Pallidal Versus Subthalamic Deep-Brain Stimulation for Generalized Isolated Dystonia: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Procedure and Programming
2.3. Clinical Evaluation
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
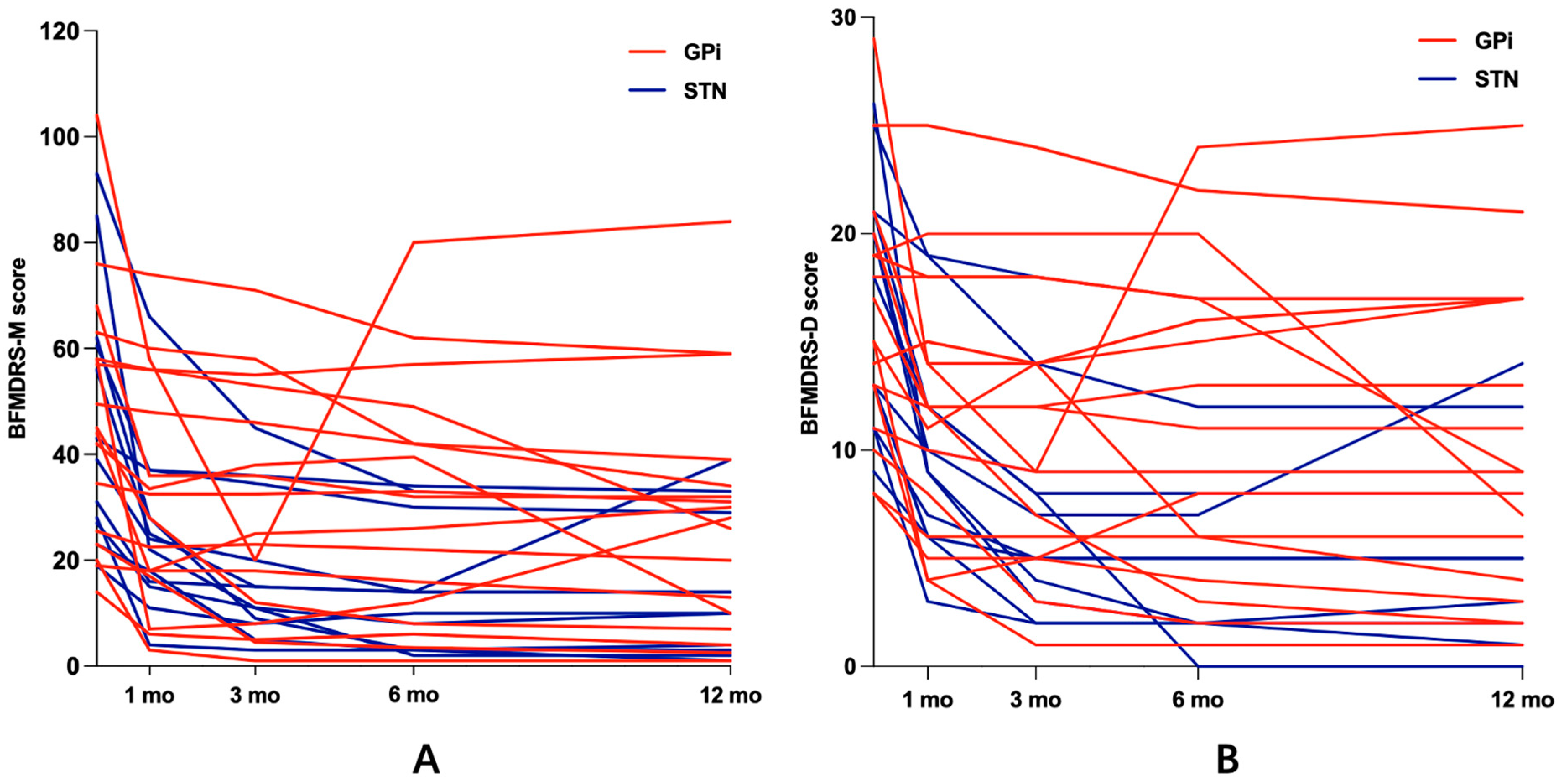
3.2. Motor Function
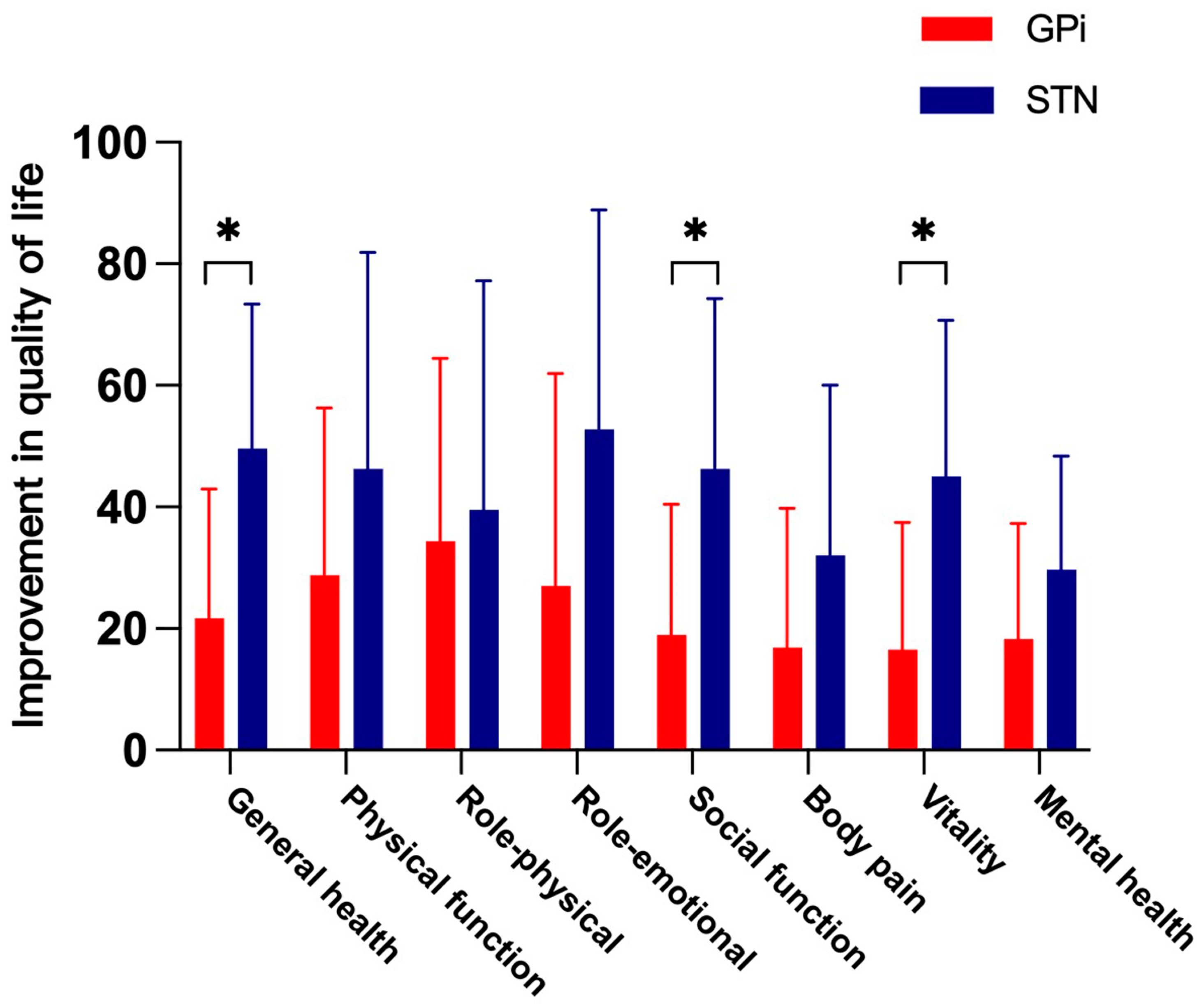
3.3. Health-Related Quality of Life
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Sadnicka, A.; Meppelink, A.M.; Kalinowski, A.; Oakeshott, P.; van den Dool, J. Dystonia. BMJ 2022, 377, e062659. [Google Scholar] [CrossRef]
- Vercueil, L.; Pollak, P.; Fraix, V.; Caputo, E.; Moro, E.; Benazzouz, A.; Xie, J.; Koudsie, A.; Benabid, A.L. Deep brain stimulation in the treatment of severe dystonia. J. Neurol. 2001, 248, 695–700. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Wang, X.; Wang, J.; Wang, X.; Wang, W. Long-Term Outcome of Subthalamic Deep Brain Stimulation for Generalized Isolated Dystonia. Neuromodulation 2022, 26, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Hock, A.N.; Jensen, S.R.; Svaerke, K.W.; Brennum, J.; Jespersen, B.; Bergdal, O.; Karlsborg, M.; Hjermind, L.E.; Lokkegaard, A. Randomised double-blind controlled study of Deep Brain Stimulation for dystonia in STN or GPi—A long term follow-up after up to 15 years. Park. Relat. Disord. 2022, 96, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Pan, Y.; Zhang, C.; Zhang, J.; Qiu, X.; Zhan, S.; Li, D.; Sun, B. Subthalamic deep brain stimulation in patients with primary dystonia: A ten-year follow-up study. Park. Relat. Disord. 2018, 55, 103–110. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Y.; Li, H.; Zhang, C.; Wang, T.; Pan, Y.; He, L.; Shen, R.; Deng, Z.; Sun, B.; et al. Deep brain stimulation of the globus pallidus internus versus the subthalamic nucleus in isolated dystonia. J. Neurosurg. 2019, 132, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Schjerling, L.; Hjermind, L.E.; Jespersen, B.; Madsen, F.F.; Brennum, J.; Jensen, S.R.; Lokkegaard, A.; Karlsborg, M. A randomized double-blind crossover trial comparing subthalamic and pallidal deep brain stimulation for dystonia. J. Neurosurg. 2013, 119, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Lozano, A.M.; Kou, N.; Munhoz, R.P.; Fasano, A. Programming Deep Brain Stimulation for Tremor and Dystonia: The Toronto Western Hospital Algorithms. Brain Stimul. 2016, 9, 438–452. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Mundinger, F. New stereotactic treatment of spasmodic torticollis with a brain stimulation system (author’s transl). Med. Klin. 1977, 72, 1982–1986. [Google Scholar]
- Kim, J.P.; Chang, W.S.; Park, Y.S.; Chang, J.W. Effects of relative low-frequency bilateral globus pallidus internus stimulation for treatment of cervical dystonia. Stereotact. Funct. Neurosurg. 2012, 90, 30–36. [Google Scholar] [CrossRef]
- Kupsch, A.; Benecke, R.; Muller, J.; Trottenberg, T.; Schneider, G.H.; Poewe, W.; Eisner, W.; Wolters, A.; Muller, J.U.; Deuschl, G.; et al. Deep-Brain Stimulation for Dystonia Study, Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 2006, 355, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J.; Wolters, A.; Kupsch, A.; Muller, J.; Kuhn, A.A.; Schneider, G.H.; Poewe, W.; Hering, S.; Eisner, W.; Muller, J.U.; et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012, 11, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, G.; Jiang, Y.; Wang, X.; Chen, Y.; Meng, F.; Zhang, K.; Yang, A.; Liu, H.; Zhang, X.; et al. Comparison of Short-Term Stimulation of the Globus Pallidus Interna and Subthalamic Nucleus for Treatment of Primary Dystonia. World Neurosurg. 2019, 123, e211–e217. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Pan, Y.; Li, D.; Zhan, S.; Zhang, J.; Sun, B. Subthalamus deep brain stimulation for primary dystonia patients: A long-term follow-up study. Mov. Disord. 2013, 28, 1877–1882. [Google Scholar] [CrossRef]
- Marsden, C.D.; Obeso, J.A. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain 1994, 117 Pt 4, 4877–4897. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 1995, 20, 128–154. [Google Scholar] [CrossRef]
- Yin, F.; Zhao, M.; Yan, X.; Li, T.; Chen, H.; Li, J.; Cao, S.; Guo, H.; Liu, S. Bilateral subthalamic nucleus deep brain stimulation for refractory isolated cervical dystonia. Sci. Rep. 2022, 12, 7678. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Czernecki, V.; Zurowski, A.M. Neuropsychological, neuropsychiatric, and quality of life issues in DBS for dystonia. Mov. Disord. 2011, 26 (Suppl. S1), S63–S78. [Google Scholar] [CrossRef]
- Soeder, A.; Kluger, B.M.; Okun, M.S.; Garvan, C.W.; Soeder, T.; Jacobson, C.E.; Rodriguez, R.L.; Turner, R.; Fernandez, H.H. Mood and energy determinants of quality of life in dystonia. J. Neurol. 2009, 256, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.H.; Doig-Beyaert, K.; Eliasziw, M.; Tsui, J.; Haffenden, A.; Suchowersky, O. Stereotactic Section of the Canadian Neurosurgical, G. Canadian Movement Disorders, The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain 2007, 130 Pt 11, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Kleiner-Fisman, G.; Liang, G.S.; Moberg, P.J.; Ruocco, A.C.; Hurtig, H.I.; Baltuch, G.H.; Jaggi, J.L.; Stern, M.B. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: Impact on severity, neuropsychological status, and quality of life. J. Neurosurg. 2007, 107, 29–36. [Google Scholar] [CrossRef] [PubMed]
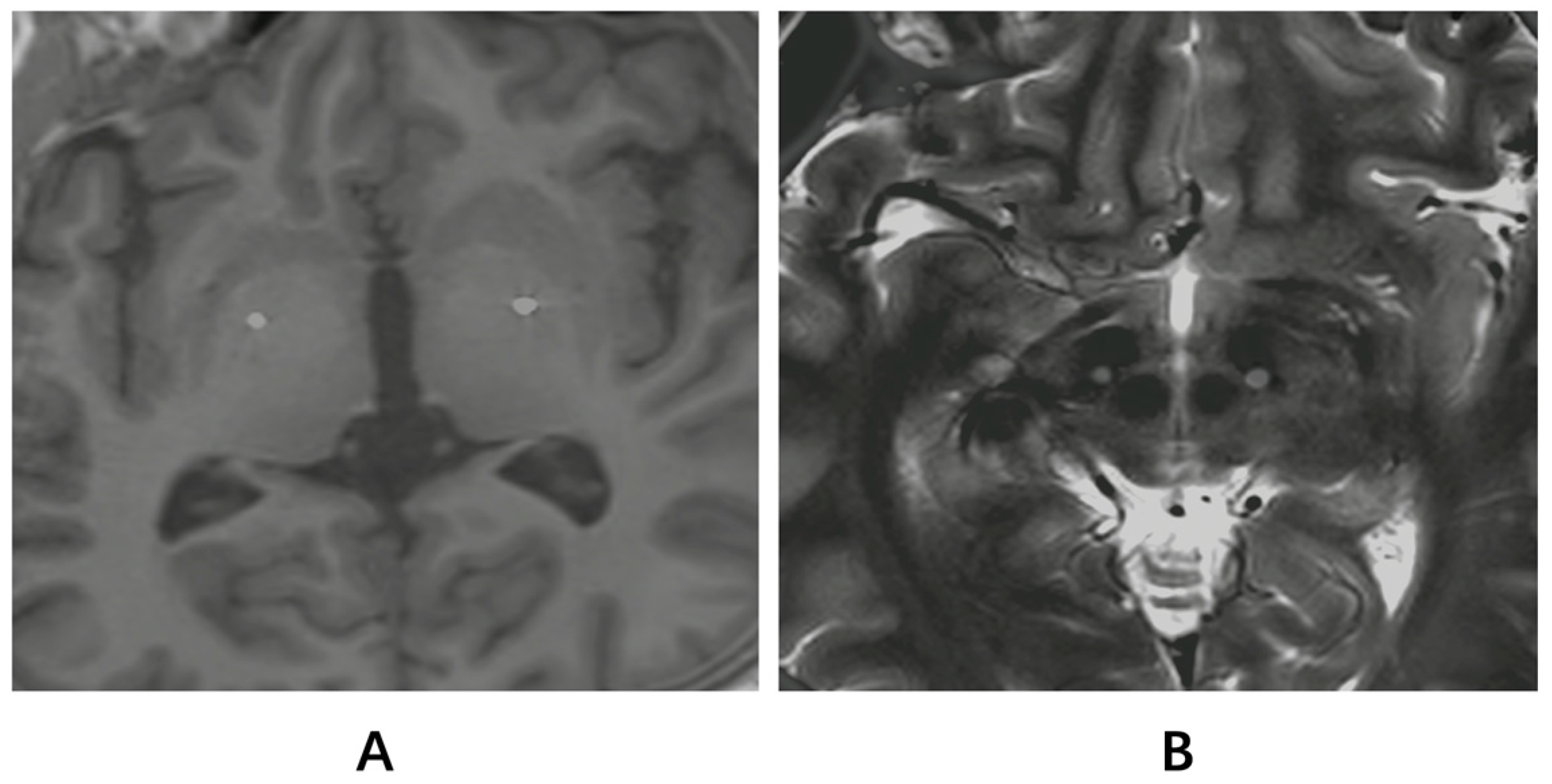
| Case | Targets | Sex | Age at Onset (yrs) | Age at Surgery (yrs) | Duration of Symptoms (mos) | Gene Mutation | Stimulation Parameters with Best Response (Contact, Pulse Width, Frequency, Amplitude, Left/Right) |
|---|---|---|---|---|---|---|---|
| 1 | GPi | F | 1 | 22 | 252 | NA | Case(+)5(−)/Case(+)2(−), 60/60, 170/170, 2.8/3.2 |
| 2 | GPi | M | 1 | 25 | 288 | No | Case(+)6(−)/Case(+)2(−), 60/60, 140/140, 3.2/3.5 |
| 3 | GPi | M | 27 | 29 | 16 | NA | Case(+)5(−)6(−)/Case(+)2(−)3(−), 60/70, 160/160, 3.5/3.0 |
| 4 | GPi | F | 40 | 45 | 60 | NA | Case(+)6(−)/Case(+)1(−), 60/60, 130/130, 3.4/3.0 |
| 5 | GPi | M | 37 | 40 | 36 | No | Case(+)6(−)/Case(+)2(−), 60/60, 150/150, 3.5/3.2 |
| 6 | GPi | F | 4 | 9 | 60 | No | Case(+)6(−)7(−)/Case(+)1(−)2(−), 90/70, 160/160, 2.9/2.8 |
| 7 | GPi | M | 5 | 29 | 288 | NA | Case(+)7(−)/Case(+)3(−), 60/60, 130/130, 3.1/2.8 |
| 8 | GPi | M | 8 | 12 | 48 | NA | Case(+)6(−)7(−)/Case(+)1(−)2(−), 100/70, 145/145, 2.95/2.85 |
| 9 | GPi | M | 9 | 10 | 6 | TOR1A | Case(+)5(−)/Case(+)1(−), 70/80, 150/150, 2.0/2.75 |
| 10 | GPi | F | 36 | 38 | 24 | NA | Case(+)5(−)6(−)/Case(+)1(−)2(−), 70/80, 140/140, 2.7/2.9 |
| 11 | GPi | M | 6 | 14 | 96 | TOR1A | Case(+)5(−)/Case(+)1(−)3(−), 80/110, 145/145, 2.65/3.55 |
| 12 | GPi | M | 8 | 12 | 48 | No | Case(+)6(−)7(−)8(−)/Case(+)2(−)4(−), 60/100, 130/130, 3.5/3.2 |
| 13 | GPi | M | 7 | 24 | 204 | GNAL | Case(+)5(−)/Case(+)2(−), 90/90, 170/170, 3.4/3.2 |
| 14 | GPi | M | 8 | 21 | 156 | TOR1A | Case(+)5(−)6(−)/Case(+)1(−)2(−), 80/80, 155/155, 2.2/2.7 |
| 15 | GPi | F | 43 | 45 | 24 | NA | Case(+)5−/Case(+)2(−), 90/70, 140/140, 3.4/3.4 |
| 16 | GPi | F | 47 | 50 | 36 | NA | Case(+)6(−)/Case(+)2(−), 90/90, 130/130, 3.4/3.2 |
| 17 | GPi | F | 4 | 19 | 180 | No | Case(+)9(−)/Case(+)1(−), 60/60, 130/130, 2.6/2.7 |
| 18 | STN | M | 8 | 13 | 60 | KMT2B | Case(+)7(−)/Case(+)2(−), 80/60, 140/140, 2.1/2 |
| 19 | STN | M | 18 | 19 | 12 | NA | Case(+)10(−)/Case(+)2(−), 60/60, 130/130, 1.9/2 |
| 20 | STN | M | 66 | 68 | 24 | No | Case(+)6(−)/Case(+)3(−), 80/70, 150/150, 2/2.25 |
| 21 | STN | F | 29 | 32 | 36 | No | Case(+)5(−)/Case(+)2(−)3(−), 80/80, 130/130, 2.5/2.4 |
| 22 | STN | F | 7 | 12 | 60 | NA | Case(+)6(−)/Case(+)2(−), 90/80, 145/145, 2.35/2.25 |
| 23 | STN | M | 4 | 13 | 108 | No | Case(+)6(−)/Case(+)2(−), 60/60, 130/130, 2.2/2.2 |
| 24 | STN | M | 8 | 10 | 24 | TOR1A | Case(+)5(−)8(−)/Case(+)1(−)4(−), 70/70, 130/130, 1.5/1.45 |
| 25 | STN | F | 5 | 6 | 12 | ANO3 | Case(+)9(−)/Case(+)1(−), 60/60, 110/110, 1.8/1.9 |
| 26 | STN | M | 3 | 23 | 240 | No | Case(+)10(−)/Case(+)2(−), 60/60, 130/130, 1.9/1.8 |
| 27 | STN | F | 6 | 15 | 84 | No | Case(+)5(−)6(−)/Case(+)1(−)2(−), 60/70, 130/130, 1.5/1.55 |
| 28 | STN | M | 9 | 14 | 60 | No | Case(+)8(−)/Case(+)4(−), 70/60, 140/140, 2.4/1.5 |
| 29 | STN | M | 6 | 11 | 60 | No | Case(+)7(−)/Case(+)3(−), 60/60, 130/130, 1.9/2.1 |
| Characteristic | GPi Group | STN Group | p Value |
|---|---|---|---|
| Demographic or clinical | |||
| Age at surgery (yrs) | 26.12 ± 13.3 | 19.67 ± 16.67 | 0.116 & |
| Age at onset (yrs) | 17.12 ± 16.75 | 14.08 ± 17.89 | 0.739 & |
| % males | 58.82% | 66.67% | 0.717 # |
| % TOR1A mutation | 17.65% | 8.33% | 0.622 # |
| Symptom duration (mos) | 107.18 ± 99.22 | 65 ± 87.18 | 0.422 & |
| Functional status | |||
| BFMDRS-M (0–120) | 47.09 ± 23.68 | 47.21 ± 24.32 | 0.989 * |
| Eye (0–8) | 2.60 ± 2.16 | 2.75 ± 1.77 | NA |
| Mouth (0–8) | 3.19 ± 2.72 | 2.33 ± 0.58 | 0.916 & |
| Speech (0–16) | 5.00 ± 4.72 | 5.20 ± 3.56 | 0.533 & |
| Neck (0–8) | 5.80 ± 2.21 | 4.64 ± 2.87 | 0.332 & |
| Trunk (0–16) | 6.00 ± 4.27 | 8.00 ± 5.38 | 0.312 & |
| Arm (0–32) | 13.88 ± 7.94 | 17.36 ± 9.85 | 0.312 * |
| Legs (0–32) | 16.50 ± 10.05 | 16.08 ± 9.06 | 0.834 & |
| BFMDRS-D (0–30) | 16.18 ± 5.74 | 17.33 ± 5.74 | 0.597 * |
| Speech (0–4) | 1.80 ± 1.08 | 2.20 ± 1.10 | 0.451 & |
| Writing (0–4) | 2.29 ± 1.10 | 2.73 ± 1.10 | 0.315 & |
| Feeding (0–4) | 2.44 ± 1.36 | 3.09 ± 0.94 | 0.177 & |
| Eating and swallowing (0–4) | 1.56 ± 0.88 | 1.20 ± 0.45 | 0.503 & |
| Hygiene (0–4) | 2.71 ± 1.10 | 3.08 ± 0.90 | 0.380 & |
| Dressing (0–4) | 2.59 ± 1.23 | 3.00 ± 1.04 | 0.367 & |
| Walking (0–6) | 4.31 ± 1.35 | 4.42 ± 1.31 | 0.718 & |
| Quality of life | |||
| SF-36 score | |||
| General health (0–100) | 35 ± 12.78 | 27.92 ± 12.70 | 0.158 * |
| Physical function (0–100) | 30.94 ± 23.40 | 18.75 ± 21.33 | 0.175 & |
| Role, physical (0–100) | 20.31 ± 24.53 | 18.75 ± 11.31 | 0.678 & |
| Role, emotional (0–100) | 27.08 ± 30.36 | 22.22 ± 32.83 | 0.544 & |
| Social function (0–100) | 35.41 ± 26.69 | 21.28 ± 18.61 | 0.212 & |
| Body pain (0–100) | 56.69 ± 31.37 | 50.17 ± 28.53 | 0.555 & |
| Vitality (0–100) | 45.32 ± 23.63 | 40 ± 24.77 | 0.569 & |
| Mental health (0–100) | 39.75 ± 12.94 | 44 ± 11.44 | 0.329 & |
| BFMDRS-M | p Value a | p Value (Mean Improvement %) b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Pre | 1 mo | 3 mo | 6 mo | 12 mo | 1 mo vs. Pre | 3 mo vs. 1 mo | 6 mo vs. 3 mo | 12 mo vs. 6 mo | 1 mo | 3 mo | 6 mo | 12 mo | |
| Total | ||||||||||||||
| GPi | 17 | 47.09 ± 23.68 | 34.09 ± 21.64 | 29.76 ± 21.25 | 30.88 ± 22.52 | 28.21 ± 22.72 | 0.000 ¥ | 0.018 ¥ | 0.153 ¥ | 0.221 ¥ | (28.8%) | (37.32%) | (38.36%) | (45.73%) |
| STN | 12 | 47.21 ± 24.32 | 25.25 ± 16.11 | 17.71 ± 13.55 | 14 ± 11.97 | 15.83 ± 13.57 | 0.001 # | 0.002 ¥ | 0.008 # | 1 ¥ | 0.069 & (45.58%) | 0.057 & (61.8%) | 0.008 & (63.91%) | 0.060 * (66.37%) |
| Eye | ||||||||||||||
| GPi | 5 | 2.6 ± 2.16 | 1.8 ± 1.68 | 1.9 ± 1.67 | 2 ± 2.55 | 1.4 ± 2.61 | 0.099 # | 0.317 ¥ | 0.854 # | 0.317 ¥ | (34.7%) | (30.67%) | (52%) | (72%) |
| STN | 2 | 2.75 ± 1.77 | 0.25 ± 0.35 | 0.25 ± 0.35 | 0 ± 0 | 0 ± 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Mouth | ||||||||||||||
| GPi | 8 | 3.19 ± 2.72 | 2.13 ± 2.10 | 1.75 ± 2.05 | 1.88 ± 2.3 | 2 ± 2.2 | 0.039 ¥ | 0.180 ¥ | 0.655 ¥ | 0.317 ¥ | (39.58%) | (55.21%) | (64.58%) | (52.08%) |
| STN | 3 | 2.33 ± 0.58 | 2 ± 1 | 1.33 ± 1.53 | 1 ± 1.73 | 1 ± 1.73 | 0.317 ¥ | 0.157 ¥ | 0.317 ¥ | NA | 0.411 & (16.67%) | 0.869 * (50%) | 0.911 & (66.67%) | 0.665 & |
| Face (eyes and mouth) | ||||||||||||||
| GPi | 9 | 4.28 ± 2.53 | 2.89 ± 2.07 | 2.61 ± 1.96 | 2.78 ± 2.39 | 2.56 ± 2.46 | 0.013 ¥ | 0.285 ¥ | 0.681 # | 0.655 ¥ | (38.70%) | (42.05%) | (49.23%) | (52.93%) |
| STN | 4 | 3.13 ± 2.02 | 1.63 ± 1.11 | 1.13 ± 1.31 | 0.75 ± 1.5 | 0.75 ± 1.5 | 0.297 # | 0.157 ¥ | 0.215 # | NA | 0.962 * (37.50%) | 0.634 * (54.17%) | 0.369 & (75%) | 0.416 & |
| Speech and swallowing | ||||||||||||||
| GPi | 15 | 5 ± 4.72 | 4.2 ± 4 | 4.07 ± 3.83 | 3.93 ± 3.58 | 4.07 ± 4.08 | 0.167 ¥ | 0.854 ¥ | 1 ¥ | 0.785 ¥ | (20%) | (18.33%) | (21.11%) | (29.44%) |
| STN | 5 | 5.2 ± 3.56 | 2.6 ± 1.34 | 2.20 ± 1.1 | 2.00 ± 1.22 | 2.00 ± 1.22 | 0.144 # | 0.317 ¥ | 0.317 ¥ | NA | 0.415 & (36.67%) | 0.348 & (41.11%) | 0.454 & (43.33%) | 0.786 & |
| Neck | ||||||||||||||
| GPi | 15 | 5.80 ± 2.21 | 4.87 ± 2.9 | 3.63 ± 2.91 | 3.5 ± 2.76 | 2.9 ± 2.61 | 0.066 ¥ | 0.016 ¥ | 0.715 ¥ | 0.131 ¥ | (20%) | (44.44%) | (45.28%) | (53.06%) |
| STN | 11 | 4.64 ± 2.87 | 2.27 ± 1.74 | 1.73 ± 1.62 | 0.73 ± 0.79 | 0.91 ± 0.83 | 0.002 # | 0.063 ¥ | 0.026 ¥ | 0.317 ¥ | 0.013 & (50%) | 0.177 & (65.15%) | 0.028 & (82.58%) | 0.215 & (73.48%) |
| Trunk | ||||||||||||||
| GPi | 17 | 6 ± 4.27 | 4.35 ± 4.24 | 3.77 ± 4.28 | 3.9 ± 3.76 | 2.65 ± 3.32 | 0.003 ¥ | 0.228 ¥ | 0.892 ¥ | 0.039 ¥ | (29.74%) | (36.60%) | (39.05%) | (54.74%) |
| STN | 12 | 8 ± 5.38 | 4.08 ± 2.39 | 2.25 ± 1.66 | 1.92 ± 1.68 | 2.08 ± 2.31 | 0.018 # | 0.002 # | 0.180 ¥ | 0.713 ¥ | 0.712 & (33.33%) | 0.087 & (60.42%) | 0.056 & (65.28%) | 0.713 ¥ (69.10%) |
| Axial (neck and trunk) | ||||||||||||||
| GPi | 17 | 11.12 ± 6.08 | 8.65 ± 6.47 | 6.97 ± 6.17 | 6.97 ± 5.52 | 5.21 ± 5.04 | 0.003 ¥ | 0.039 ¥ | 0.438 ¥ | 0.075 ¥ | (25.04%) | (38.78%) | (40.93%) | (52.29%) |
| STN | 12 | 12.25 ± 7.4 | 6.17 ± 3.66 | 3.83 ± 2.92 | 2.58 ± 2.02 | 2.92 ± 2.78 | 0.002 # | 0.001 # | 0.016 ¥ | 0.713 ¥ | 0.061 & (40.86%) | 0.105 & (60.15%) | 0.039 & (69.56%) | 0.296 & (68.08%) |
| Arms | ||||||||||||||
| GPi | 17 | 13.88 ± 7.94 | 8.85 ± 6.11 | 7.651 ± 5.92 | 8.5 ± 7.39 | 8.24 ± 7.44 | 0.003 ¥ | 0.072 ¥ | 0.726 ¥ | 0.436 ¥ | (29.49%) | (36.75%) | (34.21%) | (38.47%) |
| STN | 11 | 17.36 ± 9.85 | 8.27 ± 5.55 | 4.64 ± 2.69 | 3.27 ± 2.61 | 4.18 ± 4.07 | 0.002 # | 0.011 ¥ | 0.027 ¥ | 0.414 ¥ | 0.123 & (45.99%) | 0.028 & (70.64%) | 0.001 & (80.66%) | 0.002 * (76.89%) |
| Legs | ||||||||||||||
| GPi | 16 | 16.5 ± 10.05 | 12.06 ± 8.65 | 10.88 ± 8.76 | 10.75 ± 8.19 | 10.63 ± 8.94 | 0.018 ¥ | 0.223 ¥ | 0.473 ¥ | 0.779 ¥ | (24.99%) | (33.88%) | (35.25%) | (40.88%) |
| STN | 12 | 16.08 ± 9.06 | 10.08 ± 8.44 | 8.33 ± 8.44 | 7.33 ± 7.54 | 7.83 ± 7.95 | 0.001 # | 0.042 ¥ | 0.089 # | 0.593 ¥ | 0.101 & (41.95%) | 0.122 & (54.57%) | 0.065 & (59.15%) | 0.260 * (57.24%) |
| Limbs (arms and legs) | ||||||||||||||
| GPi | 17 | 29.41 ± 16.64 | 20.21 ± 14.14 | 17.88 ± 13.92 | 18.61 ± 14.61 | 18.24 ± 15.77 | 0.002 ¥ | 0.123 ¥ | 0.288 ¥ | 0.770 # | (28.99%) | (36.64%) | (36.39%) | (41.75%) |
| STN | 12 | 32.17 ± 18.35 | 17.67 ± 13.08 | 12.58 ± 10.86 | 10.33 ± 9.55 | 11.67 ± 11.02 | 0.001 # | 0.012 ¥ | 0.015 # | 0.680 ¥ | 0.092 & (46.08%) | 0.073 & (60.29%) | 0.024 & (65.58%) | 0.057 & (63.38%) |
| BFMDRS-D | p Value a | p Value (Mean Improvement %) b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Pre | 1 mo | 3 mo | 6 mo | 12 mo | 1 mo vs. Pre | 3 mo vs. 1 mo | 6 mo vs. 3 mo | 12 mo vs. 6 mo | 1 mo | 3 mo | 6 mo | 12 mo | |
| Total | ||||||||||||||
| GPi | 17 | 16.18 ± 5.74 | 12.24 ± 5.89 | 11.24 ± 6.43 | 11.41 ± 7.25 | 10.12 ± 7.19 | 0.002 ¥ | 0.082 ¥ | 0.670 ¥ | 0.227 ¥ | (24.46%) | (30.62%) | (30.73%) | (37.79%) |
| STN | 12 | 17.33 ± 5.74 | 10.17 ± 4.88 | 7.08 ± 4.85 | 5.92 ± 4.98 | 6.50 ± 5.52 | 0.000 # | 0.000 # | 0.041 ¥ | 0.414 ¥ | 0.051 * (41.62%) | 0.009 * (59.73%) | 0.005 * (65.56%) | 0.05 * (63.08%) |
| Speech | ||||||||||||||
| GPi | 15 | 1.80 ± 1.08 | 1.47 ± 0.99 | 1.47 ± 1.06 | 1.60 ± 1.18 | 1.47 ± 1.25 | 0.059 ¥ | 1 ¥ | 0.458 ¥ | 0.157 ¥ | (18.89%) | (17.22%) | (13.89%) | (23.89%) |
| STN | 5 | 2.20 ± 1.10 | 1.40 ± 1.14 | 1 ± 1.22 | 1 ± 1.23 | 1 ± 1.22 | 0.099 # | 0.157 ¥ | NA | NA | 0.218 & (40%) | 0.151 & (53.33%) | 0.204 & (53.33%) | 0.05 * (53.33%) |
| Writing | ||||||||||||||
| GPi | 17 | 2.29 ± 1.10 | 1.76 ± 0.97 | 1.65 ± 0.93 | 1.71 ± 0.99 | 1.71 ± 1.10 | 0.024 ¥ | 0.480 ¥ | 0.655 ¥ | 1 ¥ | (20.10%) | (15.20%) | (13.73%) | (5.88%) |
| STN | 11 | 2.73 ± 1.10 | 1.45 ± 0.82 | 1 ± 1.22 | 0.91 ± 0.54 | 1 ± 0.63 | 0.00 ¥ | 0.025 ¥ | 0.317 ¥ | 0.317 ¥ | 0.03 & (46.21%) | 0.002 & (66.67%) | 0.001 & (68.94%) | 0.003 & (65.91%) |
| Feeding | ||||||||||||||
| GPi | 16 | 2.44 ± 1.36 | 1.50 ± 1.21 | 1.38 ± 1.26 | 1.44 ± 1.46 | 1.06 ± 1.12 | 0.017 ¥ | 0.157 ¥ | 1 ¥ | 0.059 ¥ | (31.25%) | (39.06%) | (36.46%) | (47.92%) |
| STN | 11 | 3.09 ± 0.94 | 1.45 ± 0.93 | 1 ± 0.89 | 0.73 ± 0.90 | 0.82 ± 0.98 | 0.003 ¥ | 0.059 ¥ | 0.18 ¥ | 0.317 ¥ | 0.095 & (56.06%) | 0.114 & (67.42%) | 0.048 & (74.24%) | 0.174 & (71.97%) |
| Eating and swallowing | ||||||||||||||
| GPi | 9 | 1.56 ± 0.88 | 1.00 ± 1.00 | 1.11 ± 0.93 | 1.22 ± 0.97 | 1.22 ± 0.97 | 0.059 ¥ | 0.317 ¥ | 0.655 ¥ | NA | (41%) | (29.63%) | (25.93%) | (25.93%) |
| STN | 5 | 1.20 ± 0.45 | 0.80 ± 0.44 | 0.80 ± 0.45 | 0.60 ± 0.55 | 0.80 ± 0.45 | 0.157 ¥ | NA | 0.317 ¥ | 0.317 ¥ | 0.708 & (30%) | 0.938 & (30%) | 0.693 & (40%) | 0.815 & (30%) |
| Hygiene | ||||||||||||||
| GPi | 17 | 2.71 ± 1.10 | 2.06 ± 1.14 | 1.82 ± 1.29 | 1.59 ± 1.33 | 1.29 ± 1.21 | 0.016 ¥ | 0.046 ¥ | 0.317 ¥ | 0.276 ¥ | (23.53%) | (31.37%) | (37.75%) | (45.10%) |
| STN | 12 | 3.08 ± 0.90 | 1.92 ± 0.90 | 1.25 ± 0.97 | 0.92 ± 0.90 | 0.92 ± 1.08 | 0.001 # | 0.005 ¥ | 0.180 ¥ | 1 ¥ | 0.175 & (36.81%) | 0.033 & (61.11%) | 0.041 & (70.14%) | 0.1 & (72.22%) |
| Dressing | ||||||||||||||
| GPi | 17 | 2.59 ± 1.23 | 1.88 ± 1.32 | 1.59 ± 1.23 | 1.65 ± 1.41 | 1.35 ± 1.27 | 0.018 ¥ | 0.025 ¥ | 0.655 ¥ | 0.059 ¥ | (31.86%) | (39.71%) | (36.72%) | (48.53%) |
| STN | 12 | 3.00 ± 1.04 | 1.58 ± 1.08 | 1 ± 1.04 | 0.67 ± 0.78 | 0.83 ± 1.03 | 0.003 ¥ | 0.008 ¥ | 0.102 ¥ | 0.317 ¥ | 0.173 & (51.39%) | 0.074 & (68.75%) | 0.031 & (77.78%) | 0.134 & (73.61%) |
| Walking | ||||||||||||||
| GPi | 16 | 4.31 ± 1.35 | 3.38 ± 1.67 | 3.13 ± 1.78 | 3 ± 1.71 | 2.69 ± 1.58 | 0.007 ¥ | 0.102 ¥ | 0.680 ¥ | 0.518 ¥ | (23.75%) | (30.52%) | (32.29%) | (35.63%) |
| STN | 12 | 4.42 ± 1.31 | 3.08 ± 1.38 | 2.33 ± 1.56 | 2.17 ± 1.70 | 2.33 ± 1.78 | 0.003 # | 0.011 ¥ | 0.157 ¥ | 0.317 ¥ | 0.567 & (28.33) | 0.087 & (50.14%) | 0.099 # (53.47%) | 0.297 * (50.14%) |
| GPi | STN | |||||
|---|---|---|---|---|---|---|
| No. of Patients | 16 | 12 | ||||
| SF-36 Subscale | Pre | 12 Month | p Value | Pre | 12 Month | p Value |
| General health (range, 0–100) | 35 ± 12.78 | 56.69 ± 22.17 | 0.001 # | 27.92 ± 12.7 | 77.5 ± 24.07 | <0.001 # |
| Physical function (range, 0–100) | 30.94 ± 23.40 | 59.69 ± 30.96 | 0.001 # | 18.7 ± 21.33 | 65 ± 36.49 | 0.002 ¥ |
| Role—physical (range, 0–100) | 20.31 ± 24.53 | 54.69 ± 36.76 | 0.003 ¥ | 18.75 ± 11.31 | 58.33 ± 37.45 | 0.004 # |
| Role—emotional (range, 0–100) | 27.08 ± 30.36 | 54.16 ± 36.27 | 0.007 # | 22.22 ± 32.83 | 75 ± 28.88 | <0.001 # |
| Social function (range, 0–100) | 35.41 ± 26.69 | 54.33 ± 29.51 | 0.003 ¥ | 21.28 ± 18.61 | 67.58 ± 30.52 | <0.001 # |
| Body pain (range, 0–100) | 56.69 ± 31.37 | 73.56 ± 28 | 0.008 ¥ | 50.17 ± 28.53 | 82.17 ± 23.33 | 0.002 # |
| Vitality (range, 0–100) | 45.31 ± 23.63 | 61.88 ± 25.55 | 0.002 ¥ | 40 ± 24.77 | 85 ± 13.98 | <0.001 # |
| Mental health (range, 0–100) | 39.75 ± 12.94 | 58 ± 23.87 | 0.002 # | 44 ± 11.44 | 73.67 ± 21.40 | <0.001 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhu, G.; Gan, Y.; Meng, F.; Yang, A.; Zhang, J. Pallidal Versus Subthalamic Deep-Brain Stimulation for Generalized Isolated Dystonia: A Retrospective Study. J. Clin. Med. 2024, 13, 4902. https://doi.org/10.3390/jcm13164902
Wu J, Zhu G, Gan Y, Meng F, Yang A, Zhang J. Pallidal Versus Subthalamic Deep-Brain Stimulation for Generalized Isolated Dystonia: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(16):4902. https://doi.org/10.3390/jcm13164902
Chicago/Turabian StyleWu, Jingchao, Guanyu Zhu, Yifei Gan, Fangang Meng, Anchao Yang, and Jianguo Zhang. 2024. "Pallidal Versus Subthalamic Deep-Brain Stimulation for Generalized Isolated Dystonia: A Retrospective Study" Journal of Clinical Medicine 13, no. 16: 4902. https://doi.org/10.3390/jcm13164902
APA StyleWu, J., Zhu, G., Gan, Y., Meng, F., Yang, A., & Zhang, J. (2024). Pallidal Versus Subthalamic Deep-Brain Stimulation for Generalized Isolated Dystonia: A Retrospective Study. Journal of Clinical Medicine, 13(16), 4902. https://doi.org/10.3390/jcm13164902






