Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment
Abstract
1. Introduction
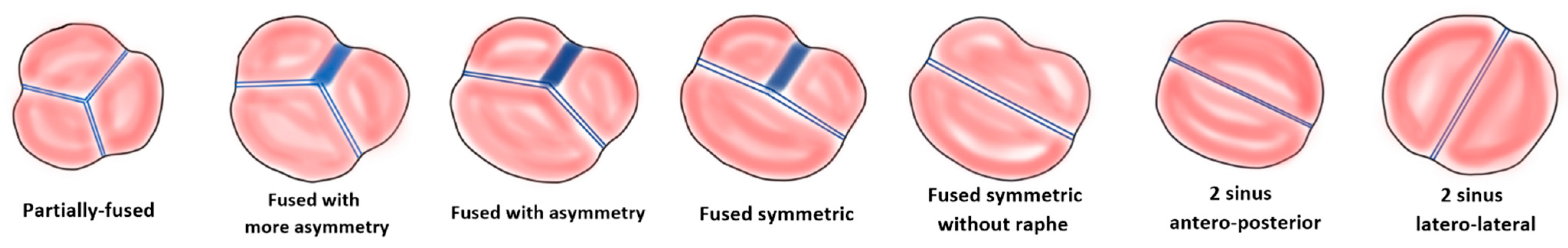
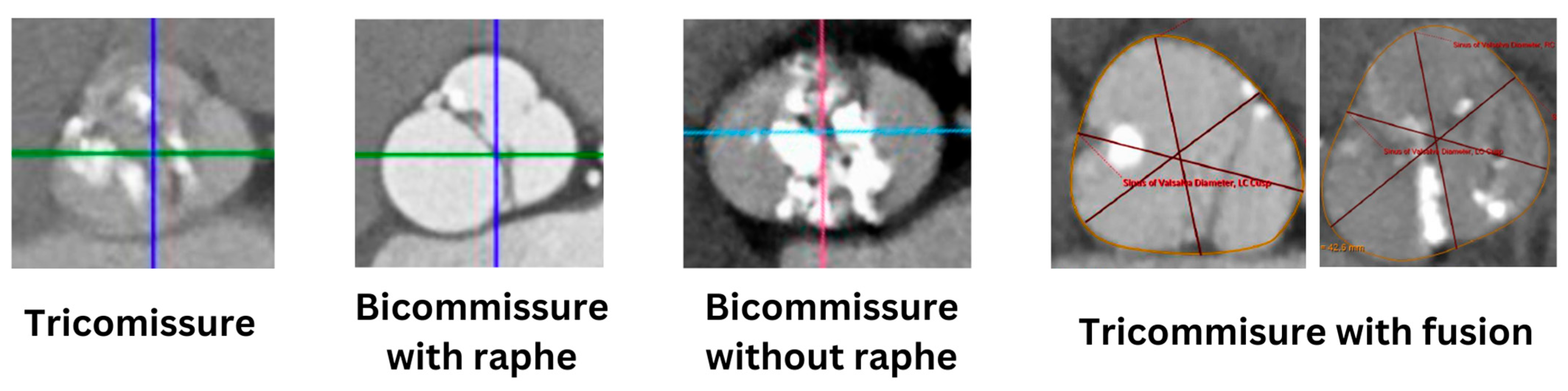
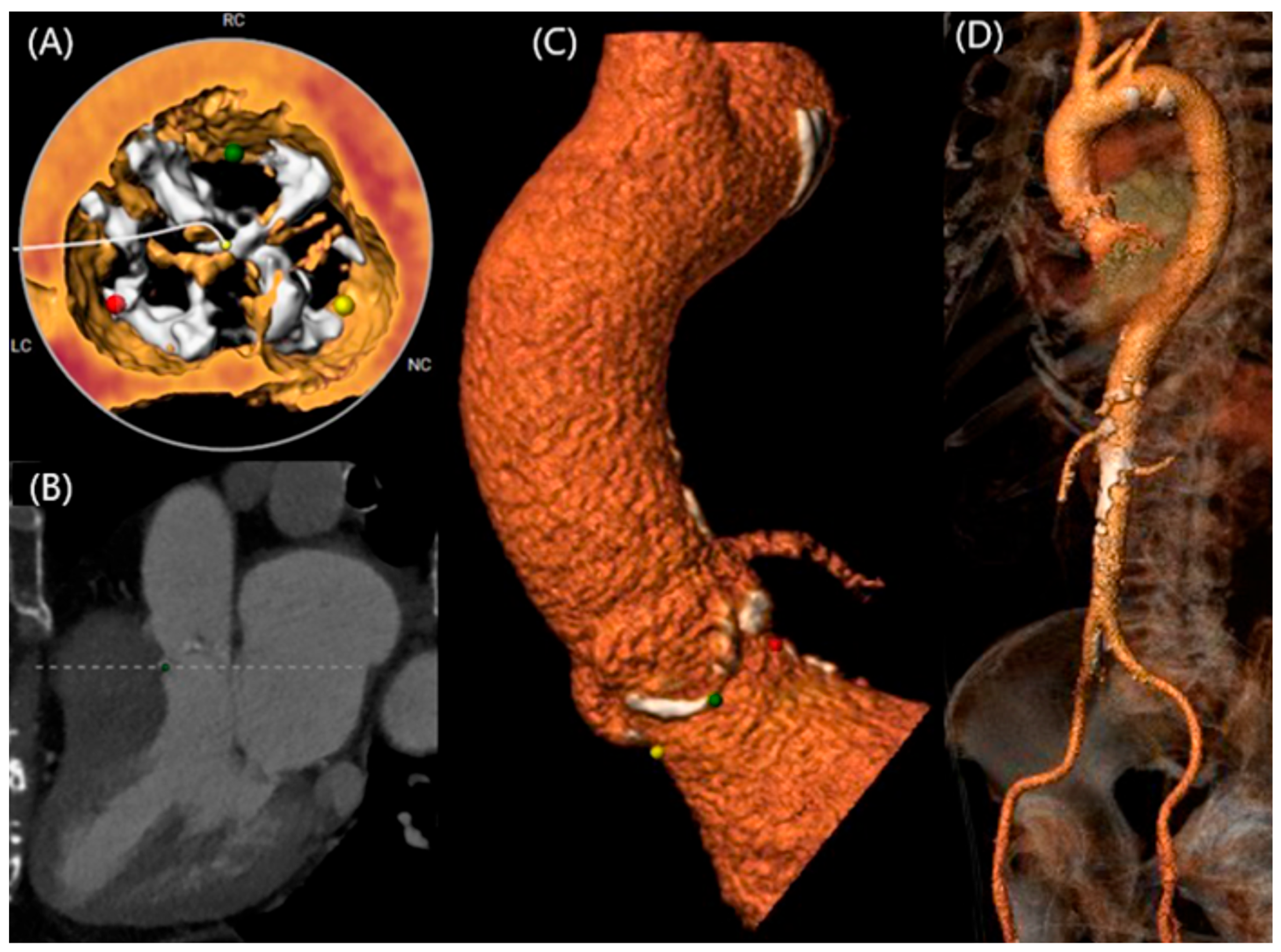
2. Anatomy of Bicuspid Aortic Valve
3. Pathogenesis and Genetic Determinants of Bicuspid Aortic Valve
4. Pathophysiology
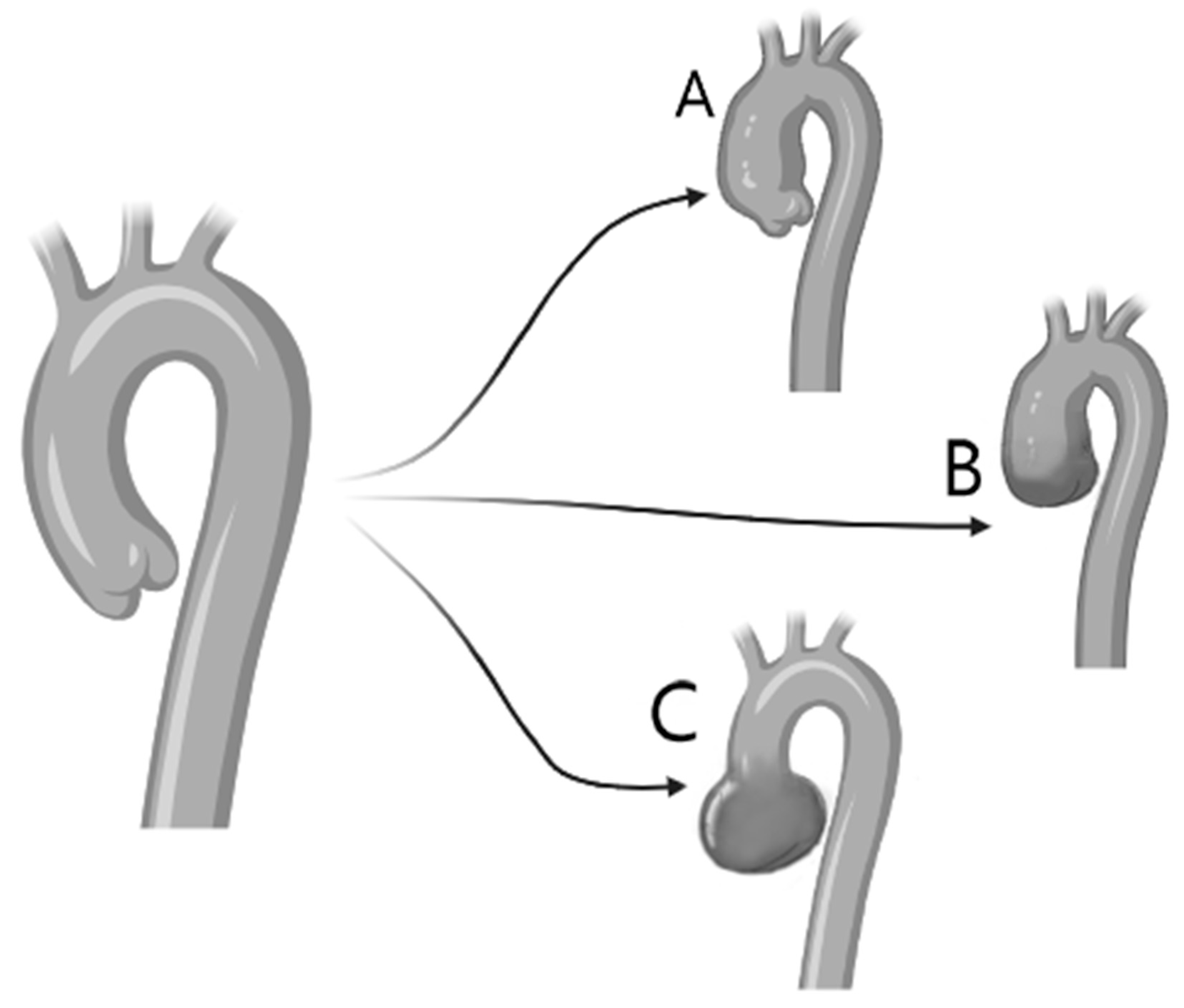
5. Aortic Root Dilatation and Aneurysm Formation
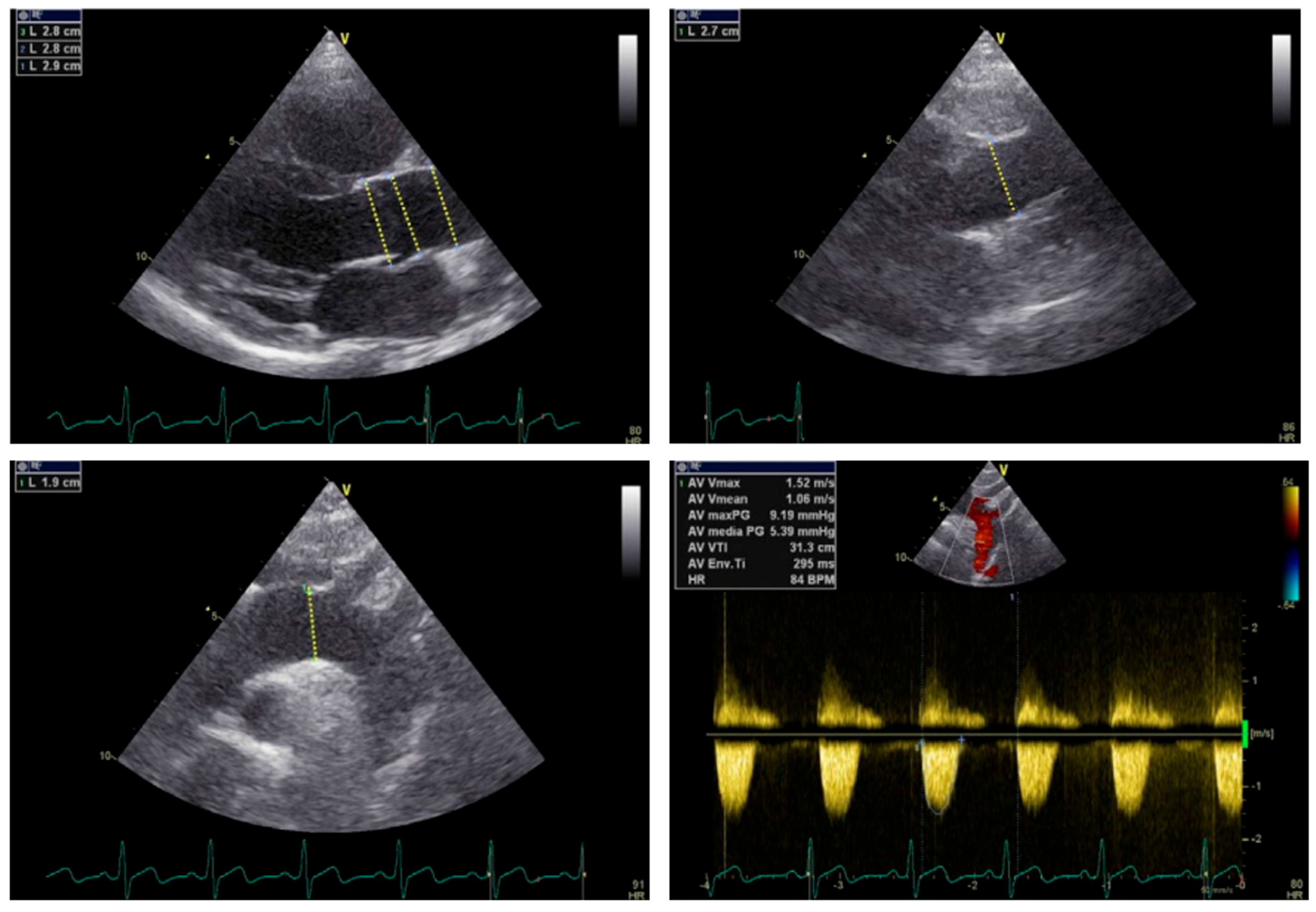
6. Treatment
6.1. Challenges in Transcatheter Interventions
6.2. Transcatheter versus Surgical Replacement
6.3. Comparison between Mechanical and Bioprosthetic Valves
6.4. Suggested Approach to Therapy Selection
7. Future Directions
8. Conclusions
Funding
Conflicts of Interest
References
- Fedak, P.W.M.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; Della, C.A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid aortic valve identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (BAVCON). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- De Kerchove, L.; Mastrobuoni, S.; Froede, L.; Tamer, S.; Boodhwani, M.; Van Dyck, M.; El Khoury, G.; Schäfers, H.-J. Variability of repairable bicuspid aortic valve phenotypes: Towards an anatomical and repair-oriented classification. Eur. J. Cardio-Thorac. Surg. 2019, 56, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur. J. Cardiothorac. Surg. 2021, 60, 448–476. [Google Scholar] [CrossRef] [PubMed]
- Jilaihawi, H.; Chen, M.; Webb, J.; Himbert, D.; Ruiz, C.E.; Rodés-Cabau, J.; Pache, G.; Colombo, A.; Nickenig, G.; Lee, M.; et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, W.K.; Dhoble, A.; Pio, S.M.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Girdauskas, E.; Borger, M.A.; Secknus, M.A.; Girdauskas, G.; Kuntze, T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur. J. Cardiothorac. Surg. 2011, 39, 809–814. [Google Scholar] [CrossRef]
- Tadros, T.M.; Klein, M.D.; Shapira, O.M. Ascending aortic dilatation associated with bicuspid aortic valve: Pathophysiology, molecular biology, and clinical implications. Circulation 2009, 119, 880–890. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef]
- Padang, R.; Bagnall, R.D.; Richmond, D.R.; Bannon, P.G.; Semsarian, C. Rare non-synonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J. Mol. Cell Cardiol. 2012, 53, 277–281. [Google Scholar] [CrossRef]
- Schaefer, B.M.; Lewin, M.B.; Stout, K.K.; Gill, E.; Prueitt, A.; Byers, P.H.; Otto, C.M. The bicuspid aortic valve: An integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008, 94, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Umetani, N.; Hisada, T.; Sugiura, S. Bicuspid aortic valves undergo excessive strain during opening: A simulation study. J. Thorac. Cardiovasc. Surg. 2013, 145, 1570–1576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Rosendael, P.J.; Kamperidis, V.; Kong, W.K.F.; van Rosendael, A.R.; Marsan, N.A.; Bax, J.J.; Delgado, V. Comparison of Quantity of Calcific Deposits by Multidetector Computed Tomography in the Aortic Valve and Coronary Arteries. Am. J. Cardiol. 2016, 118, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, H.; Yoon, S.H.; Chakravarty, T.; Maeno, Y.; Kashif, M.; Israr, S.; Abramowitz, Y.; Mangat, G.; Miyasaka, M.; Rami, T.; et al. Computed tomography characteristics of the aortic valve and the geometry of SAPIEN 3 transcatheter heart valve in patients with bicuspid aortic valve disease. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1408–1418. [Google Scholar] [CrossRef]
- Hope, M.D.; Wrenn, J.; Sigovan, M.; Foster, E.; Tseng, E.E.; Saloner, D. Imaging biomarkers of aortic disease: Increased growth rates with eccentric systolic flow. J. Am. Coll Cardiol. 2012, 60, 356–357. [Google Scholar] [CrossRef]
- Bissell, M.M.; Hess, A.T.; Biasiolli, L.; Glaze, S.J.; Loudon, M.; Pitcher, A.; Davis, A.; Prendergast, B.; Markl, M.; Barker, A.J.; et al. Aortic Dilation in Bicuspid Aortic Valve Disease. Circ. Cardiovasc. Imaging 2013, 6, 499–507. [Google Scholar] [CrossRef]
- Naito, S.; Petersen, J.; Reichenspurner, H.; Girdauskas, E. The impact of coronary anomalies on the outcome in aortic valve surgery: Comparison of bicuspid aortic valve versus tricuspid aortic valve morphotype. Interact Cardiovasc. Thorac Surg. 2018, 26, 617–622. [Google Scholar] [CrossRef]
- Michałlowska, I.M.; Hryniewiecki, T.; Kwiatek, P.; Stokłosa, P.; Swoboda-Rydz, U.; Szymański, P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J. Thorac. Imaging 2016, 31, 156–162. [Google Scholar] [CrossRef]
- Tchetche, D.; De Biase, C.; Van Gils, L.; Parma, R.; Ochala, A.; Lefevre, T.; Hovasse, T.; De Backer, O.; Sondergaard, L.; Bleiziffer, S.; et al. Bicuspid Aortic Valve Anatomy and Relationship With Devices: The BAVARD Multicenter Registry. Circ. Cardiovasc. Interv. 2019, 12, e007107. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Siu, S.C. Aortic dilatation in patients with bicuspid aortic valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar]
- Devereux, R.B.; De Simone, G.; Arnett, D.K.; Best, L.G.; Boerwinkle, E.; Howard, B.V.; Kitzman, D.; Lee, E.T.; Mosley, T.H., Jr.; Weder, A.; et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 2012, 110, 1189–1194. [Google Scholar] [CrossRef]
- Longobardo, L.; Jain, R.; Carerj, S.; Zito, C.; Khandheria, B.K. Bicuspid Aortic Valve: Unlocking the Morphogenetic Puzzle. Am. J. Med. 2016, 129, 796–805. [Google Scholar] [CrossRef]
- Braverman, A.C.; Harris, K.M.; Kovacs, R.J.; Maron, B.J. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 7: Aortic Diseases, Including Marfan Syndrome: A Scientific Statement From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 2398–2405. [Google Scholar]
- Detaint, D.; Michelena, H.I.; Nkomo, V.T.; Vahanian, A.; Jondeau, G.; Sarano, M.E. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: A comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014, 100, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am. J. Cardiol. 1970, 26, 72–83. [Google Scholar] [CrossRef]
- Hope, M.D.; Hope, T.A.; Meadows, A.K.; Ordovas, K.G.; Urbania, T.H.; Alley, M.T.; Higgins, C.B. Bicuspid aortic valve: Four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010, 255, 53–61. [Google Scholar] [CrossRef]
- Russo, C.F.; Cannata, A.; Lanfranconi, M.; Vitali, E.; Garatti, A.; Bonacina, E. Is aortic wall degeneration related to bicuspid aortic valve anatomy in patients with valvular disease? J. Thorac. Cardiovasc. Surg. 2008, 136, 937–942. [Google Scholar] [CrossRef]
- Schaefer, B.M.; Lewin, M.B.; Stout, K.K.; Byers, P.H.; Otto, C.M. Usefulness of bicuspid aortic valve phenotype to predict elastic properties of the ascending aorta. Am. J. Cardiol. 2007, 99, 686–690. [Google Scholar] [CrossRef]
- Plonek, T.; Berezowski, M.; Bochenek, M.; Filip, G.; Rylski, B.; Golesworthy, T.; Jasinski, M. A comparison of aortic root measurements by echocardiography and computed tomography. J. Thorac. Cardiovasc. Surg. 2019, 157, 479–486. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll Cardiol. 2022, 80, e223–e393. [Google Scholar]
- Nardi, F.; Pino, P.G.; De Luca, L.; Riccio, C.; Cipriani, M.; Corda, M.; Francese, G.M.; Gabrielli, D.; Oliva, F.; Gulizia, M.M.; et al. ANMCO position paper: 2022 focused update of appropriate use criteria for multimodality imaging: Aortic valve disease. Eur. Heart J. Suppl. 2022, 24, C289. [Google Scholar] [CrossRef]
- Della Corte, A.; Bancone, C.; Quarto, C.; Dialetto, G.; Covino, F.E.; Scardone, M.; Caianiello, G.; Cotrufo, M. Predictors of ascending aortic dilatation with bicuspid aortic valve: A wide spectrum of disease expression. Eur. J. Cardiothorac. Surg. 2007, 31, 397–405. [Google Scholar] [CrossRef]
- Katsi, V.; Magkas, N.; Antonopoulos, A.; Trantalis, G.; Toutouzas, K.; Tousoulis, D. Aortic valve: Anatomy and structure and the role of vasculature in the degenerative process. Acta Cardiol. 2021, 76, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin III, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll Cardiol. 2021, 77, 450–500. [Google Scholar]
- Neuburger, P.J.; James, L.; Ibrahim, H. Trends and Outcomes of Bicuspid Aortic Valve Stenosis in the TAVI Era. J. Cardiothorac. Vasc. Anesth 2023, 37, 3–5. [Google Scholar] [CrossRef]
- Philip, F.; Faza, N.N.; Schoenhagen, P.; Desai, M.Y.; Tuzcu, E.M.; Svensson, L.G.; Kapadia, S.R. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: Implications for transcatheter aortic valve therapies. Catheter. Cardiovasc. Interv. 2015, 86, E88–E98. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Suh, W.; Fischman, D.L.; Banning, A.; Martinez, S.C.; Potts, J.; Kwok, C.S.; Ratib, K.; Nolan, J.; Bagur, R.; et al. Transcatheter aortic valve replacement outcomes in bicuspid compared to trileaflet aortic valves. Cardiovasc. Revasc. Med. 2019, 20, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, A.; Saad, M.; Elgendy, I.Y.; Barssoum, K.; Omer, M.A.; Soliman, A.; Almahmoud, M.F.; Ogunbayo, G.O.; Mentias, A.; Gilani, S.; et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2019, 12, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Sanaiha, Y.; Hadaya, J.E.; Tran, Z.; Shemin, R.J.; Benharash, P. Transcatheter and Surgical Aortic Valve Replacement in Patients with Bicuspid Aortic Valve Stenosis. Ann. Thorac. Surg. 2023, 115, 611–618. [Google Scholar] [CrossRef]
- Drakopoulou, M.; Oikonomou, G.; Apostolos, A.; Karmpalioti, M.; Simopoulou, C.; Koliastasis, L.; Latsios, G.; Synetos, A.; Benetos, G.; Trantalis, G.; et al. The Role of ECG Strain Pattern in Prognosis after TAVI: A Sub-Analysis of the DIRECT Trial. Life 2023, 13, 1234. [Google Scholar] [CrossRef]
- Vincent, F.; Ternacle, J.; Denimal, T.; Shen, M.; Redfors, B.; Delhaye, C.; Simonato, M.; Debry, N.; Verdier, B.; Shahim, B.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation 2021, 143, 1043–1061. [Google Scholar] [CrossRef]
- Moscarella, E.; Ielasi, A.; Mussayev, A.; Montorfano, M.; Mullassari, A.; Martin, P.; Testa, L.; Jose, J.; Ninios, V.; Toutouzas, K.; et al. Transcatheter valve-in-valve or valve-in-ring implantation with a novel balloon-expandable device in patients with bioprosthetic left side heart valves failure: 1-year follow-up from a multicenter experience. Int. J. Cardiol. 2023, 376, 35–45. [Google Scholar] [CrossRef]
- Ehrlich, T.; de Kerchove, L.; Vojacek, J.; Boodhwani, M.; El-Hamamsy, I.; De Paulis, R.; Lansac, E.; Bavaria, J.E.; El Khoury, G.; Schäfers, H.-J. State-of-the art bicuspid aortic valve repair in 2020. Prog. Cardiovasc. Dis. 2020, 63, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Stathogiannis, K.; Synetos, A.; Latsios, G.; Karanasos, A.; Trantalis, G.; Toskas, P.; Drakopoulou, M.; Xanthopoulou, M.; Karmpalioti, M.; Simopoulou, C.; et al. Long-Term Outcomes and Valve Performance in Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 147, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, I.; Stevens, L.M.; Mazine, A.; Poirier, N.; Cartier, R.; Demers, P.; El-Hamamsy, I. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J. Thorac. Cardiovasc. Surg. 2014, 148, 1341–1346.e1. [Google Scholar] [CrossRef] [PubMed]
- Kvidal, P.; Bergström, R.; Hörte, L.G.; Ståhle, E. Observed and relative survival after aortic valve replacement. J. Am. Coll Cardiol. 2000, 35, 747–756. [Google Scholar] [CrossRef]
- Aicher, D.; Holz, A.; Feldner, S.; Köllner, V.; Schäfers, H.-J. Quality of life after aortic valve surgery: Replacement versus reconstruction. J. Thorac. Cardiovasc. Surg. 2011, 142, e19–e24. [Google Scholar] [CrossRef]
- Drakopoulou, M.; Soulaidopoulos, S.; Oikonomou, G.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Tousoulis, D.; Toutouzas, K. Novel Perspective for Antithrombotic Therapy in TAVI. Curr. Pharm. Des. 2020, 26, 2789–2803. [Google Scholar] [CrossRef] [PubMed]
- Mihaljevic, T.; Nowicki, E.R.; Rajeswaran, J.; Blackstone, E.H.; Lagazzi, L.; Thomas, J.; Lytle, B.W.; Cosgrove, D.M. Survival after valve replacement for aortic stenosis: Implications for decision making. J. Thorac. Cardiovasc. Surg. 2008, 135, 1270–1279.e12. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- Latsios, G.; Toutouzas, K.; Karanasos, A.; Synetos, A.; Drakopoulou, M.; Aggeli, C.; Tousoulis, D. Trans-femoral TAVI: Successful hemostasis of a totally calcified femoral artery (“calcium tube”) with the Manta© device. Hellenic J. Cardiol. 2020, 62, 158–160. [Google Scholar] [CrossRef]
- Poh, C.L.; Buratto, E.; Larobina, M.; Wynne, R.; O’keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. The Ross procedure in adults presenting with bicuspid aortic valve and pure aortic regurgitation: 85% freedom from reoperation at 20 years. Eur. J. Cardiothorac. Surg. 2018, 54, 420–426. [Google Scholar] [CrossRef]
- Halim, S.A.; Edwards, F.H.; Dai, D.; Li, Z.; Mack, M.J.; Holmes, D.R.; Tuzcu, M.E.; Thourani, V.H.; Harrison, J.K.; Brennan, J.M.; et al. Outcomes of Transcatheter Aortic Valve Replacement in Patients with Bicuspid Aortic Valve Disease: A Report From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation 2020, 141, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Okuno, T.; Unbehaun, A.; Mack, M.; Kapadia, S.; Falk, V. Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation? Eur. Heart J. 2022, 43, 2729–2750. [Google Scholar] [CrossRef]
- Borger, M.A.; David, T.E. Management of the valve and ascending aorta in adults with bicuspid aortic valve disease. Semin. Thorac Cardiovasc. Surg. 2005, 17, 143–147. [Google Scholar] [CrossRef]
- Forrest, J.K.; Kaple, R.K.; Ramlawi, B.; Gleason, T.G.; Meduri, C.U.; Yakubov, S.J.; Jilaihawi, H.; Liu, F.; Reardon, M.J. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2020, 13, 1749–1759. [Google Scholar] [CrossRef]
- Makkar, R.R.; Yoon, S.H.; Chakravarty, T.; Kapadia, S.R.; Krishnaswamy, A.; Shah, P.B.; Kaneko, T.; Skipper, E.R.; Rinaldi, M.; Babaliaros, V.; et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs. Tricuspid Aortic Stenosis and Mortality or Stroke Among Patients at Low Surgical Risk. JAMA 2021, 326, 1034–1044. [Google Scholar] [CrossRef]
- Conrotto, F.; D’Ascenzo, F.; Franchin, L.; Bruno, F.; Mamas, M.A.; Toutouzas, K.; Cuisset, T.; Leclercq, F.; Dumonteil, N.; Latib, A.; et al. Transcatheter Aortic Valve Implantation with or Without Predilation: A Meta-Analysis. J. Invasive Cardiol. 2022, 34, E104–E113. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Benetos, G.; Voudris, V.; Drakopoulou, M.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Antonopoulos, A.; Kosmas, E.; Iakovou, I.; et al. Pre-Dilatation Versus No Pre-Dilatation for Implantation of a Self-Expanding Valve in All Comers Undergoing TAVR: The DIRECT Trial. JACC Cardiovasc. Interv. 2019, 12, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Oyama-Manabe, N.; Aikawa, T.; Tsuneta, S.; Manabe, O. Clinical Applications of 4D Flow MR Imaging in Aortic Valvular and Congenital Heart Disease. Magn. Reson. Med. Sci. 2022, 21, 319. [Google Scholar] [CrossRef]
- Bissell, M.M.; Raimondi, F.; Ait Ali, L.; Allen, B.D.; Barker, A.J.; Bolger, A.; Burris, N.; Carhäll, C.-J.; Collins, J.D.; Ebbers, T.; et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J. Cardiovasc. Magn. Reson. 2023, 25, 1–24. [Google Scholar] [CrossRef]
- Costa, G.; Angelillis, M.; Petronio, A.S. Bicuspid Valve Sizing for Transcatheter Aortic Valve Implantation: The Missing Link. Front. Cardiovasc. Med. 2022, 8, 770924. [Google Scholar] [CrossRef]
- Drakopoulou, M.; Toutouzas, K.; Stathogiannis, K.; Latsios, G.; Sideris, S.; Xanthopoulou, M.; Penesopoulou, V.; Trantalis, G.; Synetos, A.; Papanikolaou, A.; et al. Impact of Valve Over-Sizing After Transcatheter Aortic Valve Implantation With a Self-Expanding Valve: A Multislice Computed Tomography Study. J. Invasive Cardiol. 2019, 31, E76–E82. [Google Scholar] [PubMed]
- Costopoulos, C.; Latib, A.; Maisano, F.; Testa, L.; Bedogni, F.; Buchanan, L.; Naganuma, T.; Sticchi, A.; Sato, K.; Miyazaki, T.; et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am. J. Cardiol. 2014, 113, 1390–1393. [Google Scholar] [CrossRef]
- Yoon, S.H.; Bleiziffer, S.; De Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll Cardiol. 2017, 69, 2579–2589. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lefèvre, T.; Ahn, J.M.; Perlman, G.Y.; Dvir, D.; Latib, A.; Barbanti, M.; Deuschl, F.; De Backer, O.; Blanke, P.; et al. Transcatheter Aortic Valve Replacement with Early- and New-Generation Devices in Bicuspid Aortic Valve Stenosis. J. Am. Coll Cardiol. 2016, 68, 1195–1205. [Google Scholar] [CrossRef]
- Koliastasis, L.; Doundoulakis, I.; Kokkinidis, D.G.; Milkas, A.; Drakopoulou, M.; Benetos, G.; Latsios, G.; Synetos, A.; Aggeli, K.; Tousoulis, D.; et al. TAVI with the ACURATE neo transcatheter heart valve in special populations: A systematic review. Hellenic J. Cardiol. 2022, 66, 67–71. [Google Scholar] [CrossRef]
- Koliastasis, L.; Doundoulakis, I.; Kokkinidis, D.G.; Milkas, A.M.; Kostopoulos, G.M.; Drakopoulou, M.; Latsios, G.; Synetos, A.; Benetos, G.; Lampropoulos, K.; et al. Study Level Meta-Analysis of Transcatheter Aortic Valve Implantation With the ACURATE neo Self-Expanding Transcatheter Heart Valve. Cardiol. Rev. 2023, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Sun, B.J.; Park, G.M.; Han, S.; Kim, D.-H.; Song, J.-M.; Kang, D.-H.; Song, J.-K. Aortopathy and bicuspid aortic valve: Haemodynamic burden is main contributor to aortic dilatation. Heart 2012, 98, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, H.K.; Park, J.B.; Lee, S.P.; Koo, B.K.; Kim, Y.J.; Kim, H.S.; Sohn, D.W. Progression of ascending aortopathy may not occur after transcatheter aortic valve replacement in severe bicuspid aortic stenosis. Korean J. Intern. Med. 2021, 36, 332–341. [Google Scholar] [CrossRef]
- Katsaros, O.; Apostolos, A.; Ktenopoulos, N.; Koliastasis, L.; Kachrimanidis, I.; Drakopoulou, M.; Korovesis, T.; Karanasos, A.; Tsalamandris, S.; Latsios, G.; et al. Transcatheter Aortic Valve Implantation Access Sites: Same Goals, Distinct Aspects, Various Merits and Demerits. J. Cardiovasc Dev. Dis. 2023, 11, 4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsaros, O.; Ktenopoulos, N.; Korovesis, T.; Benetos, G.; Apostolos, A.; Koliastasis, L.; Sagris, M.; Milaras, N.; Latsios, G.; Synetos, A.; et al. Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment. J. Clin. Med. 2024, 13, 4970. https://doi.org/10.3390/jcm13174970
Katsaros O, Ktenopoulos N, Korovesis T, Benetos G, Apostolos A, Koliastasis L, Sagris M, Milaras N, Latsios G, Synetos A, et al. Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment. Journal of Clinical Medicine. 2024; 13(17):4970. https://doi.org/10.3390/jcm13174970
Chicago/Turabian StyleKatsaros, Odysseas, Nikolaos Ktenopoulos, Theofanis Korovesis, Georgios Benetos, Anastasios Apostolos, Leonidas Koliastasis, Marios Sagris, Nikias Milaras, George Latsios, Andreas Synetos, and et al. 2024. "Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment" Journal of Clinical Medicine 13, no. 17: 4970. https://doi.org/10.3390/jcm13174970
APA StyleKatsaros, O., Ktenopoulos, N., Korovesis, T., Benetos, G., Apostolos, A., Koliastasis, L., Sagris, M., Milaras, N., Latsios, G., Synetos, A., Drakopoulou, M., Tsalamandris, S., Karanasos, A., Tsioufis, K., & Toutouzas, K. (2024). Bicuspid Aortic Valve Disease: From Pathophysiology to Treatment. Journal of Clinical Medicine, 13(17), 4970. https://doi.org/10.3390/jcm13174970













