Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Macrovascular Assessment
2.4. Microvascular Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar]
- Matheus AS, D.M.; Tannus LR, M.; Cobas, R.A.; Palma CC, S.; Negrato, C.A.; Gomes MD, B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef]
- Haffner, S.M.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998, 339, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef]
- Aikaeli, F.; Njim, T.; Gissing, S.; Moyo, F.; Alam, U.; Mfinanga, S.G.; Okebe, J.; Ramaiya, K.; Webb, E.L.; Jaffar, S.; et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: A systematic review and meta-analysis. PLoS Glob. Public Health 2022, 2, e0000599. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Rubin, J.; Nambi, V.; Chambless, L.E.; Steffes, M.W.; Juraschek, S.P.; Coresh, J.; Sharrett, A.R.; Selvin, E. Hyperglycemia and arterial stiffness: The Atherosclerosis Risk in the Communities study. Atherosclerosis 2012, 225, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, T.Z.; Lee, M.S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc. Imaging 2014, 7, 1025–1038. [Google Scholar] [CrossRef]
- Liao, M.; Chen, S.; Guo, R. Association between carotid ultrasonographic parameters and microvascular and macrovascular complications in diabetes: A systematic review and meta-analysis. J. Diabetes Complicat. 2023, 37, 108554. [Google Scholar] [CrossRef]
- Brohall, G.; Odén, A.; Fagerberg, B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: A systematic review. Diabet. Med. 2006, 23, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2019, 18, 117–124. [Google Scholar] [CrossRef]
- Levy, B.I.; Ambrosio, G.; Pries, A.R.; Struijker-Boudier, H.A.J. Microcirculation in hypertension: A new target for treatment? Circulation 2001, 104, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Cheung, N.; Rogers, S.; Couper, D.J.; Klein, R.; Sharrett, A.R.; Wong, T.Y. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007, 38, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Cheug, N.; Wang, J.J.; Klein, R.; Couper, D.; Sharrett, A.R.; Wong, T.Y. Diabetic Retinopathy and the Risk of Coronary Heart Disease. Diabetes Care 2007, 30, 1742–1746. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Z. Diabetic Retinopathy and Cardiovascular Disease: A Literature Review. Diabetes Metab. Syndr. Obes. 2024, 17, 489–490. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Van Sloten, T.T.; Czernichow, S.; Houben, A.J.; Protogerou, A.D.; Henry, R.M.; Muris, D.M.; Schram, M.T.; Sep, S.J.; Dagnelie, P.C.; Van Der Kallen, C.J.; et al. Association between arterial stiffness and skin microvascular function: The SUVIMAX2 study and the Maastricht study. Am. J. Hypertens. 2015, 28, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Dunn, A.K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 2010, 15, 011109. [Google Scholar] [CrossRef]
- Lazaridis, A.; Triantafyllou, A.; Mastrogiannis, K.; Malliora, A.; Doumas, M.; Gkaliagkousi, E. Assessing skin microcirculation in patients at cardiovascular risk by using laser speckle contrast imaging. A narrative review. Clin. Physiol. Funct. Imaging 2023, 43, 211–222. [Google Scholar] [CrossRef]
- Mahé, G.; Humeau-Heurtier, A.; Durand, S.; Leftheriotis, G.; Abraham, P. Assessment of skin microvascular function and dysfunction with laser speckle contrast imaging. Circ. Cardiovasc. Imaging 2012, 5, 155–163. [Google Scholar] [CrossRef]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef]
- Koletsos, N.; Gkaliagkousi, E.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Dolgyras, P.; DIpla, K.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology 2021, 60, 2834–2841. [Google Scholar] [CrossRef]
- Dolgyras, P.; Lazaridis, A.; Anyfanti, P.; Gavriilaki, E.; Koletsos, N.; Triantafyllou, A.; Nikolaidou, B.; Galanapoulou, V.; Douma, S.; Gkaliagkousi, E. Microcirculation dynamics in systemic vasculitis: Evidence of impaired microvascular response regardless of cardiovascular risk factors. Rheumatology 2023, 62, 2510–2516. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gavriilaki, E.; Dolgyras, P.; Nikolaidou, B.; Dimitriadou, A.; Lazaridis, A.; Mastrogiannis, K.; Koletsos, N.; Triantafyllou, A.; Dimitroulas, T.; et al. Skin microcirculation dynamics are impaired in patients with rheumatoid arthritis and no cardiovascular comorbidities. Clin. Exp. Rheumatol. 2023, 41, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Markus, M.R.P.; Rospleszcz, S.; Ittermann, T.; Baumeister, S.E.; Schipf, S.; Siewert-Markus, U.; Lorbeer, R.; Storz, C.; Ptushkina, V.; Peters, A.; et al. Glucose and insulin levels are associated with arterial stiffness and concentric remodeling of the heart. Cardiovasc. Diabetol. 2019, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Pauling, J.D.; Shipley, J.A.; Hart, D.J.; McGrogan, A.; McHugh, N.J. Use of laser speckle contrast imaging to assess digital microvascular function in primary raynaud phenomenon and systemic sclerosis: A comparison using the raynaud condition score diary. J. Rheumatol. 2015, 42, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Lazaridis, A.; Anyfanti, P.; Stavropoulos, K.; Imprialos, K.; Triantafyllou, A.; Mastrogiannis, K.; Douma, S.; Doumas, M. Assessment of skin microcirculation in primary aldosteronism: Impaired microvascular responses compared to essential hypertensives and normotensives. J. Hum. Hypertens. 2022, 36, 1066–1071. [Google Scholar] [CrossRef]
- Lazaridis, A.; Triantafyllou, A.; Dipla, K.; Dolgyras, P.; Koletsos, N.; Anyfanti, P.; Aslanidis, S.; Douma, S.; Gkaliagkousi, E. Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens. Res. 2022, 45, 445–454. [Google Scholar] [CrossRef]
- de Matheus, A.S.; Clemente, E.L.S.; de Lourdes Guimarães Rodrigues, M.; Torres Valença, D.C.; Gomes, M.B.; Alessandra, A.S.; Clemente, E.L.S.; de Lourdes Guimarães Rodrigues, M.; Torres Valença, D.C.; Gomes, M.B. Assessment of microvascular endothelial function in type 1 diabetes using laser speckle contrast imaging. J. Diabetes Complicat. 2017, 31, 753–757. [Google Scholar] [CrossRef]
- Fuchs, D.; Dupon, P.P.; Schaap, L.A.; Draijer, R. The association between diabetes and dermal microvascular dysfunction non-invasively assessed by laser Doppler with local thermal hyperemia: A systematic review with meta-analysis. Cardiovasc. Diabetol. 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Canto, E.D.; van Deursen, L.; Hoek, A.G.; Elders, P.J.M.; den Ruijter, H.M.; van der Velden, J.; van Empel, V.; Serné, E.H.; Eringa, E.C.; Beulens, J.W.J. Microvascular endothelial dysfunction in skin is associated with higher risk of heart failure with preserved ejection fraction in women with type 2 diabetes: The Hoorn Diabetes Care System Cohort. Cardiovasc. Diabetol. 2023, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Golberg, M.; Califa, R.; Polani, S.; Goldstein, O.; Aviram, Z.; Niska, M.; Zalevsky, Z. Analysis of peripheral arterial disease (PAD) patients by laser speckle measurement techniques. Opt. Express 2022, 30, 18189. [Google Scholar] [CrossRef] [PubMed]
- Mennes, O.A.; Van Netten, J.J.; Van Baal, J.G.; Steenbergen, W. Assessment of microcirculation in the diabetic foot with laser speckle contrast imaging. Physiol. Meas. 2019, 40, 065002. [Google Scholar] [CrossRef] [PubMed]
- Mennes, O.A.; Selles, M.; van Netten, J.J.; van Baal, J.G.; Steenbergen, W.; Slart, R.H.J.A. Semi-automatic tracking of laser speckle contrast images of microcirculation in diabetic foot ulcers. Diagnostics 2020, 10, 1054. [Google Scholar] [CrossRef]
- Care, D.; Suppl, S.S. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Roustit, M.; Millet, C.; Blaise, S.; Dufournet, B.; Cracowski, J.L. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc. Res. 2010, 80, 505–511. [Google Scholar] [CrossRef]
- Roustit, M.; Cracowski, J.L. Non-invasive Assessment of Skin Microvascular Function in Humans: An Insight Into Methods. Microcirculation 2012, 19, 47–64. [Google Scholar] [CrossRef]
- Laurent, S.; Agabiti-Rosei, E. The Cross-Talk Between the Macro- and the Microcirculation. In Early Vascular Aging; Acedemic Press: Cambridge, MA, USA, 2015; pp. 105–116. [Google Scholar]
- Vithian, K.; Hurel, S. Microvascular complications: Pathophysiology and management. Clin. Med. J. R. Coll. Physicians Lond. 2010, 10, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Milman, S.; Crandall, J.P. Mechanisms of Vascular Complications in Prediabetes. Med. Clin. N. Am. 2011, 95, 309–325. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Suwaidi, J.A.; Khalil, C.A. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res. Int. 2017, 1, 7839101. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.Y.; Li, C. Diabetes and hypertension: Is there a common metabolic pathway? Curr. Atheroscler. Rep. 2012, 14, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.S.; Coady, S.A.; Goff, D.C.; Brancati, F.L.; Levy, D.; Selvin, E.; Vasan, R.S.; Fox, C.S. Blood pressure and the risk of developing diabetes in African Americans and Whites: ARIC, CARDIA, and the Framingham Heart Study. Diabetes Care 2011, 34, 873–879. [Google Scholar] [CrossRef]
- Chen, G.; McAlister, F.A.; Walker, R.L.; Hemmelgarn, B.R.; Campbell, N.R.C. Cardiovascular outcomes in framingham participants with diabetes: The importance of blood pressure. Hypertension 2011, 57, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef]

| DM (n = 18) | Control (n = 22) | p Value | |
|---|---|---|---|
| Age (years), mean ± S.D. | 58.6 ± 7.3 | 53.8 ± 8.2 | 0.097 |
| BMI (kg/m2), mean ± S.D. | 30.1 ± 5.3 | 27.7 ± 5.6 | 0.186 |
| Male sex, (%) | 50.0 | 45.5 | 0.775 |
| Smoking yes, (%) | 44.4 | 36.4 | 0.604 |
| Hypertension history, yes (%) | 77.8 | 31.8 | 0.004 |
| Office SBP (mmHg), mean ± S.D. | 130.7 ± 18.9 | 127.1 ± 18.0 | 0.544 |
| Office DBP (mmHg), mean ± S.D. | 81.3 ± 9.1 | 83.1 ± 11.6 | 0.592 |
| Office HR (pulses/min), mean ± S.D. | 74.1 ± 8.2 | 68.9 ± 8.5 | 0.062 |
| Glucose (mg/dL), mean ± S.D. | 130.1 ± 48.4 | 90.5 ± 6.2 | 0.005 |
| HbA1c (%), mean ± S.D. | 6.5 ± 1.0 | 5.4 ± 0.2 | 0.003 |
| Total Cholesterol (mg/dL), mean ± S.D. | 187.2 ± 51.8 | 209.3 ± 41.8 | 0.154 |
| Triglycerides (mg/dL), median (IQR) | 128.0 (73) | 93.0 (44) | 0.096 |
| HDL Cholesterol (mg/dL), mean ± S.D. | 40.3 ± 10.7 | 48.0 ± 12.2 | 0.050 |
| LDL Cholesterol (mg/dL), mean ± S.D. | 116.8 ± 42.2 | 138.2 ± 37.8 | 0.109 |
| DM (n = 18) | Control (n = 22) | p Value | |
|---|---|---|---|
| Supine SBP (mmHg), mean ± S.D. | 130.8 ± 19.8 | 124.3 ± 20.4 | 0.405 |
| Supine DBP (mmHg), mean ± S.D. | 80.8 ± 8.9 | 79.6 ± 13.2 | 0.773 |
| Baseline flux (PU), mean ± S.D. | 41.8 ± 12.6 | 38.5 ± 8.3 | 0.328 |
| Baseline to occlusion change (%), median (IQR) | −81.0 ± 6.3 | −80.1 ± 9.7 | 0.728 |
| Peak flux (PU), mean ± S.D. | 99.7 ± 29.4 | 110.4 ± 25.3 | 0.224 |
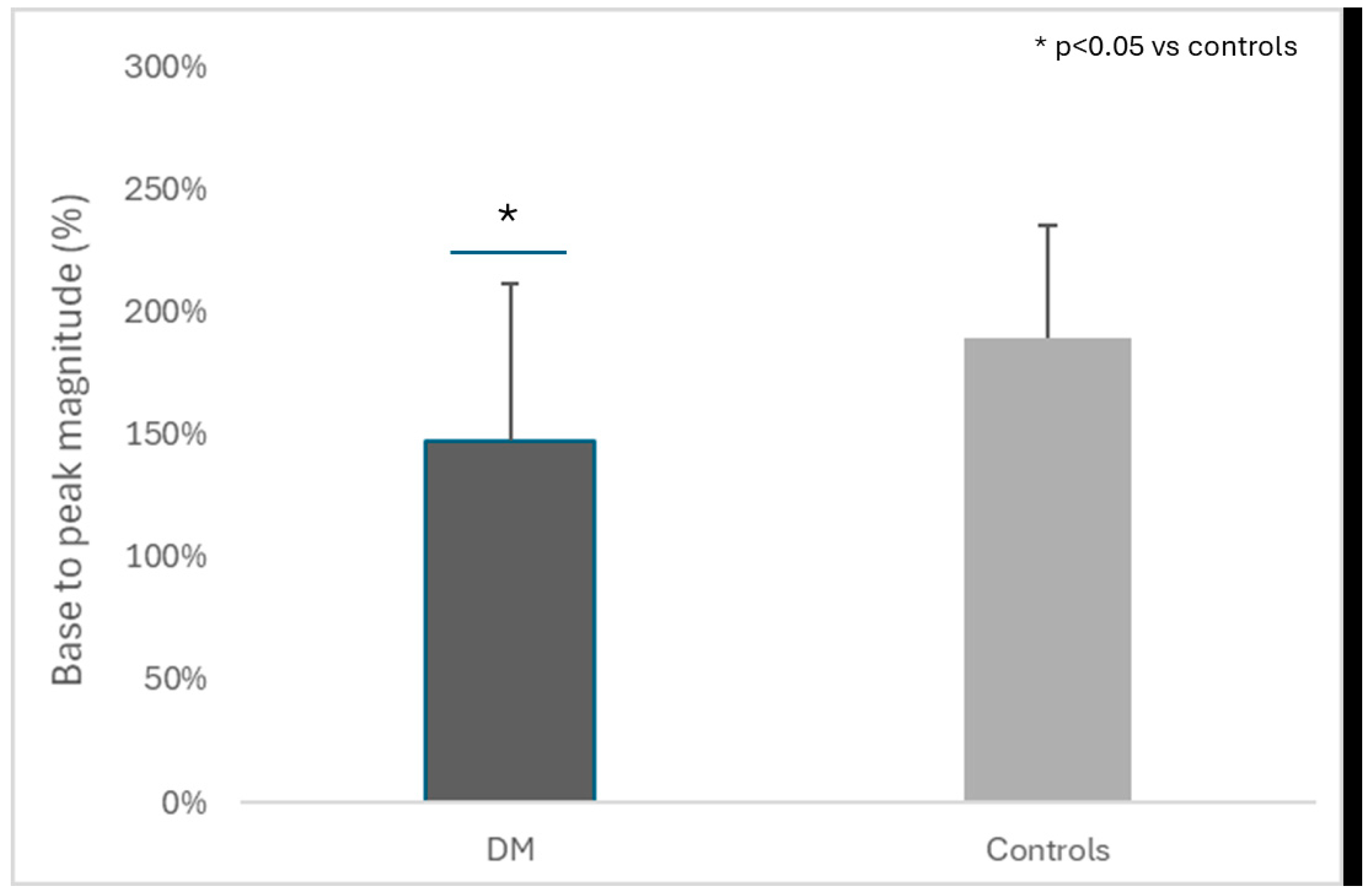
| Peak magnitude (%), mean ± S.D. | 147.0 ± 64.7 | 189.4 ± 46.0 | 0.021 |
| IMT (mm), mean ± S.D. | 0.68 ± 0.09 | 0.60 ± 0.08 | 0.006 |
| Variables | Beta | p Value |
|---|---|---|
| Model 1, Adjusted R2 = 0.263 | ||
| Age (years) | 0.076 | 0.742 |
| Office SBP (mmHg) | −0.334 | 0.038 |
| Glucose levels (mg/dL) | −0.380 | 0.019 |
| Triglycerides (mg/dL) | −0.076 | 0.369 |
| Lipid-lowering drug use | −0.190 | 0.278 |
| Antihypertensive drug use | 0.218 | 0.340 |
| IMT (mm) | −0.146 | 0.573 |
| Model 2, Adjusted R2 = 0.263 | ||
| Age (years) | 0.076 | 0.745 |
| Office SBP (mmHg) | −0.334 | 0.038 |
| Glucose levels (mg/dL) | −0.380 | 0.019 |
| Triglycerides (mg/dL) | −0.083 | 0.698 |
| Lipid-lowering drug use | −0.213 | 0.277 |
| Antihypertensive drug use | 0.210 | 0.371 |
| IMT (mm) | −0.125 | 0.648 |
| DM history | 0.069 | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamprou, S.; Koletsos, N.; Zografou, I.; Lazaridis, A.; Mintziori, G.; Trakatelli, C.M.; Kotsis, V.; Gkaliagkousi, E.; Doumas, M.; Triantafyllou, A. Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness. J. Clin. Med. 2024, 13, 4957. https://doi.org/10.3390/jcm13164957
Lamprou S, Koletsos N, Zografou I, Lazaridis A, Mintziori G, Trakatelli CM, Kotsis V, Gkaliagkousi E, Doumas M, Triantafyllou A. Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness. Journal of Clinical Medicine. 2024; 13(16):4957. https://doi.org/10.3390/jcm13164957
Chicago/Turabian StyleLamprou, Stamatina, Nikolaos Koletsos, Ioanna Zografou, Antonios Lazaridis, Gesthimani Mintziori, Christina Maria Trakatelli, Vasilios Kotsis, Eugenia Gkaliagkousi, Michael Doumas, and Areti Triantafyllou. 2024. "Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness" Journal of Clinical Medicine 13, no. 16: 4957. https://doi.org/10.3390/jcm13164957
APA StyleLamprou, S., Koletsos, N., Zografou, I., Lazaridis, A., Mintziori, G., Trakatelli, C. M., Kotsis, V., Gkaliagkousi, E., Doumas, M., & Triantafyllou, A. (2024). Skin Microvascular Dysfunction in Type 2 Diabetes Mellitus Using Laser Speckle Contrast Analysis and Association with Carotid Intima-Media Thickness. Journal of Clinical Medicine, 13(16), 4957. https://doi.org/10.3390/jcm13164957






