69–74. [Google Scholar] [CrossRef]
- Waikakul, S. Serum 25-Hydroxy-Calciferol Level and Failed Back Surgery Syndrome. J. Orthop. Surg. 2012, 20, 18–22. [Google Scholar] [CrossRef]
- Rodriguez, W.J.; Gromelski, J. Vitamin D Status and Spine Surgery Outcomes. ISRN Orthop. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.H.; Tseng, Y.K.; Chung, Y.H.; Wu, N.Y.; Li, C.H.; Lee, P.Y. The Efficacy of Oral Vitamin D Supplements on Fusion Outcome in Patients Receiving Elective Lumbar Spinal Fusion—A Randomized Control Trial. BMC Musculoskelet. Disord. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Krasowska, K.; Skrobot, W.; Liedtke, E.; Sawicki, P.; Flis, D.J.; Dzik, K.P.; Libionka, W.; Kloc, W.; Kaczor, J.J. The Preoperative Supplementation With Vitamin D Attenuated Pain Intensity and Reduced the Level of Pro-Inflammatory Markers in Patients After Posterior Lumbar Interbody Fusion. Front. Pharmacol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Kanayama, M.; Togawa, D.; Hashimoto, T.; Minami, A. Does Alendronate Disturb the Healing Process of Posterior Lumbar Interbody Fusion? A Prospective Randomized Trial. J. Neurosurg. Spine 2011, 14, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dai, Z.; Kang, Y.; Lv, G.; Keller, E.T.; Jiang, Y. Effects of Zoledronic Acid on Bone Fusion in Osteoporotic Patients after Lumbar Fusion. Osteoporos. Int. 2016, 27, 1469–1476. [Google Scholar] [CrossRef]
- Tu, C.W.; Huang, K.F.; Hsu, H.T.; Li, H.Y.; Yang, S.S.D.; Chen, Y.C. Zoledronic Acid Infusion for Lumbar Interbody Fusion in Osteoporosis. J. Surg. Res. 2014, 192, 112–116. [Google Scholar] [CrossRef]
- Kang, T.; Park, S.Y.; Hong, S.H.; Lee, J.H.; Lee, S.H.; Park, J.H. Bone Union after Spinal Fusion Surgery Using Local Bone in Long-Term Bisphosphonate Users: A Prospective Comparative Study. Arch. Osteoporos. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.; Diaz, A.; Perez-Roman, R.J.; Burks, S.S.; Wang, M.Y.; Levi, A.D. Osteoporosis Treatment in Patients Undergoing Spinal Fusion: A Systematic Review and Meta-Analysis. Neurosurg. Focus 2021, 50, 9. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhao, W.T.; Shen, P.; Zhang, F.J. The Effects of Bisphosphonates on Osteoporotic Patients after Lumbar Fusion: A Meta-Analysis. Drug Des. Dev. Ther. 2018, 12, 2233–2240. [Google Scholar] [CrossRef]
- Buerba, R.A.; Sharma, A.; Ziino, C.; Arzeno, A.; Ajiboye, R.M. Bisphosphonate and Teriparatide Use in Thoracolumbar Spinal Fusion a Systematic Review and Meta-Analysis of Comparative Studies. Spine 2018, 43, 1014–1023. [Google Scholar] [CrossRef]
- Mei, J.; Song, X.; Guan, X.; Wu, D.; Wang, J.; Liu, Q. Postoperative Bisphosphonate Do Not Significantly Alter the Fusion Rate after Lumbar Spinal Fusion: A Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Kuo, Y.J.; Chen, C.; Kang, Y.N. Effects of Teriparatide and Bisphosphonate on Spinal Fusion Procedure: A Systematic Review and Network Meta-Analysis. PLoS ONE 2020, 15, 0237566. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J. Comparison of Teriparatide and Bisphosphonate Treatment to Reduce Pedicle Screw Loosening after Lumbar Spinal Fusion Surgery in Postmenopausal Women with Osteoporosis from a Bone Quality Perspective. Spine 2013, 38, E487–E492. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Yamada, K.; Kaneko, K.; Sekiya, T.; Kanai, K.; Higashi, T.; Saito, T. Combined Teriparatide and Denosumab Therapy Accelerates Spinal Fusion Following Posterior Lumbar Interbody Fusion. Orthop. Traumatol. Surg. Res. 2018, 104, 1043–1048. [Google Scholar] [CrossRef]
- Tani, S.; Ishikawa, K.; Kudo, Y.; Tsuchiya, K.; Matsuoka, A.; Maruyama, H.; Emori, H.; Yamamura, R.; Hayakawa, C.; Sekimizu, M.; et al. The Effect of Denosumab on Pedicle Screw Fixation: A Prospective 2-Year Longitudinal Study Using Finite Element Analysis. J. Orthop. Surg. Res. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- McClung, M.R. Romosozumab for the treatment of osteoporosis. Osteoporos. Sarcopenia 2018, 4, 11–15. [Google Scholar] [CrossRef]
- Okuyama, K.; Inage, K.; Kim, G.; Mukaihata, T.; Tajiri, I.; Shiga, Y.; Inoue, M.; Eguchi, Y.; Suzuki-Narita, M.; Otagiri, T.; et al. Bone union-promoting effect of romosozumab in an ovariectomized rat posterolateral lumbar fusion model. J. Orthop. Res. 2024, 42, 1831–1840. [Google Scholar] [CrossRef]
- Mikula, A.L.; Lakomkin, N.; Hamouda, A.M.; Everson, M.C.; Pennington, Z.; Kumar, R.; Pinter, Z.W.; Martini, M.L.; Bydon, M.; Kennel, K.A.; et al. Change in spinal bone mineral density as estimated by Hounsfield units following osteoporosis treatment with romosozumab, teriparatide, denosumab, and alendronate: An analysis of 318 patients. J. Neurosurg. Spine 2024, 1–7. [Google Scholar] [CrossRef]
- Zhang, A.S.; Khatri, S.; Balmaceno-Criss, M.; Alsoof, D.; Daniels, A.H. Medical optimization of osteoporosis for adult spinal deformity surgery: A state-of-the-art evidence-based review of current pharmacotherapy. Spine Deform. 2023, 11, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Kostenuik, P.J.; Binkley, N.; Anderson, P.A. Advances in Osteoporosis Therapy: Focus on Osteoanabolic Agents, Secondary Fracture Prevention, and Perioperative Bone Health. Curr. Osteoporos. Rep. 2023, 21, 386–400. [Google Scholar] [CrossRef]
- Bryant, J.-P.; Perez-Roman, R.J.; Burks, S.S.; Wang, M.Y. Antiresorptive and anabolic medications used in the perioperative period of patients with osteoporosis undergoing spine surgery: Their impact on the biology of fusion and systematic review of the literature. Neurosurg. Focus 2021, 50, E13. [Google Scholar] [CrossRef] [PubMed]
- Ebata, S.; Takahashi, J.; Hasegawa, T.; Mukaiyama, K.; Isogai, Y.; Ohba, T.; Shibata, Y.; Ojima, T.; Yamagata, Z.; Matsuyama, Y.; et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months after Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J. Bone Jt. Surg.-Am. Vol. 2017, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Miyagi, M.; Suzuki, M.; et al. More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery. Asian Spine J. 2015, 9, 573. [Google Scholar] [CrossRef]
- Fatima, N.; Massaad, E.; Hadzipasic, M.; Shankar, G.M.; Shin, J.H. Assessment of the Efficacy of Teriparatide Treatment for Osteoporosis on Lumbar Fusion Surgery Outcomes: A Systematic Review and Meta-Analysis. Neurosurg. Rev. 2021, 44, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Maruo, K.; Tachibana, T.; Arizumi, F.; Kusuyama, K.; Kishima, K.; Yoshiya, S. Effect of Teriparatide on Subsequent Vertebral Fractures after Instrumented Fusion Surgery for Osteoporotic Vertebral Fractures with Neurological Deficits. Asian Spine J. 2019, 13, 283–289. [Google Scholar] [CrossRef]
- Inoue, G.; Ueno, M.; Nakazawa, T.; Imura, T.; Saito, W.; Uchida, K.; Ohtori, S.; Toyone, T.; Takahira, N.; Takaso, M. Teriparatide Increases the Insertional Torque of Pedicle Screws during Fusion Surgery in Patients with Postmenopausal Osteoporosis: Clinical Article. J. Neurosurg. Spine 2014, 21, 425–431. [Google Scholar] [CrossRef]
- Shibuya, Y.; Katsumi, K.; Ohashi, M.; Tashi, H.; Makino, T.; Yamazaki, A.; Hirano, T.; Sawakami, K.; Kikuchi, R.; Kawashima, H.; et al. Effect of Adjuvant Therapy with Teriparatide in Patients with Thoracolumbar Osteoporotic Vertebral Fractures Who Underwent Vertebroplasty with Posterior Spinal Fusion. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Seki, S.; Hirano, N.; Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Suzuki, K.; Watanabe, K.; Makino, H.; Kanamori, M.; Kimura, T. Teriparatide versus Low-Dose Bisphosphonates before and after Surgery for Adult Spinal Deformity in Female Japanese Patients with Osteoporosis. Eur. Spine J. 2017, 26, 2121–2127. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Teriparatide Accelerates Lumbar Posterolateral Fusion in Women with Postmenopausal Osteoporosis: Prospective Study. Spine 2012, 37, E1464–E1468. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.W.; Kim, Y.B.; Ko, M.J. The Effect of Postoperative Use of Teriparatide Reducing Screw Loosening in Osteoporotic Patients. J. Korean Neurosurg. Soc. 2018, 61, 494–502. [Google Scholar] [CrossRef]
- Dimar, J.; Bisson, E.F.; Dhall, S.; Harrop, J.S.; Hoh, D.J.; Mohamed, B.; Wang, M.C.; Mummaneni, P.V. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Perioperative Spine: Preoperative Osteoporosis Assessment. Neurosurgery 2021, 89, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Sardar, Z.M.; Coury, J.R.; Cerpa, M.; DeWald, C.J.; Ames, C.P.; Shuhart, C.; Shuhart, C.; Watkins, C.; Polly, D.W.; Dirschl, D.R.; et al. Best Practice Guidelines for Assessment and Management of Osteoporosis in Adult Patients Undergoing Elective Spinal Reconstruction. Spine 2022, 47, 128–135. [Google Scholar] [CrossRef]
- Arlt, H.; Besschetnova, T.; Ominsky, M.S.; Fredericks, D.C.; Lanske, B. Effects of Systemically Administered Abaloparatide, an Osteoanabolic PTHrP Analog, as an Adjuvant Therapy for Spinal Fusion in Rats. JOR Spine 2021, 4, e1132. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sone, T.; Soen, S.; Tanaka, S.; Yamashita, A.; Inoue, T. Abaloparatide Increases Lumbar Spine and Hip BMD in Japanese Patients with Osteoporosis: The Phase 3 ACTIVE-J Study. J. Clin. Endocrinol. Metab. 2022, 107, e4222–e4231. [Google Scholar] [CrossRef]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.; Harris, A.G.; et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef] [PubMed]

| Class | Agent | Brand Name | Mechanism of Action |
|---|---|---|---|
| Vitamin and mineral supplements | Calcium | N/A | The main mineral component of bone. Forms calcium salts (mostly calcium phosphate) by osteoblasts which harden cartilaginous bone matrices and thus bone building |
| Vitamin D | N/A | Activates intestinal absorption of calcium and maintaining calcium homeostasis | |
| Antiresorptive agents | Bisphosphonates | Reclast, Boniva, Fosamax, Zoneta, Actonel Aclasta | Inhibits osteoclast function, thus allowing osteoblasts to more efficiently build bone mass |
| Anabolic agents | Denosumab | Prolia | Monoclonal antibody that inhibits receptor activator of nuclear factor kappa-B ligand (RANKL), resulting in decreased osteoclast development |
| Romosozumab | Evenity | Monoclonal antibody that binds and inhibits sclerostin, a protein secreted by osteocytes that inhibits osteoblast function increases RANKL which activates osteoclasts (PMID: 30775535). Thus, romosozumab is unique in that it increases bone formation and decreases bone resorption | |
| Parathyroid hormone (PTH) analogs | Teriparatide | Forteo | Regulates calcium and phosphate metabolism in bone and the kidneys. Counterintuitively increases bone resorption, thus resulting in increased serum calcium levels. However, low-dose and intermittent exposure (i.e., once daily) disproportionately activate osteoblasts with increased serum calcium more than osteoclast function, thus having a net effect of increased bone mineral density |
| Abaloparatide | Tymlos | Similar to teriparatide, but with different pharmacokinetics that may confer some advantages in bone mineral density improvements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Najjar, Y.A.; Quraishi, D.A.; Kumar, N.; Hussain, I. Bone Health Optimization in Adult Spinal Deformity Patients: A Narrative Review. J. Clin. Med. 2024, 13, 4891. https://doi.org/10.3390/jcm13164891
Al-Najjar YA, Quraishi DA, Kumar N, Hussain I. Bone Health Optimization in Adult Spinal Deformity Patients: A Narrative Review. Journal of Clinical Medicine. 2024; 13(16):4891. https://doi.org/10.3390/jcm13164891
Chicago/Turabian StyleAl-Najjar, Yousef A., Danyal A. Quraishi, Neerav Kumar, and Ibrahim Hussain. 2024. "Bone Health Optimization in Adult Spinal Deformity Patients: A Narrative Review" Journal of Clinical Medicine 13, no. 16: 4891. https://doi.org/10.3390/jcm13164891






