Associations between Late Lactate Clearance and Clinical Outcomes in Adults with Hyperlactataemia in the Setting of Diabetic Ketoacidosis
Abstract
1. Introduction
2. Materials and Methods
Data Management and Statistical Analysis
3. Results
Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Predictor | β (95% CI) | p-Value |
|---|---|---|
| Early lactate clearance | +18.23 (+1.03, +35.43) | 0.039 |
| Age (years) | −0.12 (−0.36, +0.13) | 0.355 |
| Female sex | +7.52 (−0.27, +15.31) | 0.059 |
| Co-morbidities | ||
| Renal | +0.16 (−37.44, +37.77) | 0.993 |
| Respiratory | −31.29 (−65.05, +2.47) | 0.062 |
| Cardiovascular | +5.64 (−21.06, +32.35) | 0.679 |
| Frailty score (referenced to 1) | ||
| 2 | −9.74 (−37.85, +18.37) | 0.497 |
| 3 | −23.63 (−52.31, +5.06) | 0.107 |
| 4 | −27.34 (−58.24, +3.56) | 0.096 |
| 5 | −31.99 (−68.02, + 4.05) | 0.102 |
| 6 | −24.09 (−55.04, +6.87) | 0.127 |
| 7 | −38.74 (−86.14, +8.66) | 0.134 |
| 8 | −58.01 (−145.77, +29.76) | 0.195 |
| BMI (kg/m2) | +0.07 (−0.62, +0.77) | 0.839 |
| APACHE-III Score | +0.70 (+0.52, +0.87) | <0.001 |
| Predictor | β (95% CI) | p-Value |
|---|---|---|
| Early lactate resolution | +8.23 (+0.03, +16.43) | 0.049 |
| Age (years) | +1.01 (−0.89, +1.13) | 0.332 |
| Female sex | +13.38 (−12.97, +39.35) | 0.713 |
| Co-morbidities | ||
| Renal | −0.48 (−2.69, +1.74) | 0.681 |
| Respiratory | −14.18 (−25.62, +2.73) | 0.064 |
| Cardiovascular | +10.16 (−8.76, +29.08) | 0.293 |
| Frailty score (referenced to 1) | ||
| 2 | +3.29 (−59.35, +65.95) | 0.918 |
| 3 | +25.39 (−38.11, +88.90) | 0.433 |
| 4 | +9.60 (−57.62, +76.81) | 0.780 |
| 5 | +82.99 (−6.93, +172.91) | 0.070 |
| 6 | +60.16 (−23.45, +143.77) | 0.158 |
| 7 | +13.69 (−2.09, +25.29) | 0.212 |
| 8 | +11.49 (−252.61, +275.58) | 0.932 |
| BMI (kg/m2) | +0.62 (−1.47, +2.71) | 0.561 |
| APACHE-III Score | +1.78 (+1.13, +2.42) | <0.001 |
| Number of Comorbidities | Early Lactate Clearance N = 427 | Late Lactate Clearance N = 84 1 | p-Value 2 |
|---|---|---|---|
| 0 | 119 (27.9%) | 17 (20.2%) | 0.423 |
| 1 | 281 (65.8%) | 62 (73.8%) | |
| 2 | 15 (3.5%) | 5 (6.0%) | |
| 3 | 9 (2.1%) | 0 | |
| 4 | 3 (0.7%) | 0 | |
| 5 | 0 | 0 |
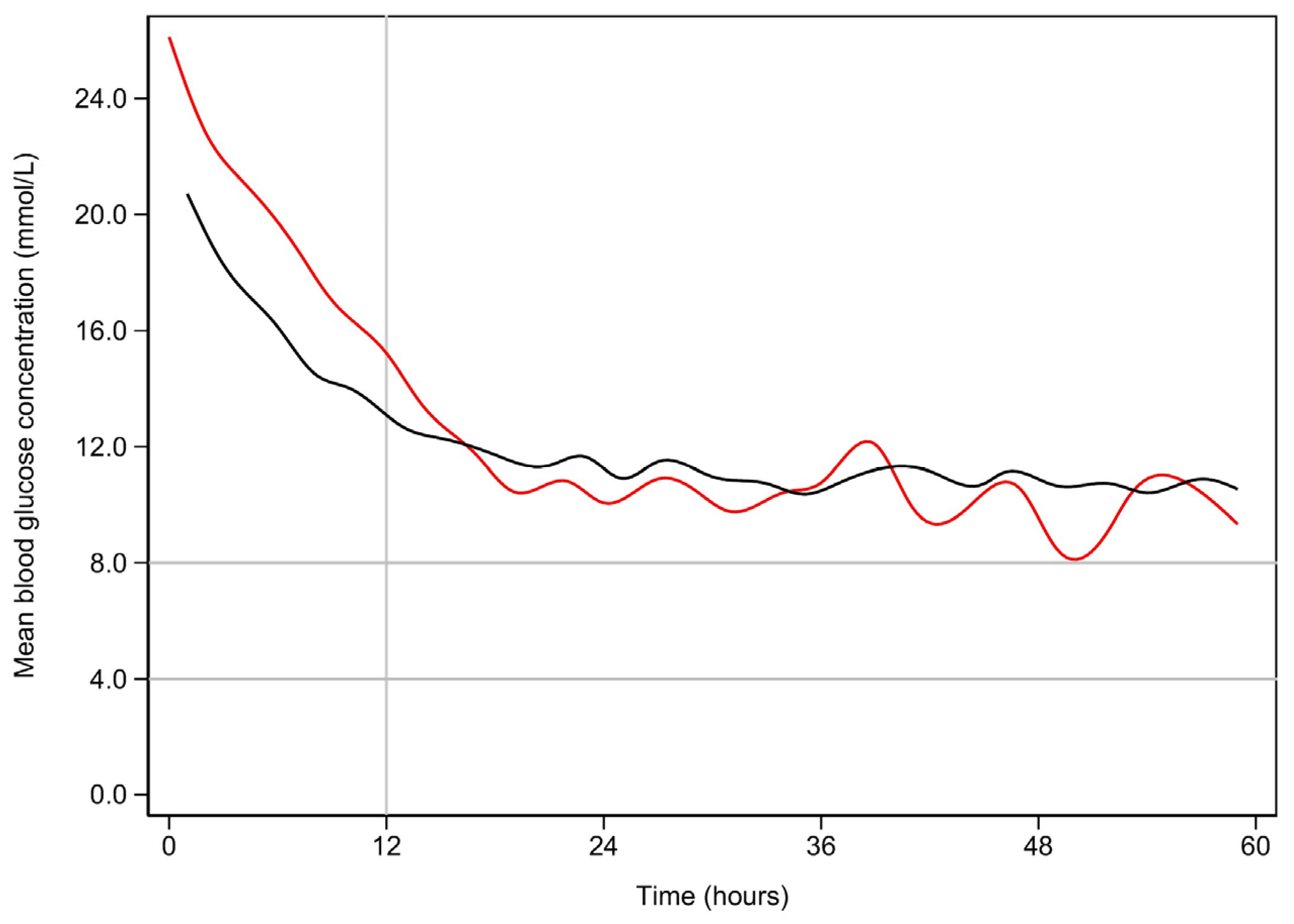

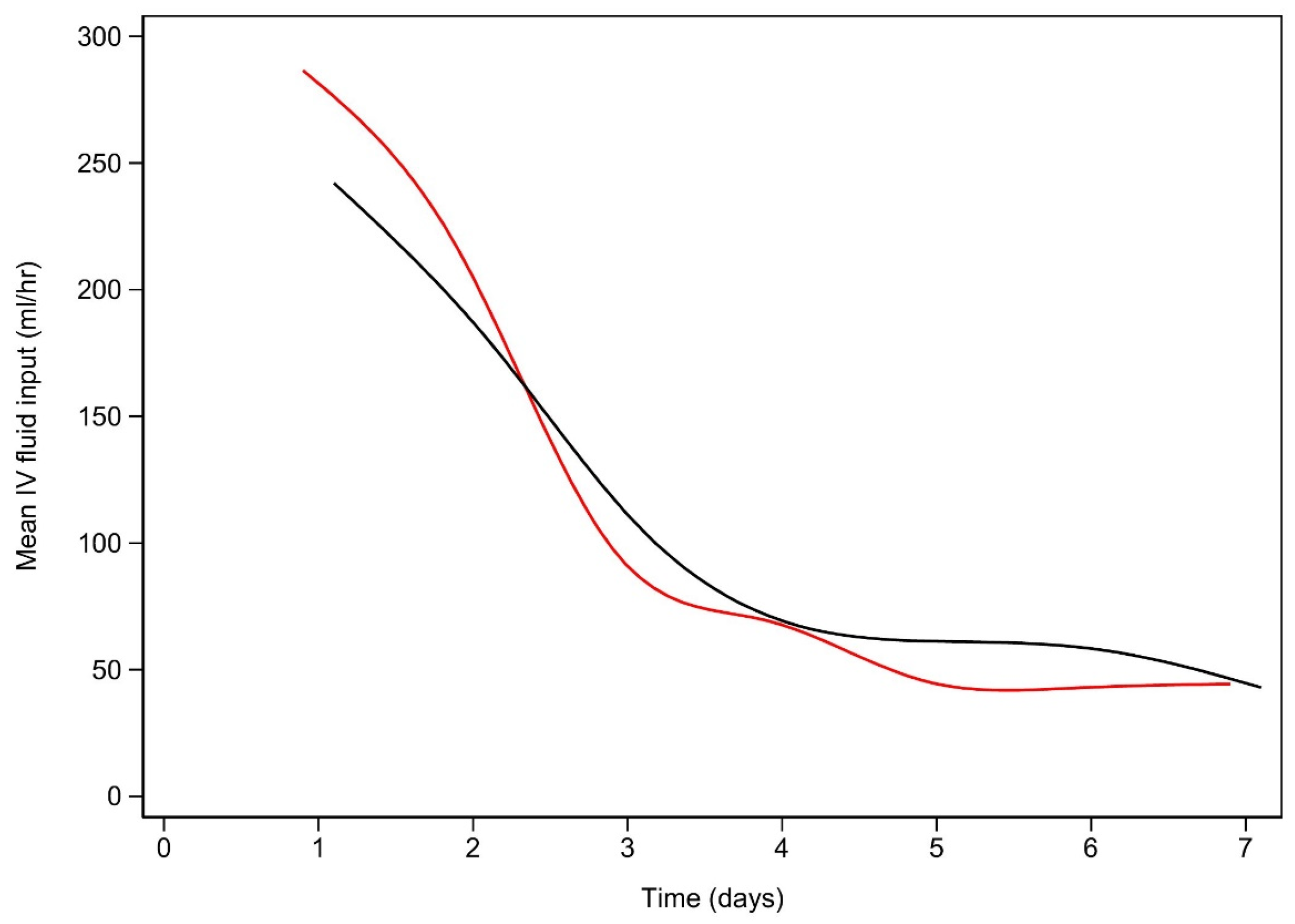
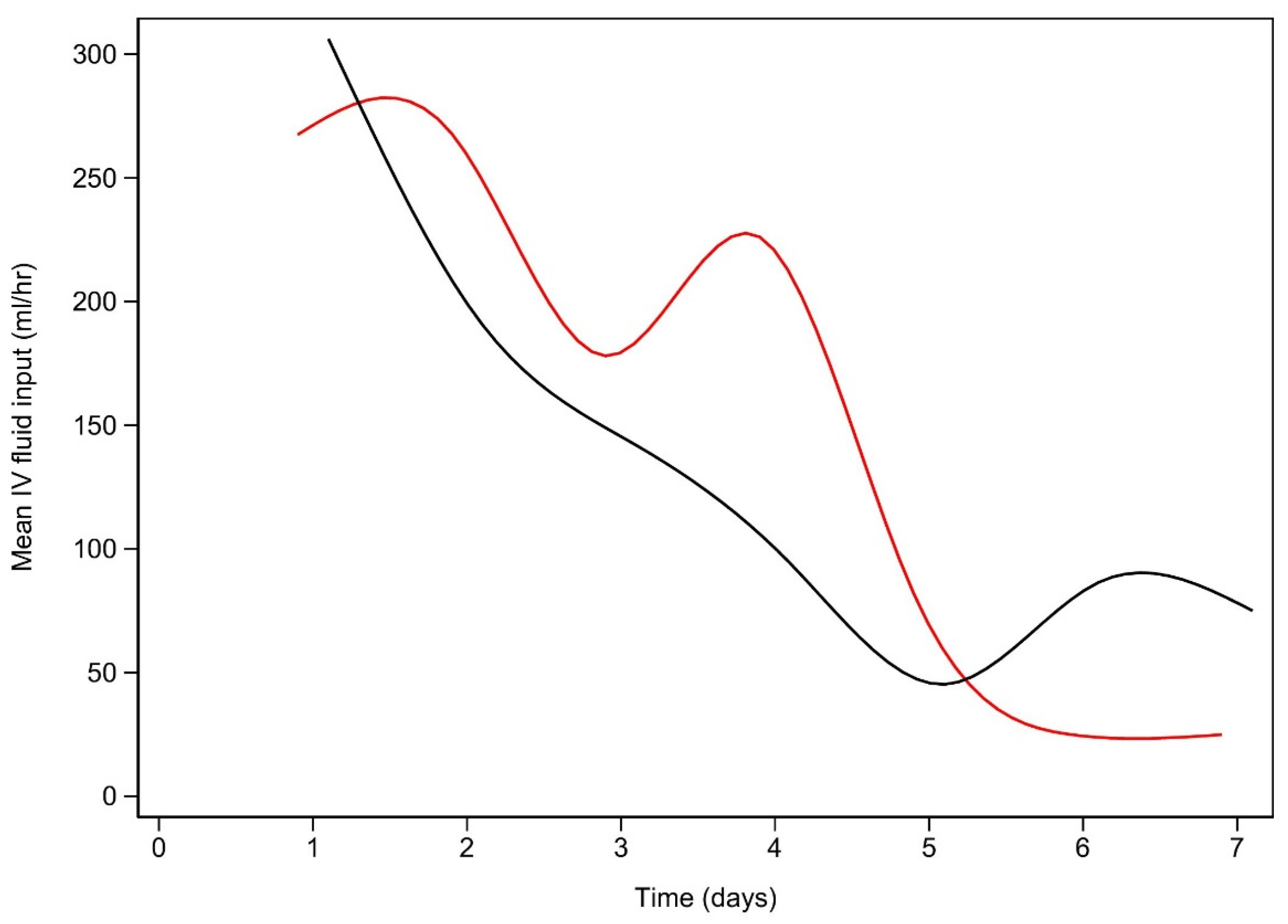
References
- Moskowitz, A.; Berg, K.; Giberson, T.; Graver, A.; Donnino, M. The relationship between lactic acid and thiamine levels in patients with diabetic ketoacidosis. Crit. Care Med. 2012, 40, 70. [Google Scholar]
- Azevedo, L.C.P.; Choi, H.; Simmonds, K.; Davidow, J.; Bagshaw, S.M. Incidence and long-term outcomes of critically ill adult patients with moderate-to-severe diabetic ketoacidosis: Retrospective matched cohort study. J. Crit. Care 2014, 29, 971–977. [Google Scholar] [PubMed]
- Cox, K.; Cocchi, M.N.; Salciccioli, J.D.; Carney, E.; Howell, M.; Donnino, M.W. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J. Crit. Care 2012, 27, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Doola, R.; Zahumensky, A.; Shaikh, A.; Tabah, A.; Laupland, K.B.; Ramanan, M. Association between elevated lactate and clinical outcomes in adults with diabetic ketoacidosis. J. Crit. Care 2023, 78, 154377. [Google Scholar] [CrossRef] [PubMed]
- Siregar, N.N.; Soewondo, P.; Subekti, I.; Muhadi, M. Seventy-Two Hour Mortality Prediction Model in Patients with Diabetic Ketoacidosis: A Retrospective Cohort Study. J. ASEAN Fed. Endocr. Soc. 2018, 33, 124–129. [Google Scholar] [PubMed]
- Ibrahim, A.; Bayramoglu, B.; Hokenek, N.M.; Tekyol, D. Lactate clearance during the first 2 hours after hospital admission: A useful biomarker for predicting 30-day mortality in patients with diabetic ketoacidosis. Int. J. Clin. Pract. 2021, 75, e14204. [Google Scholar] [PubMed]
- Vieira, I.H.; Petrova, M.; Moura, J.P. Does the Same Hyperlactatemia Cut-Off in the Context of Acute Diseases Hold the Same Meaning in Diabetes Mellitus? Cureus 2022, 14, e25163. [Google Scholar] [CrossRef] [PubMed]
- Masharani, U.; Strycker, L.A.; Lazar, A.A.; Wu, K.; Brooks, G.A. Hyperlactatemia in diabetic ketoacidosis. Diabet. Med. 2021, 39, e14723. [Google Scholar]
- Cetin, M.; Kilic, T.Y.; Yesilaras, M.; Uz, I. Clinical utiliy of serum lactate levels in diabetic ketoacidosis in adult patients admitted to emergency department. Ann. Med. Res. 2022, 29, 827–830. [Google Scholar] [CrossRef]
- Moskowitz, A.; Graver, A.; Giberson, T.; Berg, K.; Liu, X.; Uber, A.; Gautam, S.; Donnino, M.W. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J. Crit. Care 2014, 29, 182.e5–182.e8. [Google Scholar] [CrossRef]
- Anwar, A.; Ahmed Azmi, M.; Siddiqui, J.A.; Panhwar, G.; Shaikh, F.; Ariff, M. Thiamine Level in Type I and Type II Diabetes Mellitus Patients: A Comparative Study Focusing on Hematological and Biochemical Evaluations. Cureus 2020, 12, e8027. [Google Scholar] [CrossRef]
- Morgan, T.J.; Scott, P.H.; Anstey, C.M.; Bowling, F.G. Hyperlactatemia in diabetic ketoacidosis is common and can be prolonged: Lactate time-series from 25 intensive care admissions. J. Clin. Monit. Comput. 2021, 35, 757–764. [Google Scholar] [CrossRef]
- Taskin, G.; Yilmaz, M.; Yilmaz, S.; Sirin, H.; Sapmaz, H.; Tasligil, S.; Gunes, I.; Yamanel, L. Lactate kinetics in intensive care unit admissions due to diabetic ketoacidosis. Gulhane Med. J. 2021, 63, 212–217. [Google Scholar] [CrossRef]
- Kaufmann, M.; Perren, A.; Cerutti, B.; Dysli, C.; Rothen, H.U. Severity-Adjusted ICU Mortality Only Tells Half the Truth-The Impact of Treatment Limitation in a Nationwide Database. Crit. Care Med. 2020, 48, e1242–e1250. [Google Scholar] [CrossRef]
- Bhat, J.A.; Masoodi, S.R.; Bhat, M.H.; Bhat, H.; Ahmad, P.O.; Sood, M. Lactic Acidosis in Diabetic Ketoacidosis: A Marker of Severity or Alternate Substrate for Metabolism. Indian J. Endocrinol. Metab. 2021, 25, 59–66. [Google Scholar]
- Liu, J.; Yan, H.; Li, Y. Hyperlactatemia associated with diabetic ketoacidosis in pediatric intensive care unit. BMC Endocr. Disord. 2021, 21, 110. [Google Scholar]
- Graham, J.; Teoh, W.L.; Lockman, K.A. Diabetes ketoacidosis: A marker of poor clinical outcomes? Retrospective study of critical care admissions. Diabet. Med. 2015, 32, 201–202. [Google Scholar]
- Suwarto, S.; Sutrisna, B.; Waspadji, S.; Pohan, H.T. Predictors of five days mortality in diabetic ketoacidosis patients: A prospective cohort study. Acta Medica Indones. 2014, 46, 18–23. [Google Scholar]
- Nunes, R.T.L.; Mota, C.F.M.G.P.; Lins, P.R.G.; Reis, F.S.; Resende, T.C.F.; Barberino, L.A.; da Silva, P.H.L.; de Gois, A.F.T. Incidence, characteristics and long-term outcomes of patients with diabetic ketoacidosis: A prospective prognosis cohort study in an emergency department. Sao Paulo Med. J. 2021, 139, 10–17. [Google Scholar]
- Pattipati, M.; Gudavalli, G.; Dhulipalla, L. The Influence of Obesity Hypoventilation Syndrome on the Outcomes of Patients with Diabetic Ketoacidosis. Cureus 2022, 14, e25157. [Google Scholar] [CrossRef]
- Simpson, A.; Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Morrison, D.S. Comorbidity and survival after admission to the intensive care unit: A population-based study of 41,230 patients. J. Intensive Care Soc. 2021, 22, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Huang, C.H.; Lin, W.R.; Lu, P.L.; Chang, K.; Tsai, J.J.; Bojang, K.S.; Lin, C.Y.; Chen, Y.H. Clinical outcomes of septic patients with diabetic ketoacidosis between 2004 and 2013 in a tertiary hospital in Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 663–671. [Google Scholar] [PubMed]
- Kamata, Y.; Takano, K.; Kishihara, E.; Watanabe, M.; Ichikawa, R.; Shichiri, M. Distinct clinical characteristics and therapeutic modalities for diabetic ketoacidosis in type 1 and type 2 diabetes mellitus. J. Diabetes Its Complicat. 2017, 31, 468–472. [Google Scholar]
- Sato, Y.; Morita, K.; Okada, A.; Matsui, H.; Fushimi, K.; Yasunaga, H. Factors affecting in-hospital mortality of diabetic ketoacidosis patients: A retrospective cohort study. Diabetes Res. Clin. Pract. 2021, 171, 108588. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Hamzaoui, O.; De Vita, N.; Monnet, X.; Teboul, J.L. Vasopressors in septic shock: Which, when, and how much? Ann. Transl. Med. 2020, 8, 794. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Griffin, R.; Judd, S.; Shapiro, N.I.; Safford, M.M. Obesity and risk of sepsis: A population-based cohort study. Obesity 2013, 21, E762–E769. [Google Scholar] [CrossRef] [PubMed]
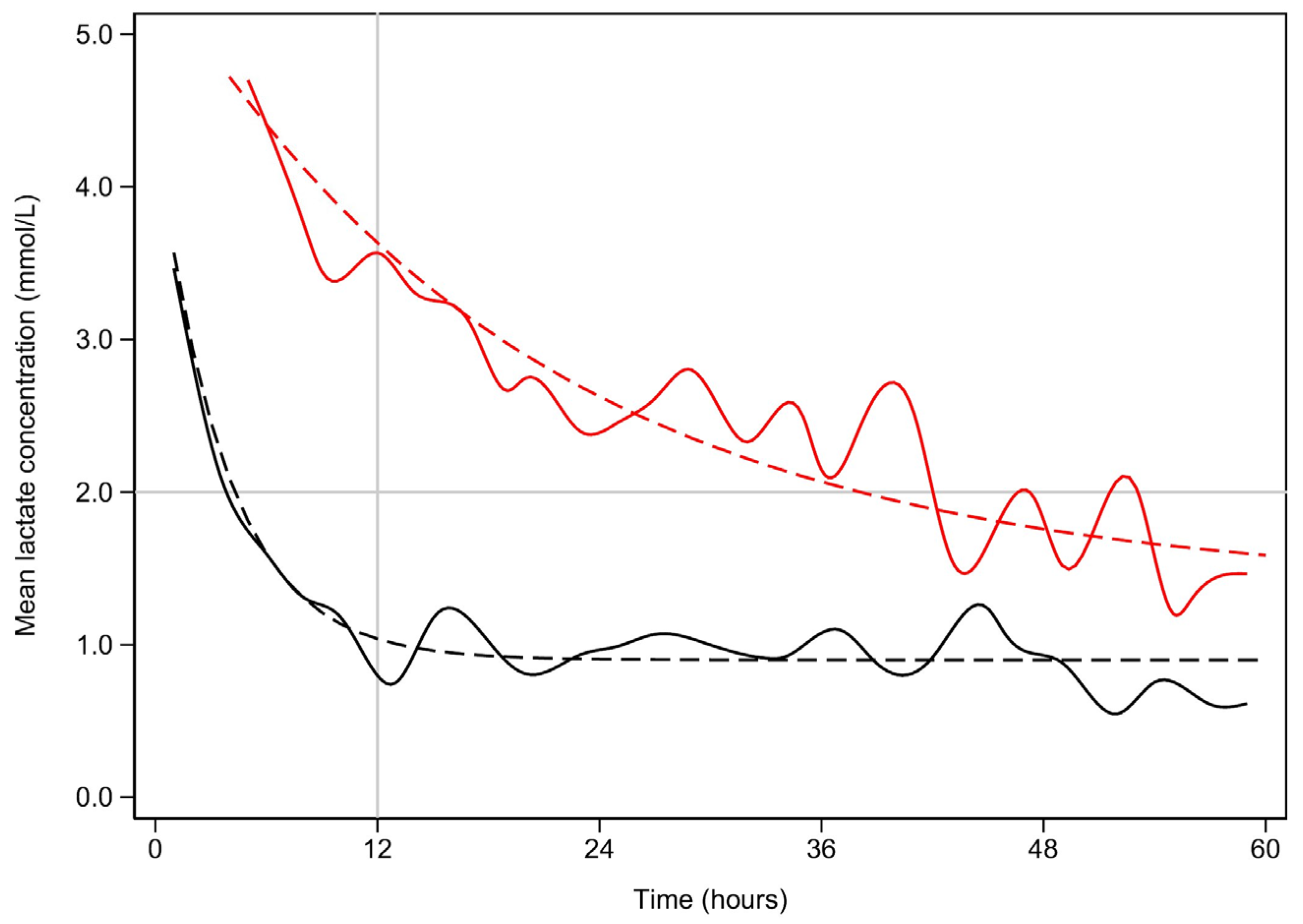
| Variable | Early Lactate Clearance 1 n = 427 | Late Lactate Clearance 1 n = 84 | p-Value 2 |
|---|---|---|---|
| Age (years) | 53 (34, 61) | 49 (30, 59) | 0.301 |
| Male sex | 219 (51.3%) | 27 (32.5%) | 0.011 |
| Comorbidities | |||
| Respiratory | 6 (1.41%) | 2 (2.41%) | 0.501 |
| Cardiovascular | 5 (1.17%) | 1 (1.20%) | 0.981 |
| Renal | 17 (3.98%) | 1 (1.20%) | 0.207 |
| IDDM | 367 (85.9%) | 68 (81.0%) | 0.975 |
| Pressure injury | 8 (1.87%) | 1 (1.82%) | 0.925 |
| Risk of Death Scores | |||
| APACHE-II Score | 21 (16, 25) | 25 (18, 28) | 0.545 |
| APACHE-III Score | 59 (47, 76) | 77 (60, 96) | 0.139 |
| APACHE-III Risk of death (%) | 0.038 (0.019, 0.057) | 0.043 (0.018, 0.068) | 0.176 |
| ANZROD (%) | 0.008 (0.003, 0.013) | 0.011 (0.004, 0.018) | 0.381 |
| Variable | Early Lactate Clearance N = 427 | Late Lactate Clearance N = 84 1 | p-Value 2 |
|---|---|---|---|
| Lactate (mmol/L) | 2.8 (2.2, 3.5) | 5.3 (4.0, 6.7) | <0.001 |
| Electrolytes | |||
| Sodium (Na+) (mmol/L) | 136 (133, 139) | 139 (133, 145) | 0.041 |
| Potassium (K+) (mmol/L) | 4.1 (3.5, 4.5) | 4.0 (3.5, 4.4) | 0.974 |
| Chloride (mmol/L) | 107 (103, 113) | 109 (105, 113) | 0.138 |
| Ionised calcium (mmol/L) | 1.20 (1.13, 1.24) | 1.19 (1.14, 1.26) | 0.539 |
| Anion gap (mmol/L) | 18 (13, 23) | 22 (15, 28) | 0.032 |
| Blood glucose (mmol/L) | 25 (14, 36) | 26 (14, 37) | 0.867 |
| Creatinine (µmol/L) | 95 (65, 123) | 115 (50, 260) | 0.062 |
| Acid-base | |||
| pH | 7.26 (7.15, 7.32) | 7.23 (7.10, 7.31) | 0.663 |
| paCO2 (mmHg) | 21 (14, 27) | 20 (15, 25) | 0.937 |
| paO2 (mmHg) | 104 (78, 123) | 116 (95, 138) | 0.798 |
| Bicarbonate (mmol/L) | 11 (6, 15) | 7 (4, 13) | 0.065 |
| Base Excess (mmol/L) | −13 (−20, −6) | −15 (−22, −11) | 0.102 |
| Oximetry | |||
| Haemoglobin (g/L) | 112 (98, 126) | 115 (99, 128) | 0.851 |
| Oxyhaemoglobin (%) | 96 (93, 97) | 96 (95, 97) | 0.935 |
| Carboxyhaemoglobin (%) | 0.8 (0.3, 1.3) | 0.8 (0.3, 1.2) | 0.946 |
| Methaemoglobin (%) | 0.5 (0.3, 1.0) | 0.5 (0.3, 1.2) | 0.829 |
| Variable | Early Lactate Clearance N = 427 1 | Late Lactate Clearance N = 84 1 | p-Value 2 |
|---|---|---|---|
| Length of stay (hours) | |||
| ICU | 53 (34, 61) | 49 (30, 59) | 0.301 |
| Hospital | 219 (51.3%) | 27 (32.5%) | 0.011 |
| IPPV | 34 (7.9%) | 13 (22.4%) | 0.034 |
| NIV | 7 (1.6%) | 4 (6.9%) | 0.079 |
| Vasopressors | 59 (13.8%) | 25 (43.1%) | <0.001 |
| RRT | 14 (3.3%) | 4 (6.9%) | 0.497 |
| Ventilation hours | |||
| IPPV | 68 (30, 137) | 73 (42, 171) | 0.736 |
| NIV | 5 (1, 8) | 11 (6, 11) | 0.002 |
| Variable | β (95% CI) | p-Value |
|---|---|---|
| ICU Length of Stay | ||
| Late lactate clearance | +15.82 (+0.05, +31.59) | 0.049 |
| APACHE-III Score | +1.05 (+0.88, +1.22) | <0.001 |
| Hospital Length of Stay | ||
| Late lactate clearance | +7.24 (+0.11, +14.37) | 0.048 |
| APACHE-III Score | +3.40 (+2.22, +4.57) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Anstey, C.; Doola, R.; Mcllroy, P.; Whebell, S.; Shekar, K.; Attokaran, A.; Marella, P.; White, K.; Luke, S.; et al. Associations between Late Lactate Clearance and Clinical Outcomes in Adults with Hyperlactataemia in the Setting of Diabetic Ketoacidosis. J. Clin. Med. 2024, 13, 4933. https://doi.org/10.3390/jcm13164933
Kumar A, Anstey C, Doola R, Mcllroy P, Whebell S, Shekar K, Attokaran A, Marella P, White K, Luke S, et al. Associations between Late Lactate Clearance and Clinical Outcomes in Adults with Hyperlactataemia in the Setting of Diabetic Ketoacidosis. Journal of Clinical Medicine. 2024; 13(16):4933. https://doi.org/10.3390/jcm13164933
Chicago/Turabian StyleKumar, Aashish, Christopher Anstey, Ra’eesa Doola, Philippa Mcllroy, Stephen Whebell, Kiran Shekar, Antony Attokaran, Prashanti Marella, Kyle White, Stephen Luke, and et al. 2024. "Associations between Late Lactate Clearance and Clinical Outcomes in Adults with Hyperlactataemia in the Setting of Diabetic Ketoacidosis" Journal of Clinical Medicine 13, no. 16: 4933. https://doi.org/10.3390/jcm13164933
APA StyleKumar, A., Anstey, C., Doola, R., Mcllroy, P., Whebell, S., Shekar, K., Attokaran, A., Marella, P., White, K., Luke, S., Tabah, A., Laupland, K., & Ramanan, M., on behalf of the Queensland Critical Care Research Network (QCCRN). (2024). Associations between Late Lactate Clearance and Clinical Outcomes in Adults with Hyperlactataemia in the Setting of Diabetic Ketoacidosis. Journal of Clinical Medicine, 13(16), 4933. https://doi.org/10.3390/jcm13164933







